Abstract
The use prevalence of the highly addictive psychostimulant methamphetamine (MA) has been steadily increasing over the past decade. MA abuse has been associated with both transient and permanent alterations in cerebral blood flow (CBF), hemorrhage, cerebrovascular accidents and death. To understand MA-induced changes in CBF, we exposed C56BL/6 mice to an acute bolus of MA (5 mg/kg MA, delivered IP). This elicited a biphasic CBF response, characterized by an initial transient increase (~5 min) followed by a prolonged decrease (~30 min) of approximately 25% relative to baseline CBF – as measured by laser Doppler flowmetry over the somatosensory cortex. To assess if this was due to catecholamine derived vasoconstriction, phentolamine, an α-adrenergic antagonist was administered prior to MA treatment. This reduced the initial increase in CBF but failed to prevent the subsequent, sustained decrease in CBF. Consistent with prior reports, MA caused a transient increase in mean arterial blood pressure, body temperature and respiratory rate. Elevated respiratory rate resulted in hypocapnia. When respiratory rate was controlled by artificially ventilating mice, blood PaCO2 levels after MA exposure remained unchanged from physiologic levels, and the MA-induced decrease in CBF was abolished. In vivo two-photon imaging of cerebral blood vessels revealed sustained MA-induced vasoconstriction of pial arterioles, consistent with laser Doppler flowmetry data. These findings show that even a single, acute exposure to MA can result in profound changes in CBF, with potentially deleterious consequences for brain function.
Keywords: cerebral blood flow, hypocapnia, methamphetamine, mice, cerebral pial arterioles, vasoconstriction, autoregulation
1. Introduction
Methamphetamine (MA), a member of the amphetamine class of stimulants, has addictive properties attributable to the activation of dopaminergic reward pathways (Volkow et al., 2001), and as a result has a high potential for abuse. The use of MA is a concerning, rapidly growing epidemic within the United States (Lineberry and Bostwick, 2006). Exposure to MA can cause long-term neurotoxicity and neuroinflammation resulting from mitochondrial dysfunction and oxidative stress due to disruption of catecholamine metabolism (Krasnova and Cadet, 2009). MA exposure produces a myriad of additional physiological responses, both centrally and peripherally, many of which can be attributed to MA induced increases in serotonin, dopamine and norepinephrine (Cruickshank and Dyer, 2009; Sulzer et al., 2005), leading to a pathophysiological increase in sympathetic tone, increased blood pressure, extreme hyperthermia and altered mental state (Schep et al., 2010).
The use of amphetamines, such as MA, is associated with cerebrovascular complications such as cerebrovascular accidents (CVA) (Perez et al., 1999; Westover et al., 2007; Yen et al., 1994), hemorrhage (Delaney and Estes, 1980; Westover et al., 2007), hypoxic damage (Kaye et al., 2008) and vasculitis (Salanova and Taubner, 1984). Interestingly, while changes to cerebral blood flow (CBF) in response to acute amphetamine exposure have been reported (Devous et al., 2001; Rose et al., 2006), there is evidence of long-term effects on CBF from MA use even in abstinent users (Chang et al., 2002; Hwang et al., 2006; Iyo et al., 1997), suggesting that the effect of MA on CBF is at least partially irreversible.
Reports on the effect of MA on global or focal CBF are controversial and incomplete as seen by the variation in published data. While some researchers have reported increases in CBF after amphetamine exposure (Devous et al., 2001; McCulloch et al., 1978; Rose et al., 2006), others have shown that CBF remains unchanged (Kimmerly et al., 2003; Moppett et al., 2008), or is decreased (Alhassoon et al., 2001; Devous et al., 2001; Wang et al., 2001; Zimmer et al., 1974). The discrepancies between studies may be attributed to the substantial variation in methodologies used to measure CBF, such as the examination of venous outflow (Zimmer et al., 1974), SPECT (Devous et al., 2001) or laser Doppler flowmetry (Saeki et al., 1990). It is difficult to compare CBF measured in blood vessels of different caliber (such as middle cerebral artery (Moppett et al., 2008) vs. pial arterioles (Saeki et al., 1990)) since vasomodulatory stimuli differentially affect large and small blood vessels (Kontos et al., 1981). In light of this, we performed a careful analysis of the effect of MA exposure on CBF, and explored possible mechanisms for MA-induced changes in CBF in C57BL/6 mice.
The mouse is not commonly used in such experiments due to the level of technical difficulty encountered during intubation and vascular manipulation in animals of this size. However, mice provide tractable genetic model system for various neurological diseases and enable facile fluorescent visualization of different cell types within the central nervous system.
2. Results
Acute exposure to MA caused sustained decrease in CBF
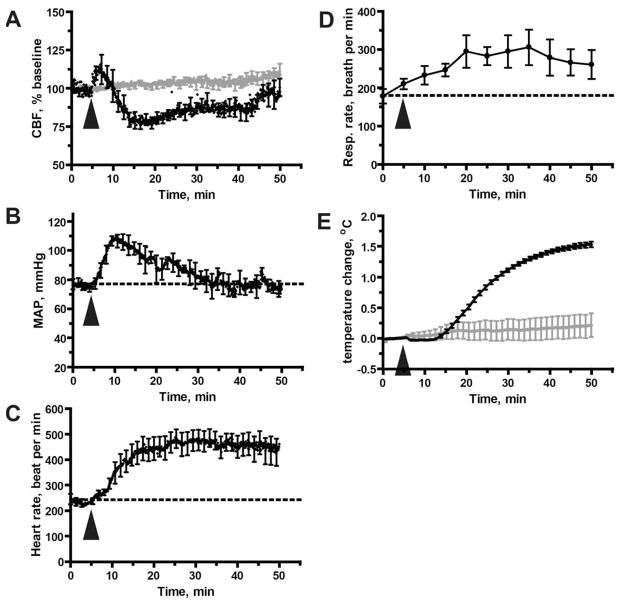
CBF was recorded by laser Doppler flowmetry over the somatosensory cortex of anesthetized C57BL/6 mice. After a baseline CBF recording for 5 min, MA (5 mg/kg) was injected intraperitoneally (i.p.), and CBF was recorded for 60 min. MA exposure caused a bi-phasic response: an initial, transient (<5min) increase in CBF was followed by a prolonged (~ 30 min) decrease, reaching a trough of about 25% relative to baseline flow (Fig. 1A). Injection of 0.9 % saline alone did not cause significant changes in CBF (Fig 1A, shown in grey). The difference between baseline and post-MA levels of CBF was statistically significant (Wilcoxon signed rank test, p < 0.001). CBF returned to approximate baseline levels within 50 min after MA injection.
Figure 1. Acute MA exposure (5 mg/kg) disregulates CBF and modulates other physiological parameters.
A. Disregulation of CBF as measured by laser Doppler flowmetry. Values are expressed as a percentage of baseline CBF, defined here as the mean CBF measured during the 5 minute period immediately preceding delivery of MA (or saline, shown in grey). Results represent mean from 5 mice treated with MA and 3 mice treated with saline. B. MA-induced hypertension. Mean arterial blood pressure was measured via a catheter inserted into the femoral artery; results shown represent mean data values from 6 mice per group. C. Increase in heart rate. Results represent data from the same mice shown in (B). D. Increase in respiratory rate; results shown are mean data values from 8 mice. E. MA-induced hyperthermia. Body temperature was measured with a rectal probe, and results are presented relative to baseline temperature (calculated as in (A)). Injection of saline did not induce hyperthermia (shown in grey). Results represent data from the same mice shown in (A). (A–E) All data represent mean ± SEM. The arrowhead indicates the time at which MA (or saline) was injected.
MA exposure also produced a transient increase in mean arterial pressure (MAP), peaking at about 10 min following MA exposure (Fig. 1B). Thus, the peak MA-induced increase in MAP was slightly delayed and prolonged, when compared with the peak initial increase in CBF (which occurred within the first 5 minutes of exposure to MA). MA exposure also produced a sustained elevation in heart rate (HR) that continued even after CBF had returned to baseline (Fig. 1C). The increase in respiratory rate (RR) (Fig. 1D) and body temperature (BT), was consistent with MA-induced stimulation of metabolic rate. The BT increase of approximately 1.5°C over the course of our experiments (Fig. 1E), is in agreement with well-characterized MA-induced hyperthermia (Cruickshank and Dyer, 2009; Kiyatkin et al., 2007; Rusyniak and Sprague, 2005). Hyperthermia was not seen with administration of saline alone (Fig 1E, shown in grey). Overall, the MA-induced physiological responses in our experimental mice closely replicated characteristic MA effects in humans, including elevations in HR and MAP (Cruickshank and Dyer, 2009; Gentry et al., 2006; Mendelson et al., 2006) and an increase in RR (Jacobs and Fornal, 1997; Mediavilla et al., 1979).
Blocking of α-adrenergic receptors does not prevent the MA-mediated decrease in CBF
MA is known to reverse the function of the norepinephrine transporter, leading to an increase in norepinephrine levels within the neuronal synapse (Cruickshank and Dyer, 2009). Since the stimulation of sympathetic nerves can cause cerebral vasoconstriction mediated by α1-adrenergic receptors (Saeki et al., 1990), we tested whether stimulation of α-adrenergic receptors might contribute to the MA-mediated decrease in CBF.
To do this, we treated mice with phentolamine (10 mg/kg, i.p., 30 min before MA injection), a nonspecific α-adrenergic antagonist. In mice pretreated with phentolamine, exposure to MA resulted in an attenuated initial increase in CBF (Fig. 2A) that was associated with a reduced rise in MAP (Fig. 2B), compared to mice exposed to MA alone (Fig. 1B). The MA-induced RR increase was not prevented by phentolamine (Fig. 2D), and neither was the MA-induced hypocapnia (Fig. 2E). The BT increase in mice pre-treated with phentolamine was also similar to the response to mice exposed to MA alone, reaching 1.4 ± 0.21°C (mean ± SEM) at 30 min following MA exposure.
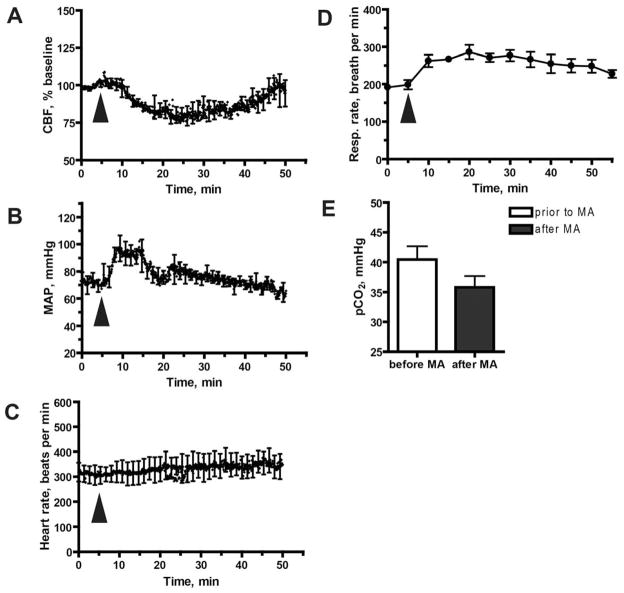
Figure 2. Blockade of α-adrenoreceptors does not prevent the MA-mediated reduction in CBF.
Mice were injected with the α-blocker phentolamine (10 mg/kg, i.p.), 30 min prior to MA exposure. A. Phentolamine pre-treatment does not prevent MA-mediated inhibition of CBF. CBF was measured with laser Doppler flowmetry in 6 mice. B. Effect of phentolamine pre-treatment on MA-mediated changes in MAP. MAP results represent mean values from 4 mice per group. C. Effect of phentolamine pre-treatment on MA-mediated changes in heart rate. Heart rate was measured in the same mice shown in (A). D. Effect of phentolamine pre-treatment on MA-mediated changes in respiratory rate. Respiratory rate measured in the same mice shown in (B). E. Effect of phentolamine pre-treatment on MA-mediated changes in PaCO2. PaCO2 was measured at baseline and 15 min after exposure to MA. Data were obtained from 5 mice. (A–E) Results represent mean ±SEM. The arrowhead indicates the time at which MA was injected.
As expected, the baseline HR in phentolamine-pretreated mice was significantly elevated in comparison to mice not treated with phentolamine (respectively: 312 ± 35 bpm vs. 223 ± 18 bpm [mean ± SEM]; p < 0.05, two-tailed t-test). However, phentolamine pre-treatment blocked the large, MA-induced rise in HR (Fig 2C). Finally, and also as expected, baseline MAP was decreased after pre-treatment with phentolamine - though not significantly (93 ± 19 mmHg without phentolamine vs. 72 ± 10 mmHg after phentolamine [mean ± SEM]; p > 0.05, two-tailed t-test).
Since phentolamine did not prevent the MA-induced, sustained inhibition of CBF (Fig. 2A), we conducted further experiments to determine whether the MA-mediated decrease in CBF could be attributed to MA-induced hypocapnia.
MA-induced inhibition of CBF is a consequence of MA-induced hypocapnia
Acute MA exposure resulted in a sustained and significant increase in RR (Fig. 1D), in agreement with previous reports (Gentry et al., 2006; Mendelson et al., 2006; Richards et al., 1995). Elevated RR can lead to hypocapnia, manifested as a reduced arterial partial pressure of CO2 (PaCO2). This is important because PaCO2 is a major regulator of cerebral blood flow: an increase in PaCO2 within the interstitial fluid of the brain parenchyma and perivascular spaces leads to vasodilation, while a decrease in PaCO2 leads to vasoconstriction of cerebral vessels (Norberg and Siesjo, 1974; Vavilala et al., 2002). To test whether hypocapnia might be an underlying cause of the MA-mediated decrease in CBF, we measured PaCO2 in arterial blood samples at 5 mins before and 15 mins after acute exposure to MA. The mice were either allowed to breathe spontaneously, or their RR was artificially controlled by mechanical ventilation without change to the pressure-determined tidal volume. Our expectation was that mechanical ventilation would prevent the MA-induced increase in RR and thus result in normalization of PaCO2 following exposure to MA.
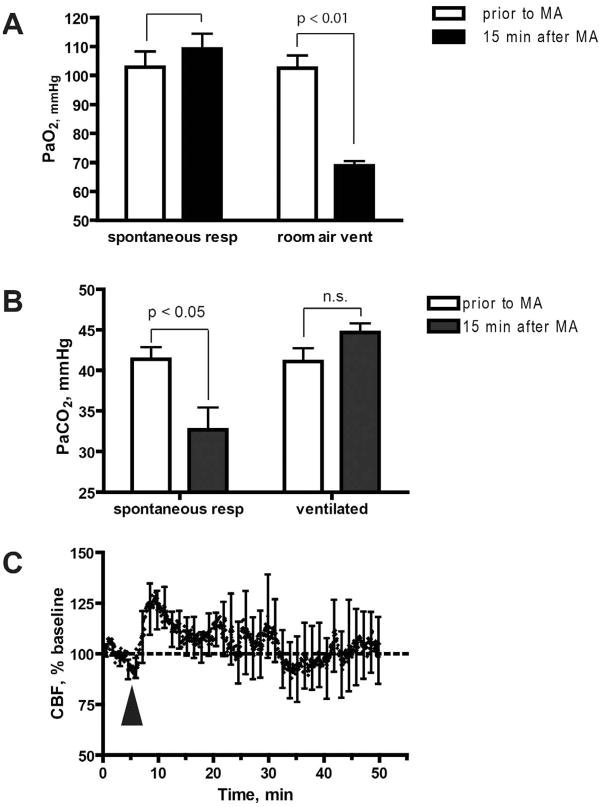
In animals that were allowed to freely breathe room air after MA administration (reaching approximately 300 breaths per min [bpm]) we observed normal PaO2 levels (Fig 3A). In experiments in which animals were subjected to artificial respiration, we used air that contained 50% O2. This was necessary to prevent the non-physiologic hypoxia that occurred when animals were mechanically ventilated using room air (21% O2), at the reduced respiratory rate of 150 bpm (Fig 3A).
Figure 3. Mechanical ventilation prevents CBF decrease in response to MA.
A. Additional O2 is needed to provide adequate oxygenation in artificially ventilated mice. Oxygenation of arterial blood was within normal range before and after exposure to MA when mice were allowed to breathe spontaneously (note that RR reached 300 bpm after exposure to MA). In contrast, exposure to MA when mice were artificially ventilated with room air at 150 bpm resulted in a significant decrease in PaO2, relative to baseline levels (p = 0.0057, two-tailed t-test). Therefore, to avoid this non-physiological hypoxia, mice were ventilated in subsequent experiments with air containing 50% oxygen. B. Mechanical ventilation prevents the MA-mediated decrease in PaCO2. PaCO2 was measured prior to MA injection or 15 min after MA injection. Results represent mean ± SEM from 7 mice (not ventilated) and 5 mice (ventilated). The asterisk denotes that pCO2 in spontaneously breathing animals was significantly reduced at 15 min following exposure to MA, as compared to baseline (p = 0.017, two-tailed t-test). C. Mechanical ventilation prevents the MA-mediated decrease in CBF. CBF was measured in mechanically ventilated mice. Value are expressed as a percentage of baseline CBF, and represent mean ± SEM from 5 mice. Arrowheads indicate MA injection.
In animals that were allowed to breathe spontaneously, PaCO2 was decreased after MA injection (Fig. 3B, p < 0.05, two-tailed t-test), as was CBF (Fig 1A). In contrast, when RR was kept constant by mechanical ventilation, PaCO2 remained unchanged following MA exposure, and the MA-induced decrease in CBF was abolished (Fig. 3C). These data suggest that the MA-induced drop in CBF is mediated by hypocapnia, which develops as a result of increased RR. This conclusion is supported by our measurements of RR in mice pretreated with phentolamine (Fig. 2), where blockade of α-adrenergic responses failed to prevent either the MA-induced increase in RR, or the MA-induced decrease in CBF.
MA exposure causes constriction of pial arterioles
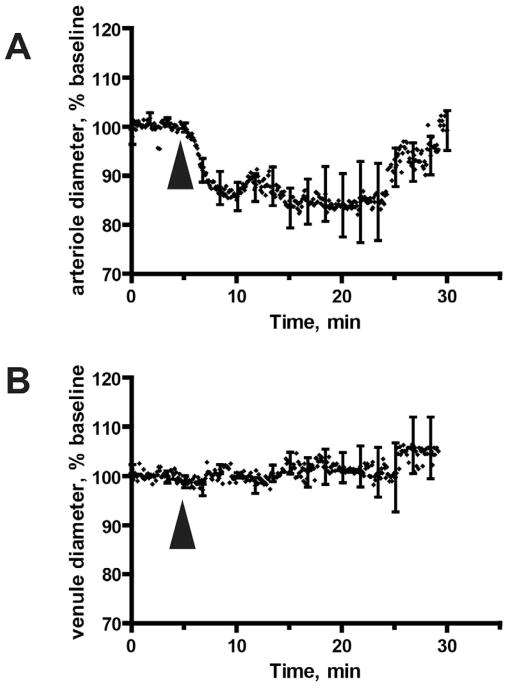
To determine the relationship between MA-induced depression of CBF, and cerebrovascular changes two-photon microscopy of pial vessels was performed in live animals acutely exposed to MA. This was done through the creation of a cranial window to visualize the pial microvasculature at a 25x magnification in animals that were injected intravenously with Texas Red dextran. The baseline diameter of pial arterioles was 29.7 ± 8.93 μm (mean ± SD) with a range of 10.0 to 45.3 μm). Acute MA exposure led to robust vasoconstriction in pial arterioles, but had no effect on cerebral venules (Fig. 4A, B). The observed reduction in arteriolar diameter to ~82% of the baseline diameter achieved statistical significance as determined by the Wilcoxon signed rank test (p-value = 0.004). According to Hagen-Poiseuille's law, the observed arterial constriction will lead to a ~2.2 fold reduction in blood flow, which is approximately 45% of the baseline blood flow. In reality, the relationship is more complex, since other mechanisms, related to blood microviscosity and turbulence, could further affect blood flow changes.
Figure 4. Acute MA exposure causes constriction of pial arterioles but does not affect pial veins.
The diameter of pial blood vessels was determined by analysis of two- photon imaging data over a 30 minute time course (5 minutes preceding MA injection, and 25 minutes thereafter). The data represent results from (A) 9 arterioles and (B) 4 veins. Images were collected from 9 animals. (A, B) Data represent mean ± SD for vessel diameter, calculated every 5 seconds. Arrowheads indicate MA injection.
Of note, the initial, transient MA-induced increase in CBF (Fig. 1A) was not associated with vasodilatation (Fig. 4), but on the contrary, was contemporaneous with vasoconstriction. This may reflect the fact that our two-photon imaging provides data only for surface pial arterioles, and not for the penetrating arterioles and larger vessels that are believed to control the perfusion of deeper brain tissues (Nishimura et al., 2007). In contrast, laser Doppler flowmetry measures cerebral blood flow in a relatively large volume of brain tissue (about 1 cubic millimeter) which contains not just pial arterioles, but also penetrating arterioles, venules, and subsurface microvessels.
The MA-induced constriction of surface pial arterioles was largely complete at 5 minutes following drug administration (Fig. 4A). However, the MA-induced decrease in CBF did not reach its nadir until 10 minutes following drug exposure (Fig. 1A). We attribute this apparent discrepancy to the differential myogenic response in vessels of varying caliber. Smaller diameter vessels, such as arterioles, are more sensitive to changes at lower pressures (Davis, 1993; Golding et al., 1998), and thus may constrict before larger caliber vessels (Golding et al., 1998), which could not be analyzed because they reside deep within tissues - and beyond the depth resolution of two-photon microscopy.
3. Discussion
MA abuse is associated with multiple cerebrovascular pathologies (Delaney and Estes, 1980; Kaye et al., 2008; Perez et al., 1999; Westover et al., 2007; Yen et al., 1994). In addition, CBF changes have been reported in human subjects after acute amphetamine exposure (Devous et al., 2001; Rose et al., 2006).
Cerebrovascular complications of MA use are often attributed to the significant rise in blood pressure resulting from increases in peripheral vasoconstriction and cardiac output. MA’s blockade of norepinephrine reuptake by presynaptic neurons, leads to accumulation of norepinephrine and prolonged pathologic stimulation of both α and β adrenoceptors (Perez et al., 1999; Simpson, 1978; Sulzer et al., 2005). While the observed peripheral cardiovascular responses to MA have been relatively consistent in the literature, the relationship between norepinephrine stimulation and changes in CBF has been more controversial. Previous studies examining the effect of catecholamines or sympathomimetics, such as MA, on CBF have yielded conflicting findings. For example, in some reports, norepinephrine injection in human subjects was shown to cause an increase in arterial pressure, but not in blood flow velocity in the middle cerebral artery (MCA) (Kimmerly et al., 2003; Moppett et al., 2008), while another study reported that norepinephrine caused an increase in arterial blood pressure and an associated decrease in blood flow velocity in MCAs (Brassard et al., 2009). In the present study, our laser Doppler probe sampled a relatively large volume of murine brain tissue (1 mm3) which contained a range of different vessel types – including small, medium and large diameter resistant vessels. We reasoned that this would provide a reliable measure of overall CBF, and thus of cerebral perfusion.
Our data show that acute MA exposure in mice causes a sustained and substantial decline in CBF that is associated with constriction of pial arterioles. Our initial hypothesis was that this decrease in CBF might be the result of vasoconstriction caused by stimulation of α1- adrenoreceptors located in the cerebral vasculature (Hartman et al., 1972). We theorized that MA, being a very potent sympathomimetic, might produce sympathetic stimulation of cerebral vessels. This hypothesis was partly based on a previous report (Saeki et al., 1990) which demonstrated that sympathetic stimulation in rats produced a transient increase of cortical CBF, which was followed by a sustained decrease in CBF; this decrease was abolished by an α-adrenergic blocker. In contrast, in our experiments, α-blockade failed to prevent the MA-induced sustained decrease in CBF, suggesting that this decrease was not due to sympathetic overstimulation; this led us to explore an alternative hypothesis relating to hypocapnia.
PaCO2 in arterial blood is a major regulator of CBF (Norberg and Siesjo, 1974; Vavilala et al., 2002). In humans hypocapnia can result from increased ventilation following MA administration (Cruickshank and Dyer, 2009; Mendelson et al., 2006), as seen in our MA-exposed mice, where RR increased from ~ 175 bpm to ~300 bpm. Blood gas analysis confirmed that PaCO2 was decreased after acute MA exposure, while PaO2 remained at physiologic levels - suggesting that the MA-mediated increase in RR might lead to hypocapnia and thereby to cerebral vasoconstriction. To test this hypothesis, we artificially ventilated mice in order to maintain a constant RR of 150 bpm, tidal volume and physiologic PaCO2. We explored ventilating animals using room air (21% O2,), at 150 bpm, but found that this led to a significant drop in PaO2 upon exposure to MA – resulting in markedly subphysiologic levels of PaO2. We therefore performed our mechanical ventilation experiments at 50% O2, to ensure that physiologic levels of PaO2 were maintained (as they were in MA-exposed animals that were allowed to breathe spontaneously). Mechanical ventilation abolished the MA-induced decrease in PaCO2 and CBF (Fig. 3C), implicating hypocapnia as the primary cause of the reduction in CBF following acute MA exposure. This conclusion is consistent with the fact that phentolamine pretreatment did not prevent the MA-induced elevation in RR, and had no effect on the MA-induced decline in CBF.
It has been suggested that catecholamines may affect the discharge rate of arterial chemoreceptors, such as those found in the carotid and aortic bodies, causing a change in RR (Folgering et al., 1982). However, catecholamines are also known to augment peripheral basal metabolic rate which in turn elicits a substantial increase in CO2 production, oxygen demand and formation of lactic acid (H+) - all of which play a role in regulation of RR both peripherally and centrally. Compensatory mechanisms would thus require increased ventilation to meet metabolic demand, resulting in a rise in RR.
Acute exposure of mice to MA led to a sustained drop in CBF within the cerebral cortex, as discussed above, as well as a transient, initial increase in CBF that occurred immediately following MA injection (Fig. 1A). A similar transient increase in CBF immediately following acute MA exposure has been reported in humans exposed to MA (Mathew and Wilson, 1989) and baboons exposed to amphetamine (McCulloch et al., 1978).
In mice subjected to α-adrenergic blockade this initial, transient MA-induced increase in CBF was greatly diminished, and was associated with a smaller MA-induced increase in MAP (Fig. 2A,B). However, these mice still experienced the MA-mediated elevation of RR, hypocapnia and consequent sustained reduction in CBF (Fig. 2A,D,E).
These data suggest that the initial, short-lived MA-induced increase in CBF may be related to MA-mediated α-adrenergic stimulation (Tsai et al., 1989), since MAP remained within autoregulatory range (Ruland and Aiyagari, 2007). However, there are conflicting reports on role of the sympathetic nervous system in CBF regulation (Busija et al., 1980; Heistad et al., 1977; Meyer et al., 1973; Skinhoj, 1972), suggesting that future followup studies may be required to precisely define the mechanism underlying the initial, transient MA-induced increase in CBF.
The baseline HR in phentolamine-pretreated mice was significantly elevated in comparison to mice not treated with phentolamine. This is a known effect of phentolamine, usually attributed to reflex tachycardia in response to decreased peripheral resistance, due to the α–adrenergic blockade (Graham and Pettinger, 1979; Kelleher et al., 1972). HR was not further increased upon exposure to MA, suggesting that the MA-mediated increase in HR may be mediated by α-adrenergic stimulation. A similar effect has been reported with the selective α1 adrenergic blocker prazosin: when conscious squirrel monkeys were pretreated with prazosin, the normal MA-induced increase in HR was abolished (Schindler et al., 1992).
Finally, and as expected, baseline MAP was decreased after pre-treatment with phentolamine - though not significantly. MAP rose in response to MA, regardless of pre-treatment with phentolamine. One possible explanation is that the MA-induced rise in MAP may be the result of β-adrenergic signaling. Alternatively, it is possible that angiotensin may contribute to the increase in MAP following MA exposure, due to its powerful vasoconstrictive activity (Reid, 1992).
An additional aspect of our findings that merits discussion is the return of CBF to baseline levels, even in the face of continuing MA-induced hyperventilation. The expectation is that persistent hyperventilation would result in continued hypocapnia, vasoconstriction and low CBF. It is unlikely that blood vessel diameter returns to normal during prolonged hypocapnia. Experiments performed in rabbits indicate that such a return may take up to 20 hours (Muizelaar et al., 1988). The CBF normalization after MA exposure can perhaps be explained as a consequence of MA-induced hyperthermia, since hyperthermia is known to lead to an increase in CBF (Katsumura et al., 1995). An additional factor which may also contribute to the normalization of CBF despite continuing hypocapnia is local metabolic demand due to ongoing catecholaminergic activity in the brain. For example, it has been observed that acute amphetamine exposure causes an increase in CBF in brain areas with dopaminergic innervation due to metabolic demand of the brain parenchyma (Devous et al., 2001; Wechsler et al., 1979).
MA-mediated increases in both cerebral and peripheral metabolic activation can also be expected to result in increased generation of [H+] and CO2, and increased oxygen demand. An initial rise in blood CO2 and decreased PaO2 due to metabolic activation would signal for an increase in ventilation (RR and tidal volume) through peripheral and central chemoreceptors. Moreover, since the buffering capacity of cerebrospinal fluid is much less than that of blood, a drop in pH due to increased CO2, H+ and lactic acid production would occur rapidly within the CNS – leading to an acidosis that would be managed quickly through respiratory compensation – again by increasing ventilation. Both pathways would potentially lead to transient hypocapnia.
Concerns arise with prolonged induced hypocapnia since this could lead to cerebral ischemia (Laffey and Kavanagh, 2002). Decreased perfusion, in the setting of an MA-induced increase in neuronal O2 demand, is likely further potentiated by decreased unloading of O2 commonly associated with hypocapnia, and could lead to a localized ischemic environment. Finally, as MA’s effects wane over time, the eventual restoration of normal tissue perfusion may have the potential to cause oxidative reperfusion injury. This will be an important topic for future investigation.
Additional studies are needed to determine whether the MA-induced decrease in CBF leads to significant, metabolically limiting tissue hypoxia. Further research is planned to determine whether MA exposure leads to the expression of hypoxia-induced genes. It is still not clear whether the observed changes in CBF are focal or global. We believe that hypocapnia-related changes are global. However, since they can be modulated by metabolic demand-related changes in CBF, mediated via dopaminergic and serotonergic circuits, it is possible that areas showing greater focal effects may also occur.
In conclusion, this report describes in detail the cerebrovascular response to MA exposure, and shows that even a single exposure to this drug can cause a sustained and significant decline in CBF. We also demonstrate that this decline in CBF can be attributed to MA-induced hyperventilation. These findings suggest additional, new concerns about the effects of even a single, one-time exposure to MA, and highlight a previously unappreciated risk of MA abuse.
4. Material and Methods
Animals and dosing
Adult (8–10 week old) c57BL/6 mice were obtained from Charles River Laboratory and housed with a 12 hour light/dark cycle. To measure the effect of acute MA exposure, mice were anesthetized with an intraperitoneal (i.p.) injection of urethane (1 g/kg) and xylazine (2 mg/kg). Urethane anesthesia was chosen because it produces minimal changes in respiration and circulation and provides immobility for a prolonged period of time (Maggi and Meli, 1986). Animals were then prepared for measurement of CBF and/or physiological parameters (see below). After recording baseline parameters for 5 min, MA (5 mg/kg) was injected i.p. in 150 μl of saline. This dose of MA exceeds the dose to which human methamphetamine users subject themselves (approx. 1 mg/kg) (Melega et al., 2007), but is comparable to doses used in previous rodent studies (Kamens et al., 2005) and was selected to take into account the much shorter plasma half-life of methamphetamine in rats compared to humans (Cook et al., 1992)(Melega et al., 1995).
Cerebral blood flow and physiological parameters
Urethane/xylazine anesthetized mice were placed on a water-perfused heating pad (37°C) throughout the duration of the experiment to prevent anesthesia-related hypothermia. Initial baseline temperature measured rectally in mice was ~ 35°C. Before the recording of physiological parameters, arterial blood gas status (see below) was measured to ensure that PaCO2, an important regulator of CBF, was in the normal physiological range (35–45 mmHg). Different physiological parameters were measured in various groups of animals of the same strain, age and gender, as specified in the Figure legends.
CBF was measured by laser Doppler flowmetry (BLF 21 D, Transonic Systems Inc., NY). The tip of the laser Doppler probe was immersed in saline at the stereotactic coordinates: bregma 5 mm, interaural 10 mm, and 0.5 mm above the intact cranium. Flow was recorded continuously for approximately 1 hour. Mice were immobilized in a stereotactic frame with ear bars to ensure stable position of the cranium throughout the experiment. Body temperature was measured with a rectal probe, and recorded continuously. Arterial blood pressure was monitored via a femoral artery catheter. Blood gas status was measured with a blood gas analyzer (Rapidlab 248, Bayer) in 40 μl microsamples of blood from the femoral artery. Blood samples were obtained prior to injection of MA. In some mice, blood samples were also obtained 15 min after the MA injection, in order to assess MA’s effects on PaCO2. Respiratory rate was measured every 5 min by using a stop-watch to determine the time taken for 30 breaths (which were counted visually). Heart rate was measured using a custom-assembled electrocardiograph. The heart rate was extracted from the electrocardiogram using a custom Matlab function.
Ventilation
An incision was made from the sternal notch to just below the submental region. Skin and musculature were retracted to expose the trachea. A transverse incision was made along anterior portion of trachea to allow placement of an endotracheal tube. After the tube was inserted into the trachea it was secured by sutures that allowed for positive pressure ventilation without loss of air. Ventilation was performed with a SAR-830, CWE ventilator. Tidal volume was set automatically by the ventilator for each respiratory cycle, based on lung capacity as determined by resistance to air flow. Ventilation was performed using either 21% O2 (in early, trial experiments) or 50% O2 (in all subsequent experiments) at a rate of 150 bpm. Our initial experiments showed that ventilation with room air (21% O2) at a fixed rate of 150 bpm resulted in a non-physiologic hypoxia following exposure to MA (Fig. 3A), which was not observed in spontaneously respiring animals treated with MA. To prevent this, we therefore performed all subsequent ventilation studies using air that contained 50% O2.
Two-photon microscopy
For cranial window surgery, a urethane/xylazine anesthetized animal was placed into a custom-made metal frame glued to the skull with dental acrylic cement. 1% ferric chloride was applied to the skull to remove any remaining periosteal layer. A craniotomy (3 mm in diameter) was performed, centered 1–2 mm posterior to the bregma and 2 mm from the midline. The dura was removed, and agarose (0.75%) in artificial cerebrospinal fluid (Harvard Apparatus) was poured into the craniotomy. Finally, the craniotomy was closed with a coverslip (thickness 0.17 mm). The frame and mouse were then transferred to the microscope stage. To visualize blood vessels, Texas Red–dextran (MW 70 kDa, Invitrogen) was injected intravenously (10 mg/kg, in 0.1 ml of saline). Imaging was performed on a Spectra Physics MaiTai HP DeepSee/Olympus Fluoview FV1000 multiphoton imaging setup with a 25x NA 1.05 water immersion microscope objective, and recorded by FluoView1000 software. The excitation wavelength was 880 nm, with the laser power at the sample set below 10 mW. The Texas Red fluorescence was detected using a 607/36 bandpass emission filter. MA was delivered during the imaging session via an intraperiteonal catheter.
Arterioles and veins were identified by their characteristic branching pattern, color under white light illumination, blood flow direction and relative position. Vessel diameter was extracted from two-photon images of pial arterioles and venules using a computer program written by one of the authors (AP). The diameter of the vessel was determined by placing a line perpendicular to the vessel and then measuring the intensity of the signal. The intensity of the signal was greater inside the vessel, because the blood contained Texas Red-dextran. The diameter of the vessel was measured automatically through all the images recorded during the imaging session.
Statistical analysis
Differences between the baseline level of CBF or blood vessel diameter, and the level of CBF or blood vessel diameter following exposure to MA were assessed using the Wilcoxon signed rank test. The difference between the baseline PaCO2 levels and the PaCO2 levels following exposure to MA was tested by two-tailed Student’s t-test. All analyses were implemented with SAS 9.2 (SAS Institute Inc., Cary, NC).
Research Highlights.
Even a single exposure to methamphetamine (MA) results in decrease in cerebral blood flow (CBF).
Phentolamine, an alpha-adrenergic receptor blocker, did not prevent the MA-mediated decrease of CBF, arguing against a norepinephrine-dependent mechanism of cerebral vasoconstriction.
Decrease in blood flow is the result of MA-induced hyperventilation and resultant hypocapnia.
These results highlight a previously unappreciated risk of methamphetamine abuse.
Acknowledgments
Sources of support:
NIH grant awards T32NS051152 (OP), R01DA026325 (OP, CS, TD, KK, CF, SD, JS), T32GM007356 (JS), T32CA009363 (CS), the American Heart Association 0635595T (KK), The DANA Foundation Brain and Immuno Imaging Program (KK).
We thank Drs. Handy Gelbard (University of Rochester) and Samuel Poloyac (University of Pittsburgh) for helpful discussions and Dr. Diane Lawrence of NIDA for encouragement and support.
Abbreviations
- CBF
cerebral blood flow
- MA
methamphetamine
- Pa
partial pressure
- i.p.
intraperitoneal
- RR
respiratory rate
- MAP
mean arterial pressure
- HR
heart rate
- BT
body temperature
- bpm
breath per minute
- SEM
standard error of the mean
- SD
standard deviation
Footnotes
Disclosure/conflict of interests: No conflict of interest is reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited literature
- Alhassoon OM, Dupont RM, Schweinsburg BC, Taylor MJ, Patterson TL, Grant I. Regional cerebral blood flow in cocaine- versus methamphetamine-dependent patients with a history of alcoholism. Int J Neuropsychopharmacol. 2001;4:105–12. doi: 10.1017/S1461145701002334. [DOI] [PubMed] [Google Scholar]
- Brassard P, Seifert T, Secher NH. Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth. 2009;102:800–5. doi: 10.1093/bja/aep065. [DOI] [PubMed] [Google Scholar]
- Busija DW, Heistad DD, Marcus ML. Effects of sympathetic nerves on cerebral vessels during acute, moderate increases in arterial pressure in dogs and cats. Circ Res. 1980;46:696–702. doi: 10.1161/01.res.46.5.696. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Sadler BM, Hill JM, Voyksner RD, Pugh DE, White WR, Perez-Reyes M. Pharmacokinetics of oral methamphetamine and effects of repeated daily dosing in humans. Drug Metab Dispos. 1992;20:856–62. [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Davis MJ. Myogenic response gradient in an arteriolar network. Am J Physiol. 1993;264:H2168–79. doi: 10.1152/ajpheart.1993.264.6.H2168. [DOI] [PubMed] [Google Scholar]
- Delaney P, Estes M. Intracranial hemorrhage with amphetamine abuse. Neurology. 1980;30:1125–8. doi: 10.1212/wnl.30.10.1125. [DOI] [PubMed] [Google Scholar]
- Devous MD, Sr, Trivedi MH, Rush AJ. Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers. J Nucl Med. 2001;42:535–42. [PubMed] [Google Scholar]
- Folgering H, Ponte J, Sadig T. Adrenergic mechanisms and chemoreception in the carotid body of the cat and rabbit. J Physiol. 1982;325:1–21. doi: 10.1113/jphysiol.1982.sp014131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, Owens SM. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM., Jr Comparison of the myogenic response in rat cerebral arteries of different calibers. Brain Res. 1998;785:293–8. doi: 10.1016/s0006-8993(97)01419-4. [DOI] [PubMed] [Google Scholar]
- Graham RM, Pettinger WA. Effects of prazosin and phentolamine on arterial pressure, heart rate, and renin activity: evidence in the conscious rat for the functional significance of the presynaptic alpha-receptor. J Cardiovasc Pharmacol. 1979;1:497–502. doi: 10.1097/00005344-197909000-00002. [DOI] [PubMed] [Google Scholar]
- Hartman BK, Zide D, Udenfriend S. The use of dopamine -hydroxylase as a marker for the central noradrenergic nervous system in rat brain. Proc Natl Acad Sci U S A. 1972;69:2722–6. doi: 10.1073/pnas.69.9.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Marcus ML, Sandberg S, Abboud FM. Effect of sympathetic nerve stimulation on cerebral blood flow and on large cerebral arteries of dogs. Circ Res. 1977;41:342–50. doi: 10.1161/01.res.41.3.342. [DOI] [PubMed] [Google Scholar]
- Hwang J, Lyoo IK, Kim SJ, Sung YH, Bae S, Cho SN, Lee HY, Lee DS, Renshaw PF. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006;82:177–81. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Iyo M, Namba H, Yanagisawa M, Hirai S, Yui N, Fukui S. Abnormal cerebral perfusion in chronic methamphetamine abusers: a study using 99MTc-HMPAO and SPECT. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:789–96. doi: 10.1016/s0278-5846(97)00079-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–5. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–25. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Katsumura H, Kabuto M, Hosotani K, Handa Y, Kobayashi H, Kubota T. The influence of total body hyperthermia on brain haemodynamics and blood-brain barrier in dogs. Acta Neurochir (Wien) 1995;135:62–9. doi: 10.1007/BF02307416. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S, Duflou J, McKetin R. Methamphetamine-related fatalities in Australia: demographics, circumstances, toxicology and major organ pathology. Addiction. 2008;103:1353–60. doi: 10.1111/j.1360-0443.2008.02231.x. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH, Herd JA. Effects of propranolol, phentolamine and methyl atropine on cardiovascular function in the squirrel monkey during behavioral experiments. J Pharmacol Exp Ther. 1972;182:204–17. [PubMed] [Google Scholar]
- Kimmerly DS, Tutungi E, Wilson TD, Serrador JM, Gelb AW, Hughson RL, Shoemaker JK. Circulating norepinephrine and cerebrovascular control in conscious humans. Clin Physiol Funct Imaging. 2003;23:314–9. doi: 10.1046/j.1475-0961.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci. 2007;26:1242–53. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Dietrich WD, Navari RM, Povlishock JT, Ghatak NR, Ellis EF, Patterson JL., Jr Mechanism of cerebral arteriolar abnormalities after acute hypertension. Am J Physiol. 1981;240:H511–27. doi: 10.1152/ajpheart.1981.240.4.H511. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- Lineberry TW, Bostwick JM. Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc. 2006;81:77–84. doi: 10.4065/81.1.77. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–14. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Changes in cerebral blood flow and mental state after amphetamine challenge in schizophrenic patients. Neuropsychobiology. 1989;21:117–23. doi: 10.1159/000118564. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Deshmukh VD, Harper AM. Indirect sympathomimetic agents and cerebral blood flow and metabolism. Eur J Pharmacol. 1978;47:11–8. doi: 10.1016/0014-2999(78)90368-0. [DOI] [PubMed] [Google Scholar]
- Mediavilla A, Feria M, Fernandez JF, Cagigas P, Pazos A, Florez J. The stimulatory action of d-amphetamine on the respiratory centre, and its mediation by a central alpha-adrenergic mechanism. Neuropharmacology. 1979;18:133–42. doi: 10.1016/0028-3908(79)90053-4. [DOI] [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–6. [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–20. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, 3rd, Everhart ET, Jones RT. Human pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther. 2006;80:403–20. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shimazu K, Okamoto S, Koto A, Ouchi T, Sari A, Ericsson AD. Effects of alpha adrenergic blockade on autoregulation and chemical vasomotor control of CBF in stroke. Stroke. 1973;4:187–200. doi: 10.1161/01.str.4.2.187. [DOI] [PubMed] [Google Scholar]
- Moppett IK, Sherman RW, Wild MJ, Latter JA, Mahajan RP. Effects of norepinephrine and glyceryl trinitrate on cerebral haemodynamics: transcranial Doppler study in healthy volunteers. Br J Anaesth. 2008;100:240–4. doi: 10.1093/bja/aem374. [DOI] [PubMed] [Google Scholar]
- Muizelaar JP, van der Poel HG, Li ZC, Kontos HA, Levasseur JE. Pial arteriolar vessel diameter and CO2 reactivity during prolonged hyperventilation in the rabbit. J Neurosurg. 1988;69:923–7. doi: 10.3171/jns.1988.69.6.0923. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–70. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg K, Siesjo BK. Quantitative measurement of blood flow and oxygen consumption in the rat brain. Acta Physiol Scand. 1974;91:154–64. doi: 10.1111/j.1748-1716.1974.tb05671.x. [DOI] [PubMed] [Google Scholar]
- Perez JA, Jr, Arsura EL, Strategos S. Methamphetamine-related stroke: four cases. J Emerg Med. 1999;17:469–71. doi: 10.1016/s0736-4679(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–78. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Richards CF, Clark RF, Holbrook T, Hoyt DB. The effect of cocaine and amphetamines on vital signs in trauma patients. J Emerg Med. 1995;13:59–63. doi: 10.1016/0736-4679(94)00123-5. [DOI] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Strudwick MW, McMahon KL, Chalk JB, Snyder P, De zubicaray GI. Assessment of dynamic susceptibility contrast cerebral blood flow response to amphetamine challenge: a human pharmacological magnetic resonance imaging study at 1.5 and 4 T. Magn Reson Med. 2006;55:9–15. doi: 10.1002/mrm.20749. [DOI] [PubMed] [Google Scholar]
- Ruland S, Aiyagari V. Cerebral autoregulation and blood pressure lowering. Hypertension. 2007;49:977–8. doi: 10.1161/HYPERTENSIONAHA.107.087502. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am. 2005;89:1277–96. doi: 10.1016/j.mcna.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Sato A, Sato Y, Trzebski A. Effects of stimulation of cervical sympathetic trunks with various frequencies on the local cortical cerebral blood flow measured by laser Doppler flowmetry in the rat. Jpn J Physiol. 1990;40:15–32. doi: 10.2170/jjphysiol.40.15. [DOI] [PubMed] [Google Scholar]
- Salanova V, Taubner R. Intracerebral haemorrhage and vasculitis secondary to amphetamine use. Postgrad Med J. 1984;60:429–30. doi: 10.1136/pgmj.60.704.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol (Phila) 2010;48:675–94. doi: 10.3109/15563650.2010.516752. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Zheng JW, Tella SR, Goldberg SR. Pharmacological mechanisms in the cardiovascular effects of methamphetamine in conscious squirrel monkeys. Pharmacol Biochem Behav. 1992;42:791–6. doi: 10.1016/0091-3057(92)90031-a. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Blood pressure and heart rate responses produced by d-amphetamine: correlation with blood levels of drug. J Pharmacol Exp Ther. 1978;205:366–73. [PubMed] [Google Scholar]
- Skinhoj E. The sympathetic nervous system and the regulation of cerebral blood flow in man. Stroke. 1972;3:711–6. doi: 10.1161/01.str.3.6.711. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tsai ML, Lee CY, Lin MT. Responses of cerebral circulation produced by adrenoceptor agonists and antagonists in rats. Neuropharmacology. 1989;28:1075–80. doi: 10.1016/0028-3908(89)90120-2. [DOI] [PubMed] [Google Scholar]
- Vavilala MS, Lee LA, Lam AM. Cerebral blood flow and vascular physiology. Anesthesiol Clin North America. 2002;20:247–64. doi: 10.1016/s0889-8537(01)00012-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayashi T, Chang CF, Chiang YH, Tsao LI, Su TP, Borlongan C, Lin SZ. Methamphetamine potentiates ischemia/reperfusion insults after transient middle cerebral artery ligation. Stroke. 2001;32:775–82. doi: 10.1161/01.str.32.3.775. [DOI] [PubMed] [Google Scholar]
- Wechsler LR, Savaki HE, Sokoloff L. Effects of d- and l-amphetamine on local cerebral glucose utilization in the conscious rat. J Neurochem. 1979;32:15–22. doi: 10.1111/j.1471-4159.1979.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry. 2007;64:495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- Yen DJ, Wang SJ, Ju TH, Chen CC, Liao KK, Fuh JL, Hu HH. Stroke associated with methamphetamine inhalation. Eur Neurol. 1994;34:16–22. doi: 10.1159/000117002. [DOI] [PubMed] [Google Scholar]
- Zimmer R, Lang R, Oberdorster G. Effects of catecholamine infusions on cerebral blood flow and oxygen consumption of the isolated perfused dog brain. Stroke. 1974;5:397–405. doi: 10.1161/01.str.5.3.397. [DOI] [PubMed] [Google Scholar]