Abstract
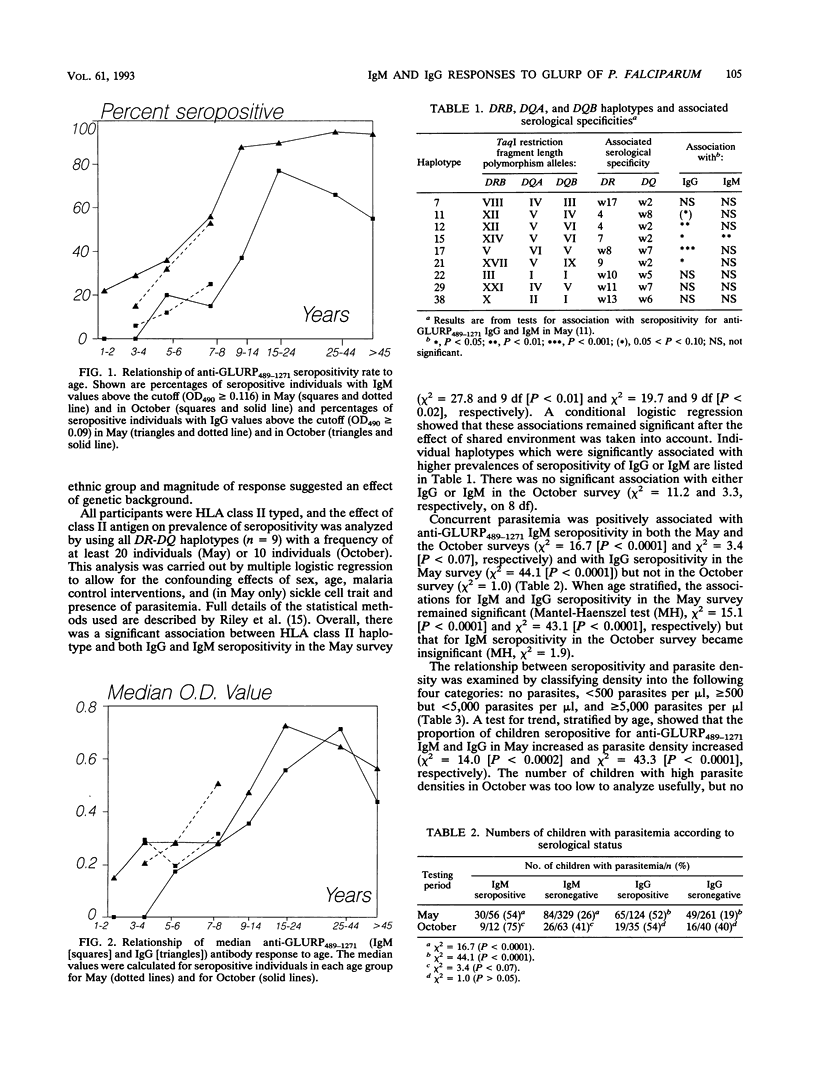
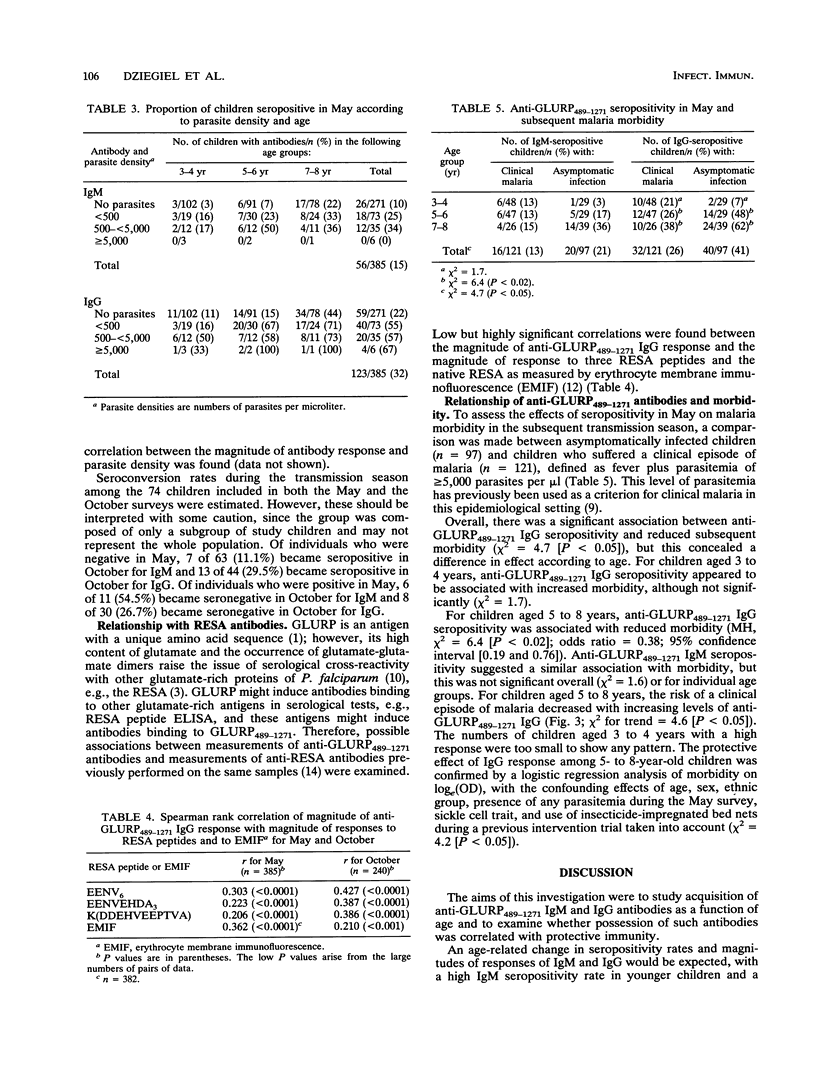
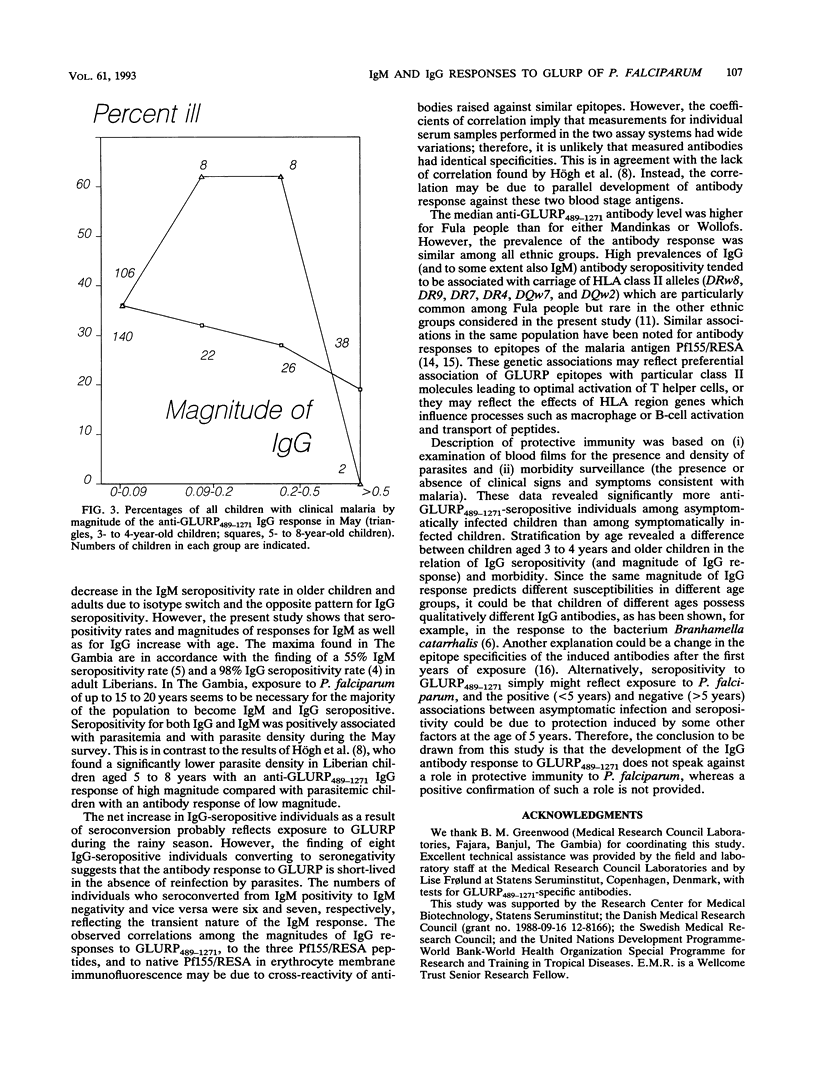
The aims of the present study were to describe the age-related immunoglobulin M (IgM) and IgG response to part of a 220-kDa glutamate-rich protein (GLURP) from Plasmodium falciparum and to determine possible correlations of possession of these antibodies with malaria morbidity. IgM and IgG levels were measured with a recombinant fusion protein consisting of the carboxy-terminal 783 amino acids of the GLURP. Samples for the study were obtained during a longitudinal malaria morbidity survey performed in The Gambia; cross-sectional surveys were performed at the beginning of the transmission season in May and in October. Seropositivity rates increased with age to a maximum of 77% for IgM and 95% for IgG in adults. High prevalences of seropositivity were associated with certain human leukocyte antigen class II alleles (DRw8, DR9, DR7, DR4, DQw7, and DQw2) or haplotypes. The relationship between anti-GLURP489-1271 antibodies and clinical immunity is not clear; asymptomatically infected children aged 5 to 8 years had significantly higher levels of IgG than clinically ill children of the same age, suggesting that antibodies to the carboxy-terminal part of the GLURP may contribute to immunity to P. falciparum. However, this was not significant for younger children.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borre M. B., Dziegiel M., Høgh B., Petersen E., Rieneck K., Riley E., Meis J. F., Aikawa M., Nakamura K., Harada M. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasitol. 1991 Nov;49(1):119–131. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- Carlsson B., Wallin J., Böhme J., Möller E. HLA-DR-DQ haplotypes defined by restriction fragment analysis. Correlation to serology. Hum Immunol. 1987 Oct;20(2):95–113. doi: 10.1016/0198-8859(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Dziegiel M., Borre M. B., Jepsen S., Hogh B., Petersen E., Vuust J. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991 Mar;44(3):306–313. doi: 10.4269/ajtmh.1991.44.306. [DOI] [PubMed] [Google Scholar]
- Goldblatt D., Turner M. W., Levinsky R. J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990 Nov;162(5):1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Bradley A. K., Greenwood A. M., Byass P., Jammeh K., Marsh K., Tulloch S., Oldfield F. S., Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81(3):478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Hogh B., Petersen E., Dziegiel M., David K., Hanson A., Borre M., Holm A., Vuust J., Jepsen S. Antibodies to a recombinant glutamate-rich Plasmodium falciparum protein: evidence for protection of individuals living in a holoendemic area of Liberia. Am J Trop Med Hyg. 1992 Mar;46(3):307–313. doi: 10.4269/ajtmh.1992.46.307. [DOI] [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Olerup O., Troye-Blomberg M., Schreuder G. M., Riley E. M. HLA-DR and -DQ gene polymorphism in West Africans is twice as extensive as in north European Caucasians: evolutionary implications. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8480–8484. doi: 10.1073/pnas.88.19.8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Bennett S., Thomas P. J., O'Donnell A., Lindsay S. W., Good M. F., Greenwood B. M. Recognition of dominant T cell-stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):648–657. doi: 10.1016/0035-9203(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Troye-Blomberg M., Bennett S., Perlmann H., Andersson G., Smedman L., Perlmann P., Greenwood B. M. Association between immune recognition of the malaria vaccine candidate antigen Pf155/RESA and resistance to clinical disease: a prospective study in a malaria-endemic region of west Africa. Trans R Soc Trop Med Hyg. 1991 Jul-Aug;85(4):436–443. doi: 10.1016/0035-9203(91)90207-f. [DOI] [PubMed] [Google Scholar]
- Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today. 1991 May;7(5):99–105. doi: 10.1016/0169-4758(91)90166-l. [DOI] [PubMed] [Google Scholar]


