Abstract
Background
Inside the body, cells lie in direct contact or in close proximity to other cell types in a tightly controlled architecture that often regulates the resulting tissue function. Therefore, tissue engineering constructs that aim to reproduce the architecture and the geometry of tissues will benefit from methods of controlling cell–cell interactions with microscale resolution.
Scope of the review
We discuss the use of microfabrication technologies for generating patterned co-cultures. In addition, we categorize patterned co-culture systems by cell type and discuss the implications of regulating cell-cell interactions in the resulting biological function of the tissues.
Major conclusions
Patterned co-cultures are a useful tool for fabricating tissue engineered constructs and for studying cell–cell interactions in vitro, because they can be used to control the degree of homotypic and heterotypic cell–cell contact. In addition, this approach can be manipulated to elucidate important factors involved in cell-matrix interactions.
General significance
Patterned co-culture strategies hold significant potential to develop biomimetic structures for tissue engineering. It is expected that they would create opportunities to develop artificial tissues in the future.
Keywords: cell adhesion, cell-cell interaction, co-culture, microfabrication, micropatterning, tissue engineering
1. Introduction
The ability to control the environment of cell culture systems is crucial for in vitro cell function studies and for optimum design of tissue constructs that mimic the organizational complexity of in vivo tissue architectures [1–3]. In vivo, cells integrate and interact with a microenvironment compromised of a milieu of biochemical, biomechanical and bioelectrical signals derived from surrounding cells, extracellular matrix (ECM), and soluble factors. These components vary in both time and space and are integral to the regulation of cellular behaviors.
Cell to cell interactions that occur primarily through direct contact or exchange of soluble factors play an important role in regulating the fate and function of individual cell types in many organ systems. In addition to their role in homeostasis in vivo, intercellular communications are also significant for regenerative processes as well as for in vitro reconstruction of tissues for tissue replacement. The lack of such cell-cell interactions is one potential reason for the loss of functional capabilities of cell types such as hepatocytes outside the body [4]. In tissue culture, much of the native cell-cell interactions present in vivo are lost due to tissue isolation, digestion, and purification of specific cell populations. To address this issue, co-cultures of multiple cell types have been used to better mimic the organization and complexity of the in vivo microenvironment. Traditionally, to study cell-cell interactions in vitro, multiple cell types were seeded on a tissue culture substrate [5–10]. However, it is difficult to control the degree of homotypic and heterotypic cellular interactions using this approach.
Recently, emerging technologies at the interface of engineering and materials science have resulted in a number of new methods to control the various aspects of the cellular microenvironment [1–3, 11–12]. One of the greatest breakthroughs in creating a controlled local cellular environment is the development of patterned co-culture systems. Patterned co-cultures are a useful tool for studying cell-cell interactions and for engineering tissue constructs because they improve control over spatial distribution of cells in culture, allow for the precise manipulation of the degree of homotypic and heterotypic contact, and maintain the function of cell types through the introduction of supportive cells.
In this review, we discuss the use of microfabrication technologies for generating patterned co-cultures. In addition, we categorize patterned co-culture systems by cell type and then discuss cell-cell interactions to be probed in the systems.
2. Tools for generating patterned co-cultures
Traditionally, co-cultures of two or more cell types were generated by randomly seeding the cells on a substrate [5–10]. Random co-culture systems have presented insight into homotypic and heterotypic cell-cell interactions but have been limited by the inability to vary local cell seeding density and the degree of cell-cell contact. To overcome these limitations, micropatterned co-culture systems have been used to enhance the control of spatial localization of multiple cell types relative to each other and to enable detailed mechanistic studies of the processes that regulate cell-cell interactions. In this section, we review several techniques available for generating patterned co-cultures.
2.1 Selective adhesion of cells to micropatterned substrates
Based on the expression levels of adhesion molecules, such as integrins and cadherins, different cell types exhibit different levels of adhesiveness against various surfaces. These differences enable researchers to localize specific cell types to micropatterned regions on a substrate for culturing multiple cell types. By using this concept, Bhatia et al. used photolithography to co-culture hepatocytes and fibroblasts on micropatterned substrates in a controlled manner [4, 13–15]. In this approach, cell-adhesive ECM (e.g., collagen) that mediates the adhesion of the first cell type (i.e. hepatocytes) was patterned by a typical photolithography process. These cells were then allowed to attach and spread to the substrate, and unattached cells were washed out. Finally, secondary cells (i.e. fibroblasts) were seeded and adhered to the unmodified regions of the substrate by nonspecific, serum-mediated attachment.
This technique was used to localize cells efficiently within patterned co-cultures. Furthermore, this approach can be easily implemented since it requires only access to well established techniques such as photolithography. However, despite these advantages, the technique is limited by several issues. First, it depends on the specific cell-cell and cell-substrate adhesiveness of each cell type. For example, the primary cells must adhere weakly to the unmodified region but strongly attach to the patterned regions. Also, secondary cells must adhere to the unmodified region of the substrate and not to the first cell type. Furthermore, the seeding order of cell type, as well as the choice of the matrix materials, is limited.
2.2 Soft lithography-based patterning
Soft lithography, developed by the Whitesides group, is a set of techniques that uses elastomeric stamps made of poly(dimethylsiloxane) (PDMS), with patterned relief features to generate micro- and nanoscale patterns [11–12]. Soft lithographic techniques have been used to generate exquisite control over the deposition of proteins and cells in spatially defined patterns. For example, microcontact printing has been used to regulate cell shape with microscale resolution [16–18], and microfluidic channels have been used to control the spatial and temporal distribution of biomolecules [19–21]. These methods have also been used to generate patterned co-cultures. Even though the examples below involve an extra level of complexity to make PDMS microstructures, the rapid preparation of patterned co-cultures is possible without the need for special equipments or a clean room.
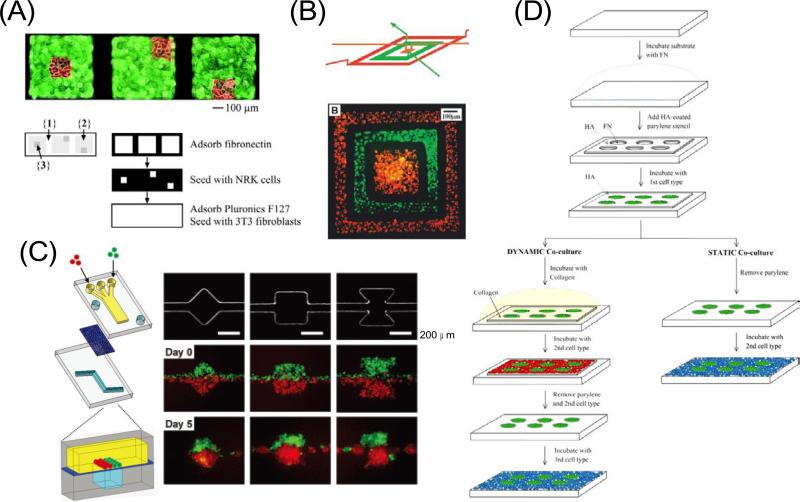
Chen et al. exploited a multilevel PDMS stamp to seed two different cell types in juxtaposition (Figure 1A) [22]. By placing a PDMS stamp on the surface, ECM was adsorbed onto regions of the substrate not blocked by the stamp. When the stamp was pushed further, another level collapsed against the substrate shielding part of the adhesive regions. The first cell type was then seeded through holes in the stamp and allowed to adhere to the unprotected regions. After removing the stamp and blocking regions not coated with ECM, the second cell type was added and attached to the adhesive, but previously obstructed regions.
Figure 1.
Patterned co-cultures based on soft lithographic methods. (A) Patterning two different cell types using a multilevel PDMS stamp. The stamp was placed against a Petri dish masking region 1. Regions 2 and 3 were then coated with fibronectin. When the stamp was pushed against the substrate, the middle level collapsed, shielding region 2. After NRK cells (red) were seeded onto region 3, the stamp was removed, the substrate was immersed in pluronics F127 to render region 1 non-cell-adhesive, and fibroblasts (green) were seeded onto region 2 [22]. Copyright (2002) National Academy of Sciences, U.S.A. (B) Two cell types deposited on a tissue culture dish in a concentric square pattern by using a 3D microfluidic system. These cells were cultured in the channel for 24 hours to grow and spread into a confluent layer and the fluorescence micrograph was taken after the PDMS channel was removed [25]. Copyright (2000) National Academy of Sciences, U.S.A. (C) Compartmentalized microfluidic system for co-culturing spheroids. Two PDMS channel layers were separated by a semi-porous polycarbonate membrane which is rendered resistant to cell adhesion. The top channel was a straight channel with a dead-end. The bottom channel consisted of a straight channel with or without chambers. Cells were introduced into the top channel using multiple laminar flows. Micrographs show the bottom layer geometry and actual cellular patterning. MDA-MB-231 cells (green) and COS7 cells (red), were juxtaposed in the top layer as fluid focuses them together into one channel in the bottom layer. Each type of cell self-aggregated to form multiple spheroids [26]. Reproduced by permission of The Royal Society of Chemistry. (D) Static and dynamic patterned co-cultures using microfabricated parylene-C stencils [30]. Reproduced by permission of The Royal Society of Chemistry.
Microfluidics also offers several approaches to pattern various cell types [23–24]. For example, Whitesides and colleagues used three-dimensional (3D) microfluidic systems to pattern two different cell types in complex, discontinuous structures (Figure 1B) [25]. Since many isolated channels can be contained in the multilayered stamp, multiple cell types can be patterned more easily than through microcontact printing, although the placement of the cell populations in contact with each other is not possible because of the presence of stamp walls separating the compartments. Recently, Takayama et al. reported a microfluidic method to form co-culture spheroids of various geometries and compositions (Figure 1C) [26]. They used a two-layered microfluidic device that sandwiches a semi-porous membrane so that flow occurs from the top channel through the membrane to the bottom channel. Arbitrary cellular arrangement was possible by regulating the geometric features of the bottom channel so that as culture media drains, the flow hydrodynamically focused cells onto the membrane only over the regions of the bottom channel. When the top channel had multiple inlets, cells could be seeded in adjacent laminar streams, allowing different cell types to be patterned simultaneously in well defined spatial arrangements.
PDMS stencils with microengineered holes can also be used to pattern cells to specific regions of a substrate [27–28]. To generate patterned co-cultures, the first cell type is seeded and attached into the holes in the membrane that has been brought into conformal contact with a substrate. The membrane is subsequently removed from the substrate to yield a patterned array of cells. Finally, the second cell type is seeded and adhered to the region not covered by the first cells. A typical problem with PDMS stencils is that they are mechanically weak and difficult to handle. To overcome this issue stencils have been fabricated from other materials such as parylene [29]. Khademhosseini and colleagues used microfabricated parylene membranes to generate static and dynamic co-cultures of multiple cell types, which can manipulate the spatial and temporal cell-cell interactions in tissue culture by changing the cell adhesiveness to parylene membrane surfaces (Figure 1D) [30]. In this system, the top surface of the parylene membrane was pretreated with hyaluronic acid (HA) to decrease nonspecific cell adhesion and the coated membranes were then placed onto a substrate. The first cell type was seeded and only adhered to the substrate through the holes in the membrane. Collagen was then deposited on the parylene membrane to change the surface properties to cell adhesive. Subsequently, the second cell type was seeded on the membrane to form a patterned co-culture. To seed the third cell type, the secondary cells were removed by peeling off the parylene membrane from the substrate. Parylene membranes can be easily removed or attached to a surface due to their mechanical robustness compared to PDMS membranes and form a reversible binding with hydrophobic surfaces. Thus, they could be used for multiple patterning processes.
2.3 Switchable surface-based patterning
Recent advances in the ability to engineer surface properties of substrates have allowed researchers to dynamically modulate the interactions between cells and the substrate surface in real time using external trigger such as light [31–36], voltage [37–42], heat [43–47] and microelectrodes [48–54]. These techniques can be used for the sequential patterning of multiple cell types and control over the adhesion and motility of individual cell types.
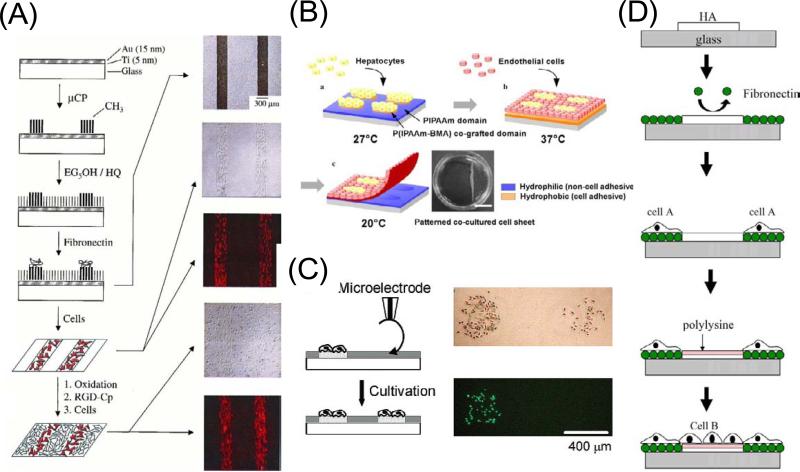
Mrksich et al. developed a monolayer that can be electrically switched to allow the immobilization of cell-adhesive molecules [41–42]. In this process an inert and non-adhesive monolayer of hydroquinone group against a background of tri(ethylene glycol) groups was used. Application of a positive electrical potential promoted the oxidation of the hydroquinone group to the corresponding benzoquinone. The latter is a reactive dienophile and was selectively added to a cyclopentadiene group to form the Diels–Alder adduct. By conjugating the diene to the RGD peptide ligand, they selectively switched on the immobilization of peptide by applying an electrical potential to the surface. This dynamic substrate was used to prepare co-cultures of two different cell types by allowing a first population of cells to attach to a patterned monolayer and then activating a second pattern for attachment of a second population (Figure 2A). Although this technique allows sophisticated control over the molecular composition of the surface, it requires extensive synthesis.
Figure 2.
Patterned co-cultures based on switchable surfaces. (A) An electroactive substrate to pattern two cell populations into a co-culture. Microcontact printing was used to pattern hexadecanethiolate onto a gold substrate. A second monolayer was assembled on the remaining regions of gold by immersing the substrate into a solution of hydroquinone-terminated alkanethiol (HQ) and penta(ethylene glycol)-terminated alkanethiol (EG5OH). The substrate was then immersed in a solution of fibronectin, followed by fibronectin adsorption only to the methyl-terminated regions of the monolayer. Fibroblasts attached only to the regions presenting fibronectin, and when cultured in serum-containing media, divided to fill these regions entirely. The surrounding inert monolayer strictly confined the cell to the rectangular regions. Electrochemical oxidation of the monolayer in the presence of media containing RGD-Cp led to the immobilization of the peptide. Micrographs show that the two cell populations are patterned on the substrate [42]. Copyright (2001) National Academy of Sciences, U.S.A. (B) Patterning co-culture and harvesting of co-cultured cell sheet using thermally responsive surfaces. First cell type was seeded and cultured at 27°C, resulting in localization of the cells onto P(IPAAm–BMA) co-grafted islands showing hydrophobic nature. Second cell type seeded and cultured at 37 °C, resulted in generation of patterned co-cultures. Decreasing temperature to 20 °C induced detachment of co-cultured cell sheet [44]. Reprinted from Biochem. Biophys. Res. Commun, 348, Y. Tsuda, A. Kikuchi, M. Yamato, G. Chen, T. Okano, Heterotypic cell interactions on a dually patterned surface, 937–944, Copyright (2006), with permission from Elsevier. (C) Overwriting cell population on a previously cell-patterned substrate using oxidation by a microelectrode. Optical and fluorescence micrographs of a couple of HeLa cell populations patterned in a stepwise fashion. The population on the left side was first cultured and stained with calcein-AM, followed by the local oxidation treatment to make the population on the right side [53]. Reprinted with permission from H. Kaji, M. Kanada, D. Oyamatsu, T. Matsue, M. Nishizawa, Microelectrochemical approach to induce local cell adhesion and growth on substrates, Langmuir, 20 (2004) 16–19. Copyright 2004 American Chemical Society. (D) Layer-by-layer deposition of ionic biomolecules to generate patterned co-cultures. Reprinted from Biomaterials, 59, A. Khademhosseini, K. Y. Suh, J. M. Yang, G. Eng, J. Yeh, S. Levenberg, R. Langer, Layer-by-layer deposition of hyaluronic acid and poly-l-lysine for patterned cell co-cultures, 3583–3592, Copyright (2004), with permission from Elsevier.
Okano et al. presented a patterned co-culture technique that used thermally responsive polymers [44–47]. In this process, poly(N-isopropylacrylamide) (PIPAAm) was covalently grafted as a thin layer onto tissue culture grade polystyrene dishes by electron beam radiation. Above the lower critical solution temperature (LCST, 32 °C) of PIPAAm, the polymer network collapses making the polymer dehydrated and relatively hydrophobic, thereby becoming cell adhesive. Under its LCST of 32 °C, the polymer is hydrated, and cell attachment is highly suppressed. PIPAAm copolymerized with other monomers can be designed to vary its LCST at which the polymer becomes cell adhesive. By using these features, patterned co-cultures were generated in which a patterned surface of PIPAAm and its copolymer was prepared to seed two cell types at different temperatures (Figure 2B). Since the entire surface became cell repellent at lower temperatures, patterned cells could be removed from the temperature responsive surface in the form of a cell sheet. This feature is of particular importance in clinical applications.
Kaji et al. developed a surface patterning technique based on an electrochemical method, which enables the localized immobilization of cells under physiological conditions [52–53]. This technique used a microelectrode to electrochemically generate an oxidizing agent HBrO, which acts on a heparin- or albumin-coated substrate, initially antibiofouling, to render these regions cell adhesive. Since this technique can be conducted under cell cultivation conditions, it facilitated the stepwise immobilization of multiphenotype cell arrays (Figure 2C) [53] and the in situ directional navigation of cell migration [52, 55]. Also, since the patterning procedure requires only small numbers of electrodes and a small dry battery, it was readily applicable to miniaturized and semiclosed systems such as microfluidic devices [49, 51] and tubing scaffolds [50].
The use of electrostatic interactions has also been applied for generating patterned co-cultures [56–59]. For example, Khademhosseini et al. developed a method that used layer-by-layer deposition of ionic biomolecules to pattern cellular co-cultures. Hyaluronic acid (HA), a biocompatible and biodegradable material, was patterned on a substrate by capillary force lithography, followed by fibronectin (FN) adsorption onto the HA-free region (Figure 2D) [59]. Then, the first cell type was seeded and only attached to the FN coated region. Subsequent ionic adsorption of poly-L-lysine (PLL) to the HA pattern was used to change its surface from cell repulsive to cell adhesive. Finally, the second cell type was seeded and attached to the PLL pattern. In this approach, care must be taken to ensure that solutions of the cell-adhesive electrolyte are not toxic to the first cell type.
2.4 Dielectrophoresis-based pattering
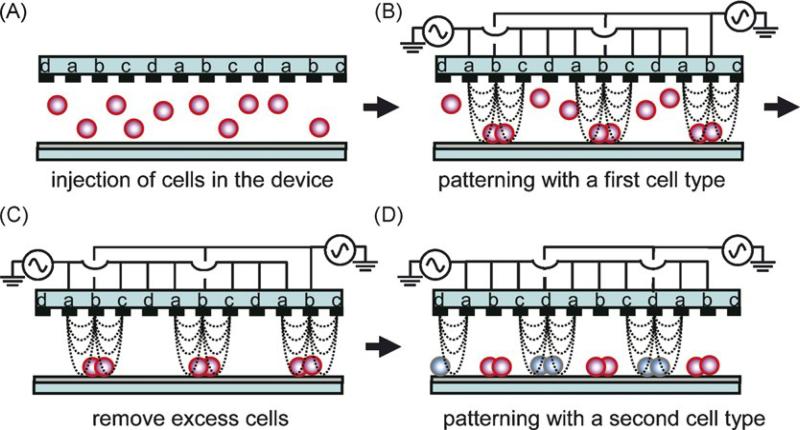
Dielectrophoresis (DEP) is a phenomenon in which particles are manipulated based upon the interactions between a nonuniform electric field and charge polarizations induced in the particles [60]. The movement of particles toward the strong electric field region is referred to as positive dielectrophoresis (p-DEP), and movement in the opposite direction is termed negative dielectrophoresis (n-DEP). DEP-based manipulation techniques have been used for patterning different cell types [61–63]. Matsue et al. demonstrated the fabrication of periodic and alternate cell lines incorporating two cell types of adhesive cells using n-DEP (Figure 3) [63]. An interdigitated array (IDA) electrode with four independent microelectrode subunits was used as a template to form cellular micropatterns. In this system, the n-DEP force was induced by applying an ac voltage (typically 12Vpp, 1 MHz) to direct cells toward a weaker region of electric field strength. After removing excess cells from the device, a second cell type was introduced into the device and, by changing the AC voltage mode, these cells were guided to other areas to form a different pattern.
Figure 3.
Pattering two different cell types based on a dielectrophoretic method. An interdigitated array (IDA) electrode with four independent microelectrode subunits was used as a template to form cellular micropatterns (A). The n-DEP force was induced by applying an AC voltage to direct cells toward a weaker region of electric field strength (B). After removing excess cells from the device (C), a second cell type was introduced into the device and, by changing the AC voltage mode, these cells were guided to other areas to form a different pattern (D) [63]. Reprinted from Biosens. Bioelectron., 24, M. Suzuki, T. Yasukawa, H. Shiku, T. Matsue, Negative dielectrophoretic patterning with different cell types, 1043–1047, Copyright (2008), with permission from Elsevier.
2.5 Mechanically configurable devices
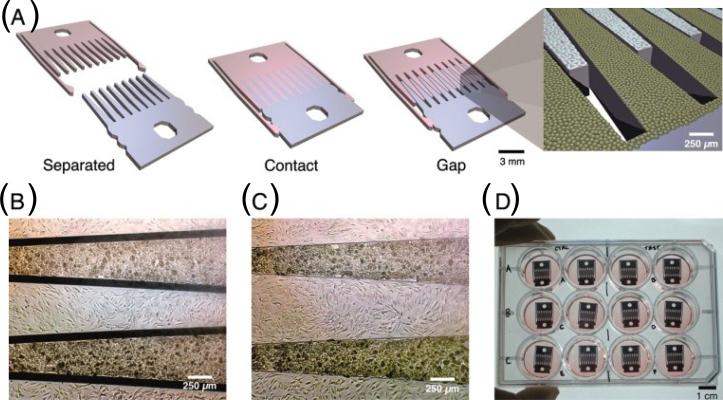
Dynamic manipulation of cell-cell contact has been achieved using mechanically configurable devices [64–65]. Hui and Bhatia developed a technique for the dynamic control of cell–cell adhesion that could affect cellular phenotype (Figure 4) [64]. In this set up, a microfabricated silicon substrate consisting of two interlocking parts was manually manipulated to bring cells in close proximity to each other. The two parts could be joined in discrete configurations such that different types of cells are adjacent to one another or are separated by a micron-scale gap. By culturing hepatocytes and supportive stromal cells on these substrates and by adjusting the placement of the interlocking components, the effects of paracrine and juxtacrine signals could be examined to derive important insight into the nature of these interactions.
Figure 4.
Patterned co-cultures based on a mechanically configurable device. (A) Microfabricated silicon parts can be fully separated (left), locked together with comb fingers in contact (center), or slightly separated (right). (B, C) Micrographs of hepatocytes (darker cells) and 3T3 fibroblasts cultured on the comb fingers. (D) Devices in a standard 12-well plate [64]. Copyright (2007) National Academy of Sciences, U.S.A.
2.6 3D patterned co-cultures
To better mimic the organization and complexity of in vivo tissue structure, a 3D structure containing heterogeneous cell types is desirable. Over the years a number of attempts have been made to control cell-cell interaction in 3D structures.
For example, Okano et al. demonstrated a double-layered co-culture system that used a PIPAAm-grafted thermo-responsive culture dish [66]. In this approach, endothelial cells cultured on the dish were recovered as contiguous cell sheet and placed directly onto a hepatocyte layer. They also fabricated multilayered cultures combining micropatterned endothelial cells as vascular precursors with fibroblast monolayer sheets as tissue matrix [67]. Stratified tissue equivalents were constructed by alternately layering fibroblast monolayer sheets with patterned endothelial cell sheets harvested from thermo-responsive micro-patterned surfaces. Cell culture substrates covalently grafted with different thermo-responsive polymers permitted spatial switching of cell adhesion and detachment using applied small temperature changes. Ito et al. utilized magnetic forces to precisely place magnetically labeled cells onto target cells to control heterotypic cell-cell adhesion in the formation of 3D tissue structures [68]. Magnetite cationic liposomes carrying a positive surface charge accumulated in endothelial cells. Subsequently, endothelial cells specifically accumulated onto hepatocyte monolayer at sites where a magnet was positioned, the adhered to form a heterotypic layered construct with tight and close contact.
Researchers have begun adopting hydrogels as platforms for generating patterned co-cultures as both natural and artificial hydrogels are 3D and have some properties that are more similar to tissues than two-dimensional (2D) substrates. Whitesides et al. demonstrated a method to control spatial distribution of multiple types of cells within 3D matrices of a biologically derived, thermally curable hydrogel, Matrigel [69]. They used laminar flow to divide a microchannel into multiple subchannels separated by microslabs of hydrogel. Bhatia et al. fabricated a 3D hepatic tissue construct embedding hepatocytes in poly(ethylene glycol) (PEG) hydrogel structures using a multilayer photolithography platform [70]. They also presented a method for the rapid formation of 3D cellular structure within a photopolymerizable PEG hydrogel using DEP forces [71]. In this system, cells were micropatterned via DEP forces, and each single hydrogel layer was incorporated into multilayer constructs for co-cultures.
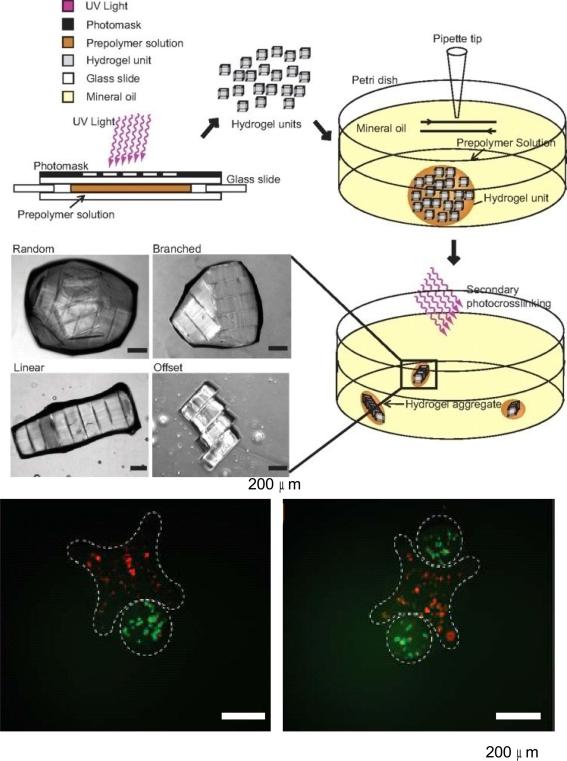
Directed assembly of microengineered gels has also been proposed as a method of controlling cell-cell interactions in 3D constructs. For example, Khademhosseini et al. presented a bottom-up approach to direct the assembly of cell-laden hydrogels to generate tissue constructs with tunable microarchitecture and complexity (Figure 5) [72]. The assembly process was driven by the tendency of multiphase liquid-liquid systems to minimize the surface area and the resulting surface free energy between the phases. First, cell-laden rectangular hydrogels were created in different aspect ratios through photopolymerization with a photomask. These microgels were then collected in hydrophobic mineral oil causing the hydrophilic hydrogels to aggregate together to form tissues of varying dimensions, which could then be solidified through a secondary UV polymerization step. More intricate structures were demonstrated with lock and key shapes, indicating the potential versatility of this technique.
Figure 5.
3D patterned co-cultures based on directed assembly of cell-laden hydrogels. Cell-laden rectangular hydrogels were created directly by photopolymerization using UV through a photomask, then allowed to aggregate and self-assemble in a hydrophobic media. Fluorescence images show microgel assembly composed of cross-shaped gels containing red-staining cells and rod-shaped gels containing green-stained cells [72]. Copyright (2008) National Academy of Sciences, U.S.A.
3. Characterization of cell-cell interactions in patterned co-cultures
Cells maintain both homotypic and heterotypic interactions with the surrounding cells in the body. For this reason, micropatterned co-cultures potentially produce biomimetic environments for cell growth in tissue engineering applications [24]. Cell-cell interactions can be determined by controlled micropatterned co-culture systems in vitro [73, 74]. In addition, this approach can be manipulated to elucidate important factors involved in cell-matrix interactions [56]. Culturing multiple types of cells (Table 1) in controlled micropatterns may also be a useful aspect of designing tissues, which could be utilized to closely mimic the natural organs in the body for a variety of biomedical applications [1].
Table 1.
Types of cells and cell sources in patterned co-culture systems.
| Type of cells | Cell source for cocultures | Reference |
|---|---|---|
| Primary human skin fibroblasts-Primary HUVEC | 80 | |
| Murine embryonic fibroblasts-Human embryonic stem cells (hESC) | 81 | |
| Connective tissue cells | ||
|
| ||
| AML12 murine hepatocytes-NIH 3T3 murine fibroblasts | 56 | |
| Lewis rat hepatocytes-NIH 3T3 J2 murine fibroblasts | 13,14,15,75,79 | |
| Sprague-Dawley rat hepatocytes-NIH 3T3 murine fibroblasts | 77 | |
| Primary hepatocytes-NIH 3T3 J2 fibroblasts | 78 | |
| Sprague-Dawley rat hepatocytes-Human aortic endothelial cells HAEC | 68 | |
| Hepatocytes from Wister rats-Human fetal lung fibroblasts TIG-1 | 46 | |
| Liver cells | ||
|
| ||
| Neural cortical embryonic stem cells-NIH3T3 murine fibroblasts | 82 | |
| Neural progenitor cells-Brain-derived immortalized microvascular endothelial cells | 83 | |
| Neurons-Rat cortical astrocytes | 84 | |
| Neural cells | ||
|
| ||
| Human cervix epithelial cells (HeLa)-Human umbilical vein endothelial cells (HUVEC) | 65 | |
| Human osteoblastic MG-63 cells-Macrophages from human U937 cells | 85 | |
| Rat mammary adenocarcinoma cells ((MTLn3 cancer cell line)-Human dermal microvascular endothelial cells (HMVEC) | 86 | |
| Human dermal microvascular endothelial cells (HMVEC)-Mouse smooth muscle cells (10T 1/2) | 86 | |
| Murine macrophage-like cells (BAC1.2F5)-Murine macrophage-like cells (LADMAC) | 69 | |
| Other cells | ||
|
| ||
| Human lung fibroblasts (IMR-90)-Human umbilical vein smooth muscle cells (HUVSMC)-Human umbilical vein endothelial cells (HUVEC) | 87,88 | |
| AML12 Mouse hepatocytes-Mouse NIH-3T3-Murine embryonic | 30,60 | |
| Three or more cell types | stem ESCs (R1 strain) | |
Here, we will discuss the biological implications of cell-cell interactions in co-cultured systems. Moreover, we will give specific examples for different types of cells in micropatterned environments.
3.1 Hepatocyte containing co-cultures
Liver is a unique organ, which is responsible for a number of functions, such as, glucose metabolization, detoxification, urea synthesis and secretion. However, despite the capacity of the liver in the body to significantly regenerate itself, in vitro maintenance of hepatocytes has been difficult. Stabilization and maintenance of liver-specific function has been shown to be improved by co-culturing hepatocytes along with other type of cells [13, 14].
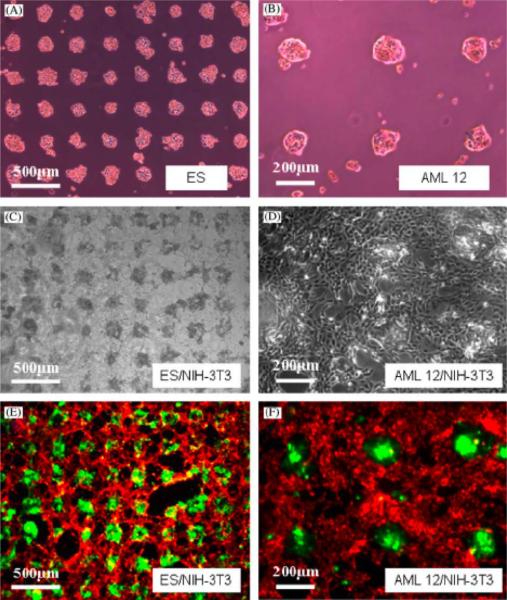
Co-cultures of fibroblasts with hepatocytes can be potentially useful in the development of artificial liver systems [75]. For example, Bhatia et al. co-cultured mouse hepatocytes and fibroblasts to enhance homotypic and heterotypic interactions related to liver function using the system explained in Section 2.1 [14]. Proximity of hepatocytes to fibroblasts as well as the amount of initial heterotypic interactions are shown to be important factors that affected the liver specific function in these micropatterned structures. This was demonstrated by changing the degree of heterotypic cell-cell contact in the co-cultures while keeping the ratio of the two cell types constant [15]. Another study carried out by Yamato et al. [46] showed that patterned co-cultures of primary rat hepatocytes and human diploid lung fibroblasts could be used to mimic native tissues. Control over cell adhesion was achieved by using thermally responsive polymers, which allowed maintenance of individual patterns while promoting confluent co-cultures. Other groups also have studied controlling heterotypic interactions in micropatterned co-cultures of rat hepatocytes and mouse fibroblasts, and reported that the liver function was preserved [76–79]. For instance, in order to find out the effects of heterotypic interactions on liver function, layered co-cultures of rat hepatocytes and human aortic endothelial cells (HAECs) were generated [68]. The enhancement in the liver function was determined by the increase in albumin expression levels for hepatocytes within this co-culture system. Another example for controlling heterotypic cellular interactions has been performed by Fukuda et al. who co-cultured either primary mouse hepatocytes or embryonic stem (ES) cells with mouse fibroblasts (Figure 6) [56]. Controlling such cellular interactions in co-cultivated systems can be useful in understanding cell-ECM interactions and cell-cell communication processes.
Figure 6.
Patterned mono and co-cultures on HA/collagen surfaces. Adhesion of (A) ES cells, (B) mouse hepatocytes on FN coated areas on HA-patterned surface after 8 h incubation period. (C) ES aggregates formed by mouse fibroblasts on collagen treated HA-patterned surface (containing previously attached hepatocytes) after 3 day incubation. (D) Co-cultivated hepatocytes and fibroblasts. (E) Fluorescent images for co-culture of ES cells/fibroblasts and (F) co-culture of hepatocytes/fibroblasts at day 3 [56]. Reprinted from Biomaterials, 27, J. Fukuda, A. Khademhosseini, J. Yeh, G. Eng, J. Cheng, O.C. Farokhzad, R. Langer, Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components, 1479–1486, Copyright (2006), with permission from Elsevier.
Temporal control of cell-cell interactions is another important parameter for improving liver tissue function. As an example, dynamic co-cultures of hepatocytes and fibroblasts were utilized to control the cell-cell interactions with the system given in Section 2.5 [64]. Soluble signaling was modulated by changing the distance between comb-shaped plates and liver specific function was quantitatively indicated by secretion of albumin from hepatocytes. Initial cell contact was shown to be a significant factor for maintaining hepatocyte function.
3.2 Fibroblast containing co-cultures
Fibroblasts are commonly found in connective tissues providing mechanical support for the structure. For this reason, they are important in tissue development and remodeling. For instance, human fibroblasts can potentially direct the differentiation of endothelial cells into capillary architectures. As an example Bianchi et al. [80] co-cultured human fibroblasts together with transfected primary endothelial cells obtained from HUVEC cells, which led to an enhancement in angiogenesis. This was achieved by controlling the surface topology and heterotypic interactions, which enhanced the metabolic activity of cells.
In another example, co-cultures of human embryonic stem (hES) cells and mitotically inactivated murine embryonic fibroblasts (MEF) were prepared to keep hES cells in undifferentiated state [81]. Expressions of octamer binding protein 4 (Oct-4) and alkaline phosphatase (ALP) were measured to determine differentiation state of hES cells. Controlling the cluster size of the hESs and their localization provided more homogeneous interactions for differentiation of co-cultured cells.
3.3 Neural cell containing co-cultures
Neural cells are responsible for electrochemical signaling processes in the nervous system. When co-cultivated with endothelial cells, proliferation of neural stem cells is stimulated and their undifferentiated state is preserved. As an example, neural stem cells were co-cultured with vascular endothelial feeder cells to observe the effects on differentiation into neural cells [82]. Neural progenitor markers LeX and Nestin were found to be expressed by neural ES cells when co-cultured with endothelial cells. This co-culturing strategy also encouraged self-renewal of neural stem cells in the mixture.
In addition to promoting neural differentiation, co-cultures of neural cells and endothelial cells can possibly induce vascularization. For instance, Ford et al. created microvasculature structures by co-cultures of neural progenitor cells and endothelial cells in vivo [83]. In this study, cell-cell interactions were controlled by stabilizing microvascular networks utilizing a co-culturing strategy. Neural progenitor cells encouraged the formation of endothelial tubular structures in microvascular networks for proper circulation.
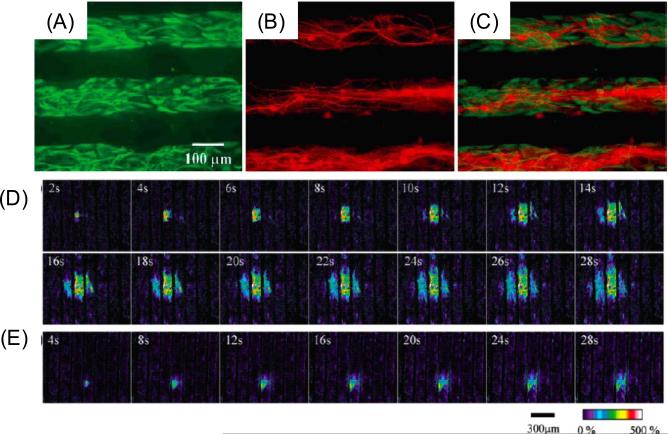
Co-cultures of neural cells can also be useful to study cell signaling events. In one example, Takano et al. co-cultured mouse astrocytes and neurons (Figure 7. A–C) to control cell growth and localization [84]. Cell-cell interactions were regulated by producing spatially separated co-cultures in this study. Physiological activity of astrocytes was shown to be preserved on these patterned substrates by testing calcium signaling (Figure 7. D–E). Moreover, cell functions for astrocytes and neurons in the co-cultures were found to be maintained by testing for the expression of specific proteins, GFAP and MAP-2, respectively. The organized networks of astrocytes and neurons could be useful to study intracellular signaling and communication pathways for central nervous system.
Figure 7.
Fluorescence images for astrocyte-neuron co-cultures. (A) FITC image for selectively labeled the astrocytes, (B) Rhodamine image for selectively labeled the neurons, (C) Overlapping images of (A) and (B). (D) Astrocytes loaded with fluorescent calcium indicator after mechanical stimulation. (E) Astrocytes loaded with both calcium indicator and purinergic receptor antagonist in the extracellular saline [84]. Reprinted with permission from H. Takano, J.-Y. Sul, M.L. Mazzanti, R.T. Doyle, P.G. Haydon, M.D. Porter, Micropatterned substrates: Approach to probing intercellular communication pathways, Anal. Chem., 74 (2002) 4640–4646. Copyright 2002 American Chemical Society.
3.4 Other co-cultures
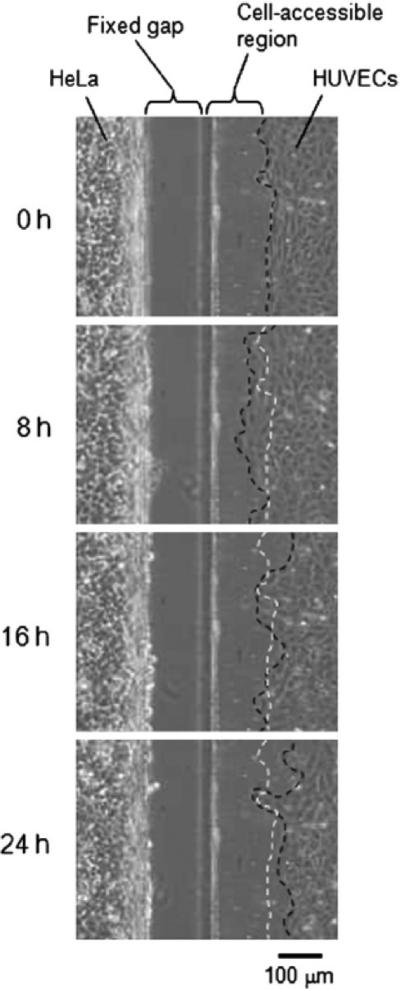
A number of other co-culture systems have been developed to study cell-cell interactions. For example, movements of HeLa cells and human umbilical vein endothelial cells (HUVECs) were studied in a co-culture system by Kaji et al. [65]. HeLa cells and HUVECs were grown separately until the confluency point and then these complementary substrates were combined. HeLa cells moved into the direction where HUVECs were located but HUVECs retreated at the same time (Figure 8). In addition, it was observed that both type of cells migrated faster compared to monoculture experiments. Directionality of such movements can be attributed to attractive and repulsive signals released by different cell types in co-culture. This controlled co-cultivation strategy could potentially be used as a model system to study tumor/endothelium interactions related to tumor metastatic processes.
Figure 8.
Phase-contrast pictures for co-cultivated HeLa cells and HUVECs that were separated by 100 um gap-type barrier. White-dotted lines represent initial HUVEC border while black-dotted lines follow the borders of moving HUVECs [65]. Reproduced by permission of The Royal Society of Chemistry.
Cell-cell interactions between osteoblasts and macrophages have also been investigated in co-culture systems [85]. In these cultures, release of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) by macrophages produced concentration gradients in microfluidic channels which then induced osteoblasts to stimulate bone resorption. These concentration gradients of cytokines were used as a measure to control cell-cell interactions.
Co-cultures of cells can also be used to study angiogenesis in cancerous tumors. For example, capillary morphogenesis was studied utilizing co-cultured cancer cells (MTLn3 cancer cell line) and human dermal microvascular endothelial cells (HMVEC) or HMVEC and mouse smooth muscle cells (10T 1/2) by providing a both biochemically and biomechanically controlled 3D microenvironment [86]. Endothelial cell activity was observed to be diminished by smooth muscle cells; whereas cancer cells promoted formation of capillary structures. In addition, this facilitated both quantitative and qualitative measurements of endothelial cell migration. This co-culture strategy can be used as an endothelial migration assay and it would be a useful method to study tumor angiogenesis, cell-cell communication and endothelial cell migration processes.
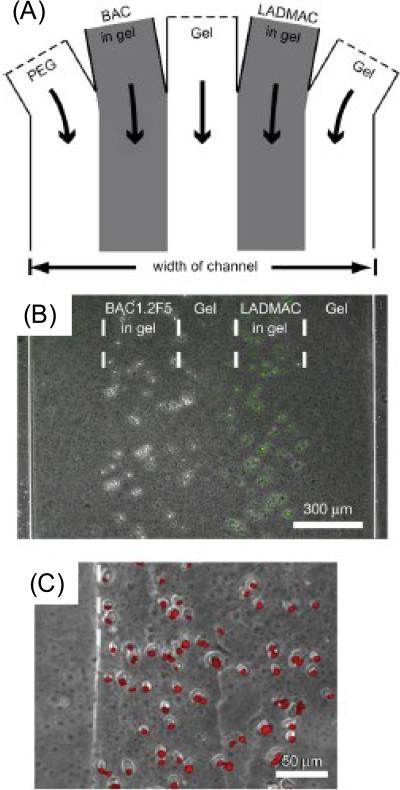
In another example, to investigate intercellular communication, two different types of murine macrophage-like cells, BAC1.2F5 and LADMAC cell lines were co-cultured [69]. The spatial distribution of co-cultures was controlled by utilizing a temperature sensitive gel as explained in Section 2.6. Figure 9 summarizes images for injection of cells and solutions in inlet channels (A), formation of hydrogels in microwells (B) and propidium iodide staining results for BAC cells (C). Intercellular communication between these cells was studied by environmental tuning, which was utilized with controlled gradients of soluble factors in their system.
Figure 9.
Cellular communication between cells. (A) Inlet channels in the hydrogel. (B) Layout of BAC and LADMAC cells in hydrogels. (C) Propidium iodide stained BAC cells (2 day culture) without CSF-1 source [69]. Reprinted from Biomaterials, 29, A.P. Wong, R. Perez-Castillejos, J.C. Love, G.M. Whitesides, Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments, 1853–1861, Copyright (2008), with permission from Elsevier.
3.5 Co-cultures containing three or more cell types
There are multiple types of cells present in native tissues. Therefore, co-cultures containing more than two types of cells would create a more biomimetic microenvironment for tissue engineering applications. For example, functioning vascular structures can be achieved by layered deposition of different types of cells [87]. In this study, human lung fibroblasts, human umbilical vein smooth muscle cells (HUVSMC) and HUVECs were cultivated together in a controlled manner to create tissue-like structures to mimic blood vessel walls. In a similar study, multilayered co-cultures of human lung fibroblasts, HUVSMCs and HUVECs were patterned to mimic vascular tissues [88]. Cell-cell communication was achieved by modulating the heterotypic interactions in vitro. Cell migration was determined to be a function of 3D matrix conditions containing different polymer mixtures. Endothelial tissue function is correlated with Inter-Cellular Adhesion Molecule 1 (ICAM-1) expression and has been enhanced with 3D architecture of cells and matrices in this work.
Another example for multitype cell containing co-culture systems has been carried out by Khademhosseini et al. who co-cultured ES cells and fibroblasts as well as hepatocytes and fibroblasts to study cellular interactions [59] with the system explained in Section 2.3. These experiments resulted in stable differentiation of primary hepatocytes in fibroblast-hepatocyte co-cultures whereas differentiation of ES cells was hindered in ES cell-fibroblast co-cultures. Based on these results, spatial orientation can be controlled using patterned co-culture approaches to enhance cellular behavior and provide better control over cell-cell interactions in microscale resolution.
Finally, Wright et al. temporally and spatially controlled multiple types of cell cultures by dynamic micropatterns using the system given in Section 2.2 [30]. They used co-cultures of mouse fibroblasts, mouse hepatocytes and mouse ES cells to investigate temporal effects on cell-cell interactions. Such dynamic co-culture systems might be useful to improve differentiation of ES cells by regulating the degree of homotypic and heterotypic cell–cell interactions.
4. Conclusions
The complexity and structural organization of native tissues are often studied by co-culture of multiple types of cells, which also improves tissue function [89]. We have discussed the strategies to generate patterned co-cultures and biological results for cell-cell interactions in co-cultured systems in the previous sections. Patterned co-cultures can be used to control the degree of homotypic or heterotypic cellular interactions as well as temporal location of cells [89]. For this reason, micropatterned co-cultures offer numerous advantages for regenerative medicine applications.
Similar strategies can be developed to produce controlled microarchitectures for tissue engineering applications. Micropatterned co-culture strategies hold significant potential to control the interactions between cell-cell, cell-ECM, cell-material and cell-microenvironment. Furthermore, understanding the communication between cells may be of benefit to developing biomimetic structures for tissue engineering. In addition, co-cultures of multiple types of cells should be developed in the future for designing better biomaterials to be used under physiological conditions. It is expected that patterned co-culture techniques would potentially create opportunities to develop artificial tissues in the future.
Acknowledgements
This paper was partially supported by the National Institutes of Health (EB008392; DE019024; HL092836), the US Army Core of Engineers. HK acknowledges support from JSPS Postdoctoral Fellowships for Research Abroad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu WF, Chen CS. Cellular and multicellular form and function. Adv. Drug Deliv. Rev. 2007;59:1319–1328. doi: 10.1016/j.addr.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khetani SR, Bhatia SN. Engineering tissues for in vitro applications. Curr. Opin. Biotechnol. 2006;17:524–531. doi: 10.1016/j.copbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [4].Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- [5].Guguenguillouzo C, Clement B, Baffet G, Beaumont C, Morelchany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult-rat hepatocytes co-cultured with another liver epithelial-cell type. Exp. Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- [6].Schrode W, Mecke D, Gebhardt R. Induction of glutamine-synthetase in periportal hepatocytes by cocultivation with a liver epithelial-cell line. Eur. J. Cell Biol. 1990;53:35–41. [PubMed] [Google Scholar]
- [7].Lawrence MB, Smith CW, Eskin SG, McIntire LV. Effect of venous shear-stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- [8].Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured-hepatocytes. Hepatology. 1988;8:1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- [9].Shimaoka S, Nakamura T, Ichihara A. Stimulation of growth of primary cultured adult-rat hepatocytes without growth-factors by coculture with nonparenchymal liver-cells. Exp. Cell Res. 1987;172:228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- [10].Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte endothelial-cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- [11].Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- [12].Xia YN, Whitesides GM. Soft lithography. Angew. Chem. Int. Ed. 1998;37:551–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [13].Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol. Prog. 1998;14:378–387. doi: 10.1021/bp980036j. [DOI] [PubMed] [Google Scholar]
- [14].Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J. Biomater. Sci. Polym. Ed. 1998;9:1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- [15].Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res. 1997;34:189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [16].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- [17].Mrksich M, Chen CS, Xia YN, Dike LE, Ingber DE, Whitesides GM. Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proc. Natl. Acad. Sci. USA. 1996;93:10775–10778. doi: 10.1073/pnas.93.20.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- [19].Jeon NL, Baskaran H, Dertinger SKW, Whitesides GM, Van de Water L, Toner M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002;20:826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- [20].Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJA, Ismagilov RF, Whitesides GM. Patterning cells and their environments using multiple laminar fluid flows in capillary networks. Proc. Natl. Acad. Sci. USA. 1999;96:5545–5548. doi: 10.1073/pnas.96.10.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Laminar flows - subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- [22].Tien J, Nelson CM, Chen CS. Fabrication of aligned microstructures with a single elastomeric stamp. Proc. Natl. Acad. Sci. USA. 2002;99:1758–1762. doi: 10.1073/pnas.042493399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khademhosseini A, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab Chip. 2005;5:1380–1386. doi: 10.1039/b508096g. [DOI] [PubMed] [Google Scholar]
- [24].Yeon JH, Park JK. Microfluidic cell culture systems for cellular analysis. Biochip J. 2007;1:17–27. [Google Scholar]
- [25].Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc. Natl. Acad. Sci. USA. 2000;97:2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Torisawa Y, Mosadegh B, Luker GD, Morell M, O'Shea KS, Takayama S. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr. Biol. 2009;1:649–654. doi: 10.1039/b915965g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ostuni E, Kane R, Chen CS, Ingber DE, Whitesides GM. Patterning mammalian cells using elastomeric membranes. Langmuir. 2000;16:7811–7819. [Google Scholar]
- [28].Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Microfabricated elastomeric stencils for micropatterning cell cultures. J. Biomed. Mater. Res. 2000;52:346–353. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [29].Wright D, Rajalingam B, Karp JM, Selvarasah S, Ling Y, Yeh J, Langer R, Dokmeci MR, Khademhosseini A. Reusable, reversibly sealable parylene membranes for cell and protein patterning. J. Biomed. Mater. Res. A. 2008;85A:530–538. doi: 10.1002/jbm.a.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wright D, Rajalingam B, Selvarasah S, Dokmeci MR, Khademhosseini A. Generation of static and dynamic patterned co-cultures using microfabricated parylene-C stencils. Lab Chip. 2007;7:1272–1279. doi: 10.1039/b706081e. [DOI] [PubMed] [Google Scholar]
- [31].Kikuchi Y, Nakanishi J, Shimizu T, Nakayama H, Inoue S, Yamaguchi K, Iwai H, Yoshida Y, Horiike Y, Takarada T, Maeda M. Arraying heterotypic single cells on photoactivatable cell-culturing substrates. Langmuir. 2008;24:13084–13095. doi: 10.1021/la8024414. [DOI] [PubMed] [Google Scholar]
- [32].Nakanishi J, Kikuchi Y, Takarada T, Nakayama H, Yamaguchi K, Maeda M. Photoactivation of a substrate for cell adhesion under standard fluorescence microscopes. J. Am. Chem. Soc. 2004;126:16314–16315. doi: 10.1021/ja044684c. [DOI] [PubMed] [Google Scholar]
- [33].Kikuchi K, Sumaru K, Edahiro J, Ooshima Y, Sugiura S, Takagi T, Kanamori T. Stepwise assembly of micropatterned co-cultures using photoresponsive culture surfaces and its application to hepatic tissue arrays. Biotechnol. Bioeng. 2009;103:552–561. doi: 10.1002/bit.22253. [DOI] [PubMed] [Google Scholar]
- [34].Edahiro J, Sumaru K, Ooshima Y, Kanamori T. Selective separation and co-culture of cells by photo-induced enhancement of cell adhesion (PIECA) Biotechnol. Bioeng. 2009;102:1278–1282. doi: 10.1002/bit.22124. [DOI] [PubMed] [Google Scholar]
- [35].Petersen S, Alonso JM, Specht A, Duodu P, Goeldner M, del Campo A. Phototriggering of cell adhesion by caged cyclic RGD peptides. Angew. Chem. Int. Ed. 2008;47:3192–3195. doi: 10.1002/anie.200704857. [DOI] [PubMed] [Google Scholar]
- [36].Ohmuro-Matsuyama Y, Tatsu Y. Photocontrolled cell adhesion on a surface functionalized with a caged arginine-glycine-aspartate peptide. Angew. Chem. Int. Ed. 2008;47:7527–7529. doi: 10.1002/anie.200802731. [DOI] [PubMed] [Google Scholar]
- [37].Fan CY, Tung YC, Takayama S, Meyhofer E, Kurabayashi K. Electrically programmable surfaces for configurable patterning of cells. Adv. Mater. 2008;20:1418–1423. [Google Scholar]
- [38].Chan EW, Park S, Yousaf MN. An electroactive catalytic dynamic substrate that immobilizes and releases patterned ligands, proteins, and cells. Angew. Chem. Int. Ed. 2008;47:6267–6271. doi: 10.1002/anie.200800166. [DOI] [PubMed] [Google Scholar]
- [39].Li Y, Yuan B, Ji H, Han D, Chen S, Tian F, Jiang X. A method for patterning multiple types of cells by using electrochemical desorption of self-assembled monolayers within microfluidic channels. Angew. Chem. Int. Ed. 2007;46:1094–1096. doi: 10.1002/anie.200603844. [DOI] [PubMed] [Google Scholar]
- [40].Jiang X, Ferrigno R, Mrksich M, Whitesides GM. Electrochemical desorption of self-assembled monolayers noninvasively releases patterned cells from geometrical confinements. J. Am. Chem. Soc. 2003;125:2366–2367. doi: 10.1021/ja029485c. [DOI] [PubMed] [Google Scholar]
- [41].Yousaf MN, Houseman BT, Mrksich M. Turning on cell migration with electroactive substrates. Angew. Chem. Int. Ed. 2001;40:1093–1096. [PubMed] [Google Scholar]
- [42].Yousaf MN, Houseman BT, Mrksich M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc. Natl. Acad. Sci. USA. 2001;98:5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials. 2009;30:5427–5432. doi: 10.1016/j.biomaterials.2009.06.033. [DOI] [PubMed] [Google Scholar]
- [44].Tsuda Y, Kikuchi A, Yamato M, Chen G, Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem. Biophys. Res. Commun. 2006;348:937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- [45].Tsuda Y, Kikuchi A, Yamato M, Nakao A, Sakurai Y, Umezu M, Okano T. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials. 2005;26:1885–1893. doi: 10.1016/j.biomaterials.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [46].Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- [47].Yamato M, Kwon OH, Hirose M, Kikuchi A, Okano T. Novel patterned cell coculture utilizing thermally responsive grafted polymer surfaces. J. Biomed. Mater. Res. 2001;55:137–140. doi: 10.1002/1097-4636(200104)55:1<137::aid-jbm180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [48].Sekine S, Kaji H, Nishizawa M. Spatiotemporal sub-cellular biopatterning using an AFM-assisted electrochemical system. Electrochem. Commun. 2009;11:1781–1784. [Google Scholar]
- [49].Hashimoto M, Kaji H, Nishizawa M. Selective capture of a specific cell type from mixed leucocytes in an electrode-integrated microfluidic device. Biosens. Bioelectron. 2009;24:2892–2897. doi: 10.1016/j.bios.2009.02.025. [DOI] [PubMed] [Google Scholar]
- [50].Kaji H, Sekine S, Hashimoto M, Kawashima T, Nishizawa M. Stepwise formation of patterned cell co-cultures in silicone tubing. Biotechnol. Bioeng. 2007;98:919–925. doi: 10.1002/bit.21505. [DOI] [PubMed] [Google Scholar]
- [51].Kaji H, Hashimoto M, Nishizawa M. On-demand patterning of protein matrixes inside a microfluidic device. Anal. Chem. 2006;78:5469–5473. doi: 10.1021/ac060304p. [DOI] [PubMed] [Google Scholar]
- [52].Kaji H, Tsukidate K, Matsue T, Nishizawa M. In situ control of cellular growth and migration on substrates using microelectrodes. J. Am. Chem. Soc. 2004;126:15026–15027. doi: 10.1021/ja045702t. [DOI] [PubMed] [Google Scholar]
- [53].Kaji H, Kanada M, Oyamatsu D, Matsue T, Nishizawa M. Microelectrochemical approach to induce local cell adhesion and growth on substrates. Langmuir. 2004;20:16–19. doi: 10.1021/la035537f. [DOI] [PubMed] [Google Scholar]
- [54].Zhao C, Zawisza I, Nullmeier M, Burchardt M, Trauble M, Witte I, Wittstock G. Microelectrochemical modulation of micropatterned cellular environments. Langmuir. 2008;24:7605–7613. doi: 10.1021/la8003432. [DOI] [PubMed] [Google Scholar]
- [55].Kaji H, Kawashima T, Nishizawa M. Patterning cellular motility using an electrochemical technique and a geometrically confined environment. Langmuir. 2006;22:10784–10787. doi: 10.1021/la0610654. [DOI] [PubMed] [Google Scholar]
- [56].Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [57].Yang IH, Co CC, Ho CC. Spatially controlled co-culture of neurons and glial cells. J. Biomed. Mater. Res. A. 2005;75:976–984. doi: 10.1002/jbm.a.30509. [DOI] [PubMed] [Google Scholar]
- [58].Co CC, Wang YC, Ho CC. Biocompatible micropatterning of two different cell types. J. Am. Chem. Soc. 2005;127:1598–1599. doi: 10.1021/ja044382a. [DOI] [PubMed] [Google Scholar]
- [59].Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- [60].Lapizco-Encinas BH, Rito-Palomares M. Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis. 2007;28:4521–4538. doi: 10.1002/elps.200700303. [DOI] [PubMed] [Google Scholar]
- [61].Ho CT, Lin RZ, Chang WY, Chang HY, Liu CH. Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap. Lab Chip. 2006;6:724–734. doi: 10.1039/b602036d. [DOI] [PubMed] [Google Scholar]
- [62].Kaji H, Hashimoto M, Sekine S, Kawashima T, Nishizawa M. Patterning adherent cells within microchannels by combination of electrochemical biolithography technique and repulsive dielectrophoretic force. Electrochemistry. 2008;76:555–558. [Google Scholar]
- [63].Suzuki M, Yasukawa T, Shiku H, Matsue T. Negative dielectrophoretic patterning with different cell types. Biosens. Bioelectron. 2008;24:1043–1047. doi: 10.1016/j.bios.2008.06.051. [DOI] [PubMed] [Google Scholar]
- [64].Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc. Natl. Acad. Sci. USA. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kaji H, Yokoi T, Kawashima T, Nishizawa M. Controlled cocultures of HeLa cells and human umbilical vein endothelial cells on detachable substrates. Lab Chip. 2009;9:427–432. doi: 10.1039/b812510d. [DOI] [PubMed] [Google Scholar]
- [66].Harimoto M, Yamato M, Hirose M, Takahashi C, Isoi Y, Kikuchi A, Okano T. Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J. Biomed. Mater. Res. 2002;62:464–470. doi: 10.1002/jbm.10228. [DOI] [PubMed] [Google Scholar]
- [67].Tsuda Y, Shimizu T, Yarnato M, Kikuchi A, Sasagawa T, Sekiya S, Kobayashi J, Chen G, Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [68].Ito A, Takizawa Y, Honda H, Hata KI, Kagami H, Ueda M, Kobayashi T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10:833–840. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- [69].Wong AP, Perez-Castillejos R, Love JC, Whitesides GM. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials. 2008;29:1853–1861. doi: 10.1016/j.biomaterials.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- [71].Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Meth. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- [72].Du YA, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- [74].Korin N, Levenberg S. Engineering human embryonic stem cell differentiation. Biotechnol. Genet. Eng. Rev. 2007;24:243–262. doi: 10.1080/02648725.2007.10648102. [DOI] [PubMed] [Google Scholar]
- [75].Li N, Tourovskaia A, Folch A. Biology on a chip: Microfabrication for studying the behavior of cultured cells. Crit. Rev. Biomed. Eng. 2003;31:423–488. doi: 10.1615/critrevbiomedeng.v31.i56.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Takahashi S, Yamazoe H, Sassa F, Suzuki H, Fukuda J. Preparation of coculture system with three extracellular matrices using capillary force lithography and layer-by-layer deposition. J. Biosci. Bioeng. 2009;108:544–550. doi: 10.1016/j.jbiosc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- [77].Nahmias Y, Arneja A, Tower TT, Renn MJ, Odde DJ. Cell patterning on biological gels via cell spraying through a mask. Tiss. Eng. 2005;11:701–708. doi: 10.1089/ten.2005.11.701. [DOI] [PubMed] [Google Scholar]
- [78].Hui EE, Bhatia SN. Microscale control of cell contact and spacing via three-component surface patterning. Langmuir. 2007;23:4103–4107. doi: 10.1021/la0630559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- [80].Bianchi F, Rosi M, Vozzi G, Emanueli C, Madeddu P, Ahluwalia A. Microfabrication of fractal polymeric structures for capillary morphogenesis: Applications in therapeutic angiogenesis and in the engineering of vascularized tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007;81B:462–468. doi: 10.1002/jbm.b.30685. [DOI] [PubMed] [Google Scholar]
- [81].Khademhosseini A, Ferreira L, Blumling J, III, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- [82].Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- [83].Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Takano H, Sul J-Y, Mazzanti ML, Doyle RT, Haydon PG, Porter MD. Micropatterned substrates: Approach to probing intercellular communication pathways. Anal. Chem. 2002;74:4640–4646. doi: 10.1021/ac0257400. [DOI] [PubMed] [Google Scholar]
- [85].Wei C-W, Cheng J-Y, Young T-H. Elucidating in vitro cell-cell interaction using a microfluidic coculture system. Biomed. Microdevices. 2006;8:65–71. doi: 10.1007/s10544-006-6384-8. [DOI] [PubMed] [Google Scholar]
- [86].Chung S, Sudo R, Mack PJ, Wan C-R, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- [87].Tan W, Desai TA. Layer-by-layer microfluidics for biomimetic three dimensional structures. Biomaterials. 2004;25:1355–1364. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- [88].Tan W, Desai TA. Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. 2005;72A:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- [89].Kim SM, Fukuda J, Khademhosseini A. Micro and Nanoengineering of the Cell Microenvironment Technologies and Applications, 2008, Ch 4. In: Khademhosseini A, Borenstein J, Toner M, Takayama S, editors. Patterned cocultures for controlling cell-cell interactions. [Google Scholar]