Abstract
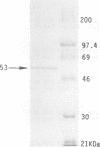
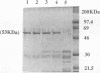
The prtT gene, coding for trypsinlike proteolytic activity, has been isolated from Porphyromonas gingivalis ATCC 53977. This gene is present immediately downstream from the sod gene on a 5.9-kb DNA fragment from the organism isolated in Escherichia coli. The complete nucleotide sequence of the gene was determined, and the deduced amino acid sequence of the enzyme corresponds to a 53.9-kDa protein with an estimated pI of 11.85. Gelatin-sodium dodecyl sulfate-polyacrylamide gel electrophoresis zymography also indicated a similar molecular size for the protease. The enzyme was purified to near homogeneity following anion-exchange and gel-filtration chromatography. The purified enzyme also exhibited a single protein species with a size of approximately 53 kDa. Enzyme activity was strongly dependent upon the presence of reducing agents (dithiothreitol, cysteine, and 2-mercaptoethanol) and was also stimulated in the presence of calcium ions. A comparison of the properties of the prtT gene product with comparable parameters of proteases previously purified from different strains of P. gingivalis suggested that the cloned protease represents a previously uncharacterized enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E., Zambon J. J., Barwa P. K., Neiders M. E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988 Jul;23(4):258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen Z. X., Potempa J., Polanowski A., Renvert S., Wikström M., Travis J. Stimulation of proteinase and amidase activities in Porphyromonas (Bacteroides) gingivalis by amino acids and dipeptides. Infect Immun. 1991 Aug;59(8):2846–2850. doi: 10.1128/iai.59.8.2846-2850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. I., Takahashi N., Kato T., Kuramitsu H. K. Isolation, expression, and nucleotide sequence of the sod gene from Porphyromonas gingivalis. Infect Immun. 1991 Apr;59(4):1564–1566. doi: 10.1128/iai.59.4.1564-1566.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo J., Otsuka M., Ohara E., Sato M., Nakamura R. Cleavage action of a trypsin-like protease from Bacteroides gingivalis 381 on reduced egg-white lysozyme. Arch Oral Biol. 1989;34(11):911–916. doi: 10.1016/0003-9969(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Purification and characterization of a 43-kDa protease of Bacteroides gingivalis. Oral Microbiol Immunol. 1990 Dec;5(6):360–362. doi: 10.1111/j.1399-302x.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D., McBride B. C. Further studies on the degradation of immunoglobulins by black-pigmented Bacteroides. Oral Microbiol Immunol. 1989 Mar;4(1):12–18. doi: 10.1111/j.1399-302x.1989.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hinode D., Hayashi H., Nakamura R. Purification and characterization of three types of proteases from culture supernatants of Porphyromonas gingivalis. Infect Immun. 1991 Sep;59(9):3060–3068. doi: 10.1128/iai.59.9.3060-3068.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Takahashi N., Kuramitsu H. K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992 Jun;174(12):3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikata M., Kanehira T., Oh H., Tani H., Tazaki M., Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem Biophys Res Commun. 1991 Jan 31;174(2):625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F. Characterization of Porphyromonas (bacteroides) gingivalis hemagglutinin as a protease. Biochem Biophys Res Commun. 1991 Jul 15;178(1):336–342. doi: 10.1016/0006-291x(91)91819-x. [DOI] [PubMed] [Google Scholar]
- Norqvist A., Norrman B., Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990 Nov;58(11):3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Okuda K., Takazoe I. Purification and characterization of a thiol-protease from Bacteroides gingivalis strain 381. Oral Microbiol Immunol. 1987 Jun;2(2):77–81. doi: 10.1111/j.1399-302x.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Endo J., Hinode D., Nagata A., Maehara R., Sato M., Nakamura R. Isolation and characterization of protease from culture supernatant of Bacteroides gingivalis. J Periodontal Res. 1987 Nov;22(6):491–498. doi: 10.1111/j.1600-0765.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Park Y., McBride B. C. Cloning of a Porphyromonas (Bacteroides) gingivalis protease gene and characterization of its product. FEMS Microbiol Lett. 1992 May 1;71(3):273–278. doi: 10.1016/0378-1097(92)90721-y. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N., Gharbia S. E. Lysis of erythrocytes by the secreted cysteine proteinase of Porphyromonas gingivalis W83. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):213–217. doi: 10.1016/0378-1097(89)90199-7. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J., Shuttleworth C. A. The degradation of type I collagen and human plasma fibronectin by the trypsin-like enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch Oral Biol. 1988;33(5):323–329. doi: 10.1016/0003-9969(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J. Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J Gen Microbiol. 1987 Oct;133(10):2883–2894. doi: 10.1099/00221287-133-10-2883. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kato T., Kuramitsu H. K. Isolation and preliminary characterization of the Porphyromonas gingivalis prtC gene expressing collagenase activity. FEMS Microbiol Lett. 1991 Nov 15;68(2):135–138. doi: 10.1016/0378-1097(91)90116-r. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]