Abstract
Background
Dendritic cell (DC)-based tumor vaccine is an attractive modality for the treatment of colon cancer because it has been recurred and produced few side effects in patients. Secretory glycoprotein 90K has been found at elevated level in various cancer tissues and sera. We investigated to establish a more effective DC vaccine for the treatment of colon cancer in which the levels of 90K are elevated.
Methods
We obtained the concentrated 90K from 293T cells stably expressing 90K. DCs were cultured from peripheral blood monocytes, and a DC vaccine pulsed with tumor lysate was compared with a DC vaccine pulsed with 90K. We measured the functional activity for CTLs by using IFN-γ-enzyme linked immunoabsorbent spot (ELISPOT) assay.
Results
DCs pulsed with tumor lysate+90K exhibited the enhanced T cell stimulation, polarization of naïve T cell toward Th1. The CTLs generated by DCs pulsed with 90K efficiently lysed HCT116 cells. The results indicate that 90K-speicifc-CTLs can recognize 90K proteins naturally presented by colon cancer cells.
Conclusion
Our study suggests that 90K-specific CTLs generated by 90K-pulsed DCs could be useful effector cells for immunotherapy in colon cancer.
Keywords: Dendritic cell, 90K, Colon cancer, Immunotherapy, CTLs
INTRODUCTION
Dendritic cells (DCs) are potent antigen presenting cells (APC), which are highly efficient in antigen presentation and stimulation of T lymphocytes (1).
Tumor vaccines must induce an immune response capable of clearing and eradicating tumor cells from cancer patients (2,3). Many attempts have been developed to induce tumor-specific immune responses, including vaccination with autologous/allogeneic tumor cells, tumor-associated proteins or peptides, and transducing DCs with viral vectors encoding tumor antigens. Especially, several recent clinical trials utilizing antigen-pulsed DCs have demonstrated increased survival of vaccinated cancer patients, the majority of tumor vaccines, including DC vaccines, can effectively induce tumor associated antigen (TAA)-specific immune responses (4,5).
90K has been reported to be a ~90 kDa secreted glycoprotein that is a ligand of galectin-3 (formerly known as Mac-2) (6,7). Numerous cell types, including hematopoietic cells and epithelial cell, synthesize and secrete 90K, thus 90K is usually present in the serum and other biologic fluids (6,8,9). High 90K serum levels have been detected in patients with inflammatory and neoplastic diseases, such as chronic hepatitis C and B (10), breast cancer (11), gastric cancer (12) and lung cancer (13). Immunohistochemical analysis of 90K expression has revealed weak or no expression in most normal tissues and strong up-regulation in tumor cells of human neoplastic tissues (14). These findings suggest that 90K can be used as a target antigen in cancer immunotherapy. It is therefore expected that 90K will be a novel target molecule in immunotherapy of colon cancer as of other 90K-positive cancers.
In this study, we investigated the possibility of immunotherapy for colon cancer using 90K-specific cytotoxic T lymphocytes (CTLs) that were stimulated in vitro by dendritic cells (DCs) pulsing with colon cancer cell lysates and 90K protein.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital.
Cell culture
HCT8, HCT116 (human colon adenocarcinoma cell lines) and 293T (human embryonic kidney cell lines) were maintained in DMEM medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum.
Generation of dendritic cells
Monocyte-derived immature dendritic cells (imDCs) were generated from human peripheral blood mononuclear cells (PBMCs) in granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin 4 (IL-4). ImDCs (day 6) were pulsed for 6 hrs with antigen, and then antigen-pulsed imDCs were matured with DC cocktail composed of IL-1β (10 ng/ml), TNF-α (50 ng/ml), IL-6 (10 ng/ml) and PGE2 (1 µg/ml) for 48 hrs. To obtain a concentrated 90K supernatant, we established 293T cells stably expressing 90K (293T/90K cells). Concentrated 90K supernatant from the 293T/90K cells was used to stimulate the DCs pulsed with the tumor lysate.
Immunophenotyping
CD86 and HLA-DR-Phycoerythrin or CD80, CD83 and HLA-ABC-conjugated with fluorescein isothiocyanate (FITC) monoclonal antibodies (BD Pharmningen) were used to analysis for immunophenotyping. Samples were analyzed using a flow cytometer (FACSCaliber; Becton Dickinson). Data anaylsis used the CELLQUEST software (Becton Dickinson).
Mixed leukocyte reaction (MLR)
Functional activity of DC was determined in the primary allogeneic MLR assay, using human T lymphocytes as responder cells. CD3+T cells were isolated using the CD3+T cell isolation kit II (Miltenyi Biotec) from human PBMCs. The MLR assays were carried out in round bottomed 96-well plates to ensure efficient DC/T cell contact. DC were added in triplicates in graded doses (105~106 cells per well) to T cells (1×105 cells per well) in a total volume of 200 µl of a CM per well. Proliferation of T cells was measured by uptake of 3H-thymidine (1 µCi/well, 5 Ci/mmol; DuPont-NEN) pulsed for 16~18 h after 5 days in culture. The cultures were harvested on GF/C glass fiber filter paper (Whatman Intl. Ltd., Maidstone, UK) using a MACH III microwell harvester (Tomtec, Hamden, CT). Incorporation of 3H-thymidine was determined on a Betacounter (Beckmann). The counts were expressed as a count per minute (cpm).
Enzyme-linked immunosorbent spot assay
The enzyme-linked immunosorbent spot (ELISPOT) assay for enumeration of antigen-specific, IFN-γ-secreting cells have been followed as manuals (BD Pharmingen). The number of IFN-γ spots was enumerated by Immuno Spot Analyzers (Cellular Technology Ltd). Data are expressed as the mean number of IFN-γ-secreting cells/105 T cells.
Cytokine release
DCs were grown in 24-well plates at 1×106 cells/well. Following supernatants were collected and stored at -80℃. IL-10, IL-12p70, IFN-γ and IL-4 concentrations were measured using ELISA kits (BD Pharmingen) according to the manufacturer-provided protocols.
RESULTS
Expression of 90K in colon cancer
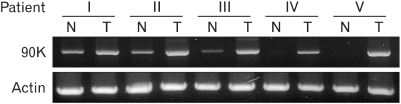
RT-PCR analysis was used to examine 90K expression in human colon cancer tissues. In contrast to the adjacent normal colon, which either lacked detectable 90K mRNA or expressed low levels of 90K mRNA, the colon cancer tissues from all 5 patients examined showed elevated 90K mRNA expression (Fig. 1). This suggests that the 90K protein is a possible candidate as a colon cancer associated tumor antigen.
Figure 1.
Expression of the 90K mRNA in colon cancer tissues. The expression of 90K was determined from extraneoplastic (Normal, N) and neoplastic regions (Tumor, T) in 5 patients with colon cancer by using RT-PCR.
Generation of 90K -pulsed DCs
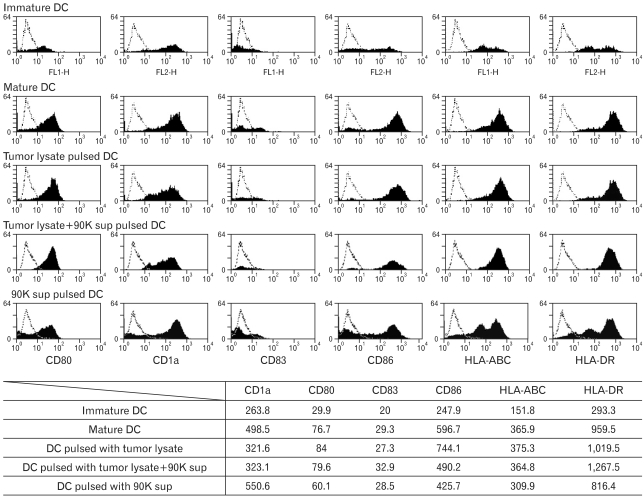
We used FACS analysis to compare the phenotype of the tumor lysate-pulsed mature DCs with the phenotype of the tumor lysate+90K supernatant (1 µg/ml)-pulsed and 90K supernatant (1 µg/ml)-pulsed mature DCs, and we found that both DCs exhibited high expression of CD80, CD83, CD86, HLA-ABC, and DR, which was characteristic of mature DCs (Fig. 2).
Figure 2.
Immunophenotype of DCs pulsed with antigens. Comparison of phenotypes of immature DC, unpulsed mDCs, tumor lysate-pulsed DCs, tumor lysate+90K-pulsed DCs and 90K-pulsed DCs. Immature DCs were obtained by culturing peripheral blood mononuclear cells (PBMCs) of healthy donors with IL-4 and GM-CSF. A DC cytokine cocktail was then added to the culture, and the cells were incubated for 48 hours to enable the immature cells to differentiate into mature DCs. Data shown as mean fluorescence intensity (MFI) increase over isotype control±with SD from three independent experiments.
T cell proliferation
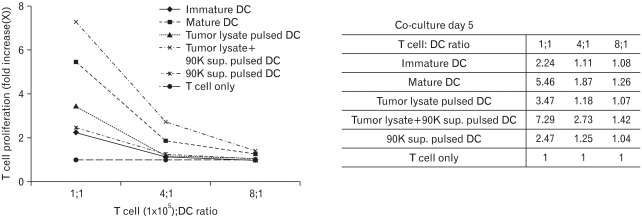
The ability of DCs to induce T cell proliferation in the mixed lymphocyte reaction (MLR) has been commonly used to evaluate their function. Therefore, we examined DC-induced CD3+T cell proliferation.
In the group of DCs sensitized with tumor lysates, T cell proliferation rate was 3.47; the T cell group without stimulation by DCs was used as the control (Fig. 3). And the group of DCs sensitized with 90K sup resulted in a lower proliferation index than tumor lysate did. On the other hand, in the DC group treated with tumor lysate+90K, the absorption rate was 7.29. This result indicates that addition of 90K increased the potential of DCs as T cell stimulators.
Figure 3.
Proliferation of allogeneic T cells stimulated with DCs pulsed by antigen. T cells were stimulated with DCs pulsed tumor lysate, tumor lysate+90K sup and 90K. T cells only were used as a negative control. DCs were co-cultured with 1×105 allogeneic T cells/well in 96-well plates for 5 days. Then, 1 µl Ci of 3H-thymidine was added to each well, and the cells were incubated for 16~18 hours before harvesting. T-cell proliferation was measured by 3[H]-thymidine uptake.
Measurement of cytokines
DCs activate T cells and regulate their differentiation into T helper cells type 1 (Th1) and/or T helper cells type 2 (Th2). So, we estimated the polarization of naive T cells toward the Th1 or Th2 subset by DCs pulsed with tumor lysate+90K. High IL-12p70 and IFN-γ levels induce the differentiation into Th1 cells. The concentrations of IL12p70 were 6.5, 9.8, and 21.2 pg/ml in the DC single group, tumor lysate-pulsed DC group, and tumor lysate+90K-pulsed DC group, respectively (Fig. 4A).
Figure 4.
Cytokine secretion assay. (A) DC function estimated by the IL-12p70 assay. Supernatants from tumor lysate-pulsed DCs and tumor lysate+90K-pulsed DCs were examined for the secretion of IL-10 and IL-12p70 by ELISA. (B) Generation of IFN-γ-producing naïve T cells coculturing with DCs. Naïve T cells stimulated by co-culture with tumor lysate-pulsed DCs and tumor lysate+90K-pulsed DCs were analyzed for the production of IL-4 and IFN-γ by ELISA.
Tumor lysate-pulsed DCs induced high IFN-γ production by T cells with minimal or no Th2 cytokine production. At day 7, the concentrations of IFN-γ were 1.9, 87.7, and 170.8 in the T cells stimulated with DCs, tumor lysate-pulsed DC group, and tumor lysate+90K pulsed DCs, respectively (Fig. 4B). T cells stimulated with tumor lysate+90K-pulsed DCs produced significantly higher levels of IFN-γ than those pulsed with tumor lysate alone. This result indicates that IL-12p70 and IFN-γ production was increased significantly with tumor lysate treatment in the presence of 90K.
Comparison of cytotoxicity
Tumor lysate-induced IFN-γ secretion by cytotoxic T cells (CTLs) is often determined by enzyme-linked immunospot (ELISPOT) assay and used as an immunological correlate of cytotoxic activity.
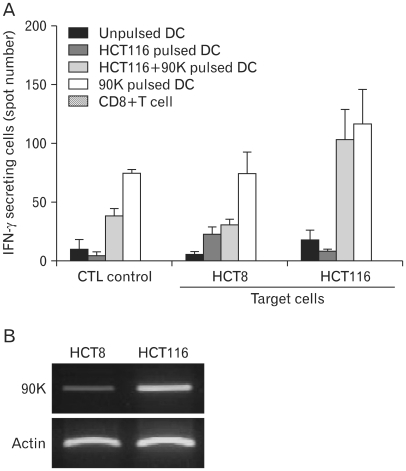
To generate antigen-specific CTLs from healthy donors, DCs pulsed with different antigens were used as antigen-presenting cells (APCs). CTLs were co-cultured with the target HCT8 or HCT116 cells, and their specific responses to antigens were examined by ELISPOT. HCT8 cells were used as target control cells, because of detection of low level 90K. The number of IFN-γ-secreting cells were increased about ten-fold among CTLs stimulated with tumor lysate+90K-pulsed DCs, against HCT116 cells than among those stimulated with tumor lysate-pulsed DCs (Fig. 5). Especially, T cells stimulated with 90K-pulsed DCs exhibited the highest amount among the CTL stimulated with antigen loaded DCs. The results indicate that the 90K protein can induce a strong antigen-specific CTL response.
Figure 5.
Production of IFN-γ-secreting cells from autologous CD8+ T cells. (A) IFN-γ-secreting cells were enumerated by the ELISPOT assay, and the results are given as the mean number of spots and SD per 1×105 T cells. T cells stimulated with HCT116 tumor lysate-pulsed DCs, tumor lysate+90K pulsed-DCs and 90K pulsed-DCs were cocultured with HCT8 or HCT116 cells in a 96-well plate for 24 hours at 37℃. Unprimed T cells (CTLs only) were used as the negative control. The results are illustrated as the mean values (with SD) of triplicate culture in two independent experiments. (B) Differential expression of the 90K mRNA in colon cancer cells were examined by RT-PCR.
DISCUSSION
DCs are potent antigen-presenting cells able to induce primary immune responses. DCs capture and process antigens into peptides (15-18). DCs then present the antigens to T cells and B cells through MHC class I and II molecules. Peptides, proteins, DNA and RNA coding these antigens and tumor lysate have been used to sensitize DCs. An alternative approach to increasing antitumor immunity is the use of fusions of DCs and tumor cells. In this study, we demonstrated that 90K was highly expressed in colon cancer, indicating that 90K might be a possible candidate as a tumor associated antigen in colon cancer. CTLs that were stimulated by 90K-pulsed DCs displayed high secretion of IFN-γ against HCT116 cells compared to those stimulated by tumor lysate-pulsed DCs (Fig. 5). These results demonstrated that the CTLs were efficiently able to lyse colon cancer cells positive for 90K molecules.
The finding that 90K is expressed in normal tissues such as breast, lung, ovary, stomach, colon, and bladder indicates the possibility of damage to normal tissues by immunotherapy targeting 90K (8). Even though the expression level is low in normal tissue, we need to prove the reactivity and impact of 90K-specific CTLs on normal tissues. Also, 90K is able to enhance not only induction of 90K-specific CTL but also the functionality of DCs. So, we need the further studies about possibility that 90K may have potential to enhance the DC function by activation the downstream signaling cascades associated with DC maturation.
In conclusion, our study suggests that 90K has the potential to be a novel target molecule in the immunotherapy of colon cancer using 90K-specific cytotoxic T lymphocytes.
ACKNOWLEDGEMENTS
This study was supported by a grant from National Research Foundation of Korea (2010-1415) and the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0720570).
Footnotes
The authors have no financial conflict of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan JM, Yu Q, Piraino ST, Pennington SE, Shankara S, Woodworth LA, Roberts BL. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J Immunol. 1999;163:699–707. [PubMed] [Google Scholar]
- 6.Iacobelli S, Arnò E, D'Orazio A, Coletti G. Detection of antigens recognized by a novel monoclonal antibody in tissue and serum from patients with breast cancer. Cancer Res. 1986;46:3005–3010. [PubMed] [Google Scholar]
- 7.Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem. 1991;266:18731–18736. [PubMed] [Google Scholar]
- 8.Koths K, Taylor E, Halenbeck R, Casipit C, Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J Biol Chem. 1993;268:14245–14249. [PubMed] [Google Scholar]
- 9.Ullrich A, Sures I, D'Egidio M, Jallal B, Powell TJ, Herbst R, Dreps A, Azam M, Rubinstein M, Natoli C, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem. 1994;269:18401–18407. [PubMed] [Google Scholar]
- 10.Iacovazzi PA, Trisolini A, Barletta D, Elba S, Manghisi OG, Correale M. Serum 90K/MAC-2BP glycoprotein in patients with liver cirrhosis and hepatocellular carcinoma: a comparison with alpha-fetoprotein. Clin Chem Lab Med. 2001;39:961–965. doi: 10.1515/CCLM.2001.155. [DOI] [PubMed] [Google Scholar]
- 11.Fusco O, Querzoli P, Nenci I, Natoli C, Brakebush C, Ullrich A, Iacobelli S. 90K (MAC-2 BP) gene expression in breast cancer and evidence for the production of 90K by peripheral-blood mononuclear cells. Int J Cancer. 1998;79:23–26. doi: 10.1002/(sici)1097-0215(19980220)79:1<23::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Park YP, Choi SC, Kim JH, Song EY, Kim JW, Yoon DY, Yeom YI, Lim JS, Kim JW, Paik SG, Lee HG. Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer. 2007;120:813–820. doi: 10.1002/ijc.22369. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki Y, Kontani K, Hanaoka J, Chano T, Teramoto K, Tezuka N, Sawai S, Fujino S, Yoshiki T, Okabe H, Ohkubo I. Expression and immunogenicity of a tumor-associated antigen, 90K/Mac-2 binding protein, in lung carcinoma. Cancer. 2002;95:1954–1962. doi: 10.1002/cncr.10899. [DOI] [PubMed] [Google Scholar]
- 14.Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG, Christian S. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. FASEB J. 2008;22:3059–3067. doi: 10.1096/fj.07-101386. [DOI] [PubMed] [Google Scholar]
- 15.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 16.Porgador A, Gillboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]