Abstract
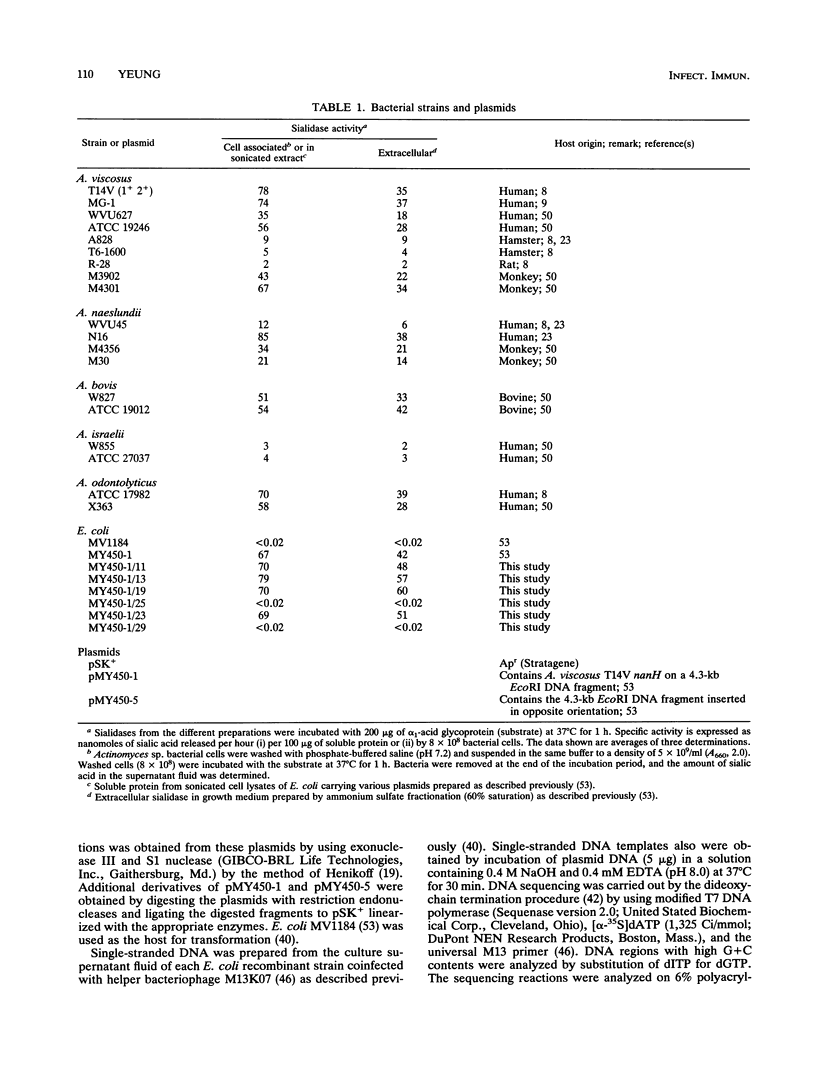
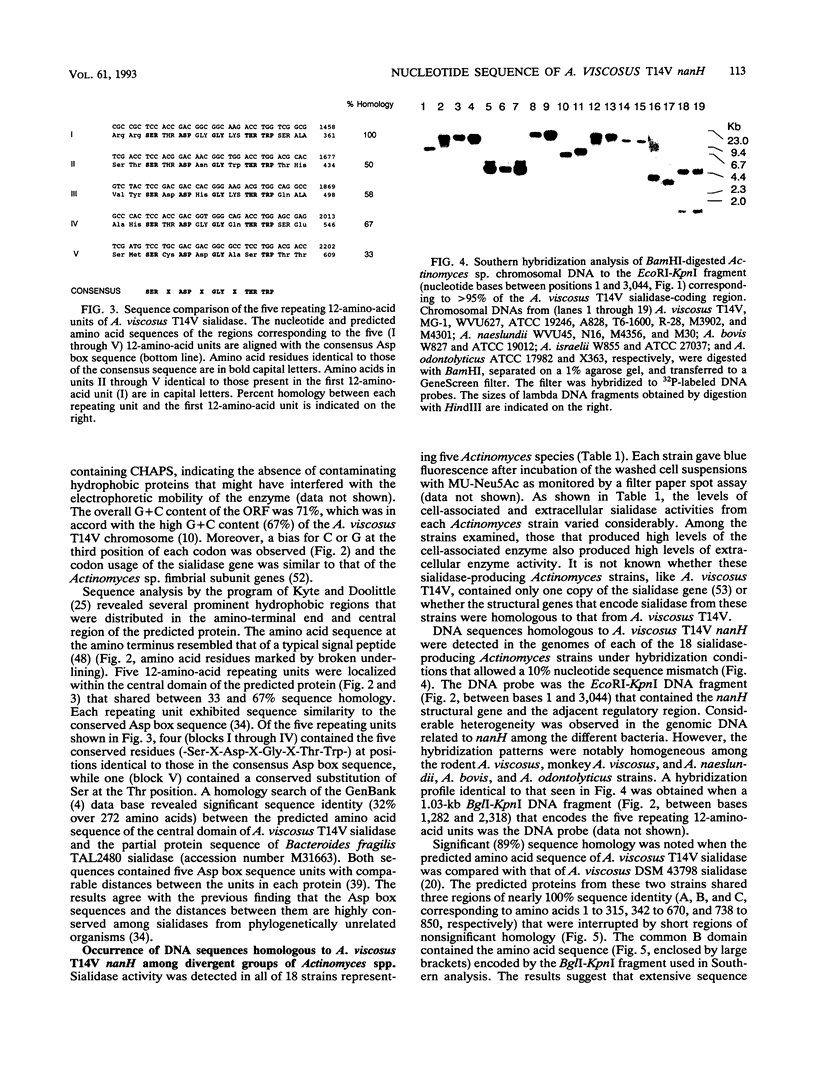
The nucleotide sequence of the Actinomyces viscosus T14V sialidase gene (nanH) and flanking regions was determined. An open reading frame of 2,703 nucleotides that encodes a predominately hydrophobic protein of 901 amino acids (M(r), 92,871) was identified. The amino acid sequence at the amino terminus of the predicted protein exhibited properties characteristic of a typical leader peptide. Five 12-amino-acid units that shared between 33 and 67% sequence identity were noted within the central domain of the protein. Each unit contained the sequence Ser-X-Asp-X-Gly-X-Thr-Trp, which is conserved among other bacterial and trypanosoma sp. sialidases. Thus, the A. viscosus T14V nanH gene and the other prokaryotic and eukaryotic sialidase genes evolved from a common ancestor. Southern hybridization analyses under conditions of high stringency revealed the existence of DNA sequences homologous to A. viscosus T14V nanH in the genomes of 18 strains of five Actinomyces species that expressed various levels of sialidase activity. The data demonstrate that the sialidase genes from divergent groups of Actinomyces spp. are highly conserved.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Webster R. G., Colman P. M., Laver W. G. Distribution of sequence differences in influenza N9 neuraminidase of tern and whale viruses and crystallization of the whale neuraminidase complexed with antibodies. Virology. 1987 Oct;160(2):346–354. doi: 10.1016/0042-6822(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Beighton D., Whiley R. A. Sialidase activity of the "Streptococcus milleri group" and other viridans group streptococci. J Clin Microbiol. 1990 Jun;28(6):1431–1433. doi: 10.1128/jcm.28.6.1431-1433.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Paton J. C., Glare E. M., Hansman D., Catcheside D. E. Cloning and expression of the pneumococcal neuraminidase gene in Escherichia coli. Gene. 1988 Nov 30;71(2):299–305. doi: 10.1016/0378-1119(88)90046-7. [DOI] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Sandberg A. L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986 Jun;52(3):840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara M., Mitchell T. J., Andrew P. W., Boulnois G. J. Streptococcus pneumoniae produces at least two distinct enzymes with neuraminidase activity: cloning and expression of a second neuraminidase gene in Escherichia coli. Infect Immun. 1991 Aug;59(8):2856–2858. doi: 10.1128/iai.59.8.2856-2858.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi. Proteases B and C are synthesized and secreted as zymogens without a signal peptide. J Biol Chem. 1989 May 25;264(15):9083–9089. [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Brown R. Neuraminidase production by Bacteroidaceae. J Med Microbiol. 1981 Feb;14(1):63–76. doi: 10.1099/00222615-14-1-63. [DOI] [PubMed] [Google Scholar]
- Galen J. E., Ketley J. M., Fasano A., Richardson S. H., Wasserman S. S., Kaper J. B. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect Immun. 1992 Feb;60(2):406–415. doi: 10.1128/iai.60.2.406-415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Henningsen M., Roggentin P., Schauer R. Cloning, sequencing and expression of the sialidase gene from Actinomyces viscosus DSM 43798. Biol Chem Hoppe Seyler. 1991 Dec;372(12):1065–1072. doi: 10.1515/bchm3.1991.372.2.1065. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Moore L. V., Kaneko B., Moore W. E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990 Jul;40(3):273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- Kahn S., Colbert T. G., Wallace J. C., Hoagland N. A., Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Fenner L. J., Antonelli P. J., Coulter M. C. Effect of neuraminidase on the adherence to salivary pellicle of Streptococcus sanguis and Streptococcus mitis. Caries Res. 1989;23(3):141–145. doi: 10.1159/000261167. [DOI] [PubMed] [Google Scholar]
- Moncla B. J., Braham P. Detection of sialidase (neuraminidase) activity in Actinomyces species by using 2'-(4-methylumbelliferyl)alpha-D-N-acetylneuraminic acid in a filter paper spot test. J Clin Microbiol. 1989 Jan;27(1):182–184. doi: 10.1128/jcm.27.1.182-184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlitsh M. J., Glickman I. Salivary neuraminidase. I. The presence of neuraminidase in human saliva. J Periodontol. 1966 Sep-Oct;37(5):368–373. doi: 10.1902/jop.1966.37.5.368. [DOI] [PubMed] [Google Scholar]
- Rogers R., Newbrun E., Tatevossian A. Neuraminidase activity in human dental plaque fluid. Arch Oral Biol. 1979;24(9):703–705. doi: 10.1016/0003-9969(79)90121-3. [DOI] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Lottspeich F., Schauer R. Cloning and sequencing of a Clostridium perfringens sialidase gene. FEBS Lett. 1988 Sep 26;238(1):31–34. doi: 10.1016/0014-5793(88)80219-9. [DOI] [PubMed] [Google Scholar]
- Rothe B., Roggentin P., Frank R., Blöcker H., Schauer R. Cloning, sequencing and expression of a sialidase gene from Clostridium sordellii G12. J Gen Microbiol. 1989 Nov;135(11):3087–3096. doi: 10.1099/00221287-135-11-3087. [DOI] [PubMed] [Google Scholar]
- Rothe B., Rothe B., Roggentin P., Schauer R. The sialidase gene from Clostridium septicum: cloning, sequencing, expression in Escherichia coli and identification of conserved sequences in sialidases and other proteins. Mol Gen Genet. 1991 Apr;226(1-2):190–197. doi: 10.1007/BF00273603. [DOI] [PubMed] [Google Scholar]
- Rowley P. T., Jacobs M., Rosecrans C., Weitkamp L. R., Doherty R. A. High resolution analysis of hemoglobins: polyacrylamide isoelectric focusing. Biochem Med. 1972 Dec;6(6):553–560. doi: 10.1016/0006-2944(72)90010-5. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Mudrick L. L., Cisar J. O., Brennan M. J., Mergenhagen S. E., Vatter A. E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986 Nov;54(2):472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Steuler H., Rohde W., Scholtissek C. Sequence of the neuraminidase gene of an avian influenza A virus (A/parrot/ulster/73, H7N1). Virology. 1984 May;135(1):118–124. doi: 10.1016/0042-6822(84)90122-3. [DOI] [PubMed] [Google Scholar]
- Tuyau J. E., Sims W. Occurrence of haemophili in dental plaque and their association with neuraminidase activity. J Dent Res. 1975 Jul-Aug;54(4):737–739. doi: 10.1177/00220345750540040701. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Westpheling J., Ranes M., Losick R. RNA polymerase heterogeneity in Streptomyces coelicolor. Nature. 1985 Jan 3;313(5997):22–27. doi: 10.1038/313022a0. [DOI] [PubMed] [Google Scholar]
- Yeung M. K., Cisar J. O. Sequence homology between the subunits of two immunologically and functionally distinct types of fimbriae of Actinomyces spp. J Bacteriol. 1990 May;172(5):2462–2468. doi: 10.1128/jb.172.5.2462-2468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K. Conservation of an Actinomyces viscosus T14V type 1 fimbrial subunit homolog among divergent groups of Actinomyces spp. Infect Immun. 1992 Mar;60(3):1047–1054. doi: 10.1128/iai.60.3.1047-1054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Fernandez S. R. Isolation of a neuraminidase gene from Actinomyces viscosus T14V. Appl Environ Microbiol. 1991 Nov;57(11):3062–3069. doi: 10.1128/aem.57.11.3062-3069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]




