Abstract
Objective
To determine effectiveness of receipt of care from podiatrist and lower extremity clinician specialists (LEC specialists) on diabetes mellitus (DM)-related lower extremity amputation.
Data Sources
Medicare 5 percent sample claims, 1991–2007.
Study Design
Individuals with DM-related lower extremity complications (LECs) were followed 6 years. Visits with podiatrists, LEC specialists, and other health professionals were tracked to ascertain whether receipt of such care reduced the hazards of an LEC amputation.
Data Collection
Individuals were stratified based on disease severity, Stage 1—neuropathy, paresthesia, pain in feet, diabetic amyotrophy; Stage 2—cellutis, charcot foot; Stage 3—ulcer; Stage 4—osteomyelitis, gangrene.
Principal Findings
Half the LEC sample died within 6 years. More severe lower extremity disease increased risk of death and amputation. Persons visiting a podiatrist and an LEC specialist within a year before developing all stage complications were between 31 percent (ulceration) and 77 percent (cellulitis and charcot foot) as likely to undergo amputation compared with individuals visiting other health professionals.
Conclusions
Individuals with an LEC had high mortality. Visiting both a podiatrist and an LEC specialist in the year before LEC diagnosis was protective of undergoing lower extremity amputation, suggesting a benefit from multidisciplinary care.
Keywords: Diabetes mellitus, amputation, podiatrist, mortality
Diabetes mellitus (DM) is a major cause of morbidity and mortality, accounting for nearly 7 percent of excess mortality in the U.S. elderly, and prevalence continues to increase (Mokdad et al. 2004; Roglic et al. 2005; Cowie et al. 2006;). Lower extremity complications (LECs) are common among persons with DM (Caputo et al. 1994; Williams, Van Gaal, and Lucioni 2002; Jeffcoate and Harding 2003; Bethel et al. 2007;); half of all amputations occur among such persons (Zoorob and Hagen 1997). Nearly 85 percent of amputations are precipitated by foot ulcers among persons with a DM diagnosis (Apelqvist and Larsson 2000).
Recommended care guidelines for DM care include foot examinations at each diabetes visit with a comprehensive foot examination performed annually and tight glycemic control (Zoorob and Hagen 1997; American Diabetes Association 2002, 2005, 2006, 2008b, 2009). Annual foot examination and glycemic control adherence rates have improved (Saaddine et al. 2002; Eliasson et al. 2005;), but many persons still do not receive adequate foot care (Apelqvist and Larsson 2000). Nonadherence is generally high and not limited to persons with LECs (Lee et al. 2003; McClellan et al. 2003; McGlynn et al. 2003; Koro et al. 2004; Sloan et al. 2004;). Diabetes education interventions have been associated with decreased risk of lesions on the feet, better self foot care, and reduced risk of ulceration and amputation by up to 50 percent (Litzelman et al. 1993; Mayfield et al. 1998; Rith-Najarian et al. 1998; Reiber and Ledoux 2002; Plank et al. 2003; Lavery, Wunderlich, and Tredwell 2005). These interventions are more effective when performed by a specialist with lower extremity care expertise (Singh, Armstrong, and Lipsky 2005).
These interventions may also decrease cost: individuals with a DM diagnosis and foot ulcers tend to incur substantially higher expenditures on personal health care services than do persons with DM without foot ulcers (Ramsey et al. 1999; O'Brien, Patrick, and Caro 2003;). Incremental expenditures of up to U.S.$46,000 per year have been attributed to foot ulcers in persons with osteomyelitis; the cost of a first lower extremity amputation is U.S.$30,000–U.S.$50,000 (Ramsey et al. 1999; Gordois et al. 2003; O'Brien, Patrick, and Caro 2003; Shearer et al. 2003;). Substantial long-term care expenditures are also incurred by individuals with DM and LECs in particular (Ramsey et al. 1999; Gordois et al. 2003; O'Brien, Patrick, and Caro 2003). There is also a cost in terms of lost productivity (American Diabetes Association 2008a).
Persons diagnosed with neuropathy have a low life expectancy (Ramsey et al. 1999; Chaturvedi et al. 2001; Faglia, Favales, and Morabito 2001; Jeffcoate and Harding 2003; Cusick et al. 2005;). Ulceration increases risk of death by 85+ percent, while amputation more than doubles mortality risk in persons with DM (Chaturvedi et al. 2001; Cusick et al. 2005;). Given pressures on public budgets, particularly for Medicare, gauging the productivity of health interventions is a high priority.
In this study, we used national longitudinal Medicare claims data to examine whether care provided by clinicians specializing in treating DM and DM-related LECs was associated with better health outcomes, measured by the probability of an amputation of part or all of a leg or foot. We studied care received from podiatrists, clinician specialists in diagnosing and treating LECs (“LEC clinician specialists”), podiatrists in combination with LEC specialists, and other clinicians who care for persons with a DM diagnosis but who are not specialized in lower extremity DM complications. We assessed productivity of receipt of services from these health provider types taken individually and in combination.
METHODS
Data
Medicare 5 percent inpatient, outpatient, Part B, and durable medical equipment claims files were used to identify a nationally representative sample of Medicare beneficiaries aged 65+ diagnosed with DM, DM-related LECs, and other related adverse outcomes (described below under “Other explanatory variables”) during 1991–2007. The data contained information on demographic characteristics and zip code of residence of beneficiaries and diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]), procedure (Current Procedural Terminology [CPT-4]; Healthcare Common Procedure Coding System [HCPCS]), U.S. Centers for Medicare and Medicaid Services (CMS) provider specialty, and provider zip codes submitted with each claim. Data on dates of death and enrollment in Medicare fee-for-services came from Medicare 5 percent annual denominator files.
Sample Selection
Individuals entered into our analysis sample after receiving a DM-related, LEC diagnosis between 1994 and 2001. We classified sample persons into five mutually exclusive stages of increasing severity based on ICD-9-CM and CPT-4 codes. We developed this severity scale based on the expert opinion of the endocrinologist on our team. This scale is based on implications for treatment at each stage (see Table 1). The incremental LEC stages, denoting increasing invasiveness of therapy and complication severity, were Stage 1 (neuropathy: 250.6, 357.2, 355.xx; paresthesia: 782.xx; pain in feet: 729.5; diabetic amyotrophy: 358.1) in which diagnoses were based largely on electrical data and individuals had neurological dysfunction but otherwise no significant physical alteration; Stage 2 (cellutis: 681.1, 682.6, 682.7; charcot foot: 094.0) in which individuals experienced major physical changes and for whom use of nonsurgical therapies is appropriate, for example, antibiotics or casting, but more invasive therapy is unlikely to be used; Stage 3 (ulcer: 707.10, 707.12-9) in which individuals would likely benefit from more extensive dermatological treatment and possibly invasive therapy, for example, debridement; Stage 4 (osteomyelitis: 730.06-7, 730.16-7, 730.26-7; gangrene: 250.7, 785.4) in which individuals would benefit from intensive intravenous antibiotic treatment and likely major surgical procedures; and Stage 5 (amputation: 84.1x; CPT-4: 27290, 27295, 27590-2, 27594-6, 27598, 27880-2, 27884, 27886, 27888, 28800, 28805, 28810, 28820, 28825) in which part of the lower extremity is removed. Being in Stage 5 was the main study outcome. We created subsamples for each of the four other stages and a combined sample including all individuals from the four subsamples. Individuals could appear in more than one stage sample if they were classified in more than one stage during 1994–2001. However, individual sample persons appeared only once in the combined sample, classified by their first diagnosed LEC stage and the associated date. The combined sample was only used for descriptive purposes.
Table 1.
Clinical Implications and Rationale for Lower Extremity Severity Stage Hierarchy and Clinician Specialists in Diagnosing and Treating Lower Extremity Complications
| Stage Diseases | Clinical Implications/Rationale |
|---|---|
| Panel A: Clinical implications and rationale for lower extremity severity stage hierarchy | |
| Stage 1: Neuropathy, paresthesia, pain in feet, diabetic amyotrophy | Stage 1 diagnoses are based on electrical data and lower extremity examinations. Neurological dysfunction but no significant physical alteration |
| Stage 2: Cellulitis, charcot foot | Now need antibiotics, or cast. Not usually a surgical problem, but need to use other therapeutic maneuvers |
| Stage 3: Ulceration | Extensive dermatology, infectious disease, and/or podiatrist input; debridement often needed |
| Stage 4: Osteomyelitis, gangrene | Extremity in danger, with extensive antibiotic therapy and likely surgical procedure |
| Panel B: Lower extremity clinician (LEC) specialists to prevent progression to a specific stage | |
| Stage 1: Neuropathy, paresthesia, pain in feet, diabetic amyotrophy | General surgeon (code 02), dermatologist (07), neurologist (13), orthopedic surgeon (20), physical medicine and rehabilitation (25), diagnostic radiology (30), physical therapist (65) |
| Stage 2: Cellulitis, charcot foot | Same as Stage 1 |
| Stage 3: Ulceration | General surgeon, orthopedic surgeon, diagnostic radiology, infectious disease (44)* |
| Stage 4: Osteomyelitis, gangrene | General surgeon, dermatologist, orthopedic surgeon, plastic and reconstructive surgeon (24), diagnostic radiology, infectious disease |
We did not include dermatologists as LEC specialists for Stage 3 individuals. While we recognize that dermatologists may be beneficial to individuals previously diagnosed with cellulitus, they would likely not be seen by individuals diagnosed with charcot foot. We therefore excluded them from this stage analysis.
For each subsample, we used a 3-year look-back period using the stage complication diagnosis date as baseline. To ensure a full 3-year look-back period, which we used to define comorbidities present at baseline, we excluded all persons initially diagnosed with the stage complication before 1994; diagnosed with the stage complication before age 68; and participating in a Medicare risk plan (HMO) or living outside of the United States for over 12 months during the look-back period. There are no data for beneficiaries in risk plans (Figure 1). We also excluded individuals lacking valid zip code data, which we needed to calculate distance to the nearest provider.
Figure 1.
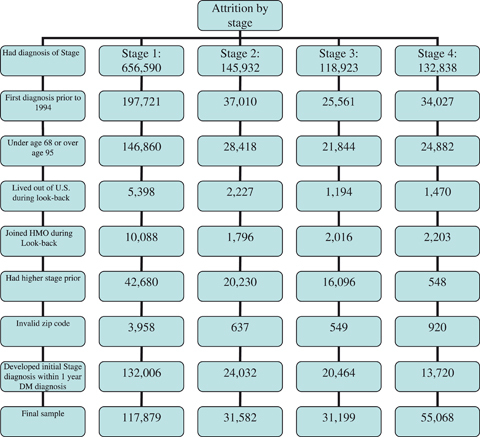
Attrition by Study Exclusion Criteria
Individuals diagnosed with a higher LEC stage before entry in a particular stage were also excluded from that sample to ensure that individuals in that analysis were at a similar level of severity in the LEC disease progression at analysis baseline. For example, the Stage 1 group consisted of all Medicare beneficiaries receiving a first diagnosis of a Stage 1 complication during the study period but who had not experienced a more severe stage complication before the Stage 1 diagnosis date. Finally, we excluded persons diagnosed with DM <1 year before the stage diagnosis date to allow a full year in which to track individuals' health care utilization, our main explanatory variables. After exclusions, there were 117,879 individuals in Stage 1, 31,582 in Stage 2, 31,199 in Stage 3, 55,068 in Stage 4 subsamples, and 189,598 in the combined analysis sample.
Sample individuals were followed for 2,190 days (6 years) after entry into the sample. Individuals were censored if, after entering the sample, they joined an HMO, moved outside the United States for more than a year, or died. Data on whether an individual resided in the United States were collected annually; thus, if a person was coded as not living in the United States during a given year, we considered the observation to be censored as of January 1 of that calendar year.
Dependent Variables
Dependent variables were hazards of a first amputation of the lower extremity within 6 years following baseline.
Types of Health Services
Key explanatory variables related to receipt of health services during the year before being diagnosed with a study stage diagnosis. We classified care received from health professionals into five mutually exclusive categories, defining binary variables for each (Table 1, panel B): (1) podiatrist (CMS provider specialty code 48) with or without care from other health professionals; (2) lower extremity clinician specialist (LEC specialist) with or without care from other health professionals; (3) podiatrist and LEC specialist with or without care from other health professionals; (4) other health professional (no care from podiatrists or LEC specialists)—general/family practitioner (01, 08), internist (11), endocrinologist (46),1 nurse practitioner (50), and physician assistant (97); and (5) no care from any of the study health professionals (but receipt of care from nonstudy health professionals, e.g., pathologists, psychiatrists).2 The omitted reference group was “other health professional.” LEC specialists were identified by using Medicare 5 percent claims data to determine which specialists were most likely to see individuals with a primary diagnosis of Stages 1–4 LECs. We classified specialists appropriate for each LEC stage according to which type of health professional would be most likely to treat individuals and prevent them from progressing to a higher stage LEC (see Table 1, panel B).
Although by definition individuals in the fifth group did not see a study health provider, most persons classified in this group had in fact received some form of care from a health professional during this time period, measured by a Medicare Part B claim. Percentages not having a Part B claim were 11.6 percent Stage 1, 14.4 percent Stage 2, 8.8 percent Stage 3, and 11.4 percent Stage 4.
Other Explanatory Variables
We included covariates for DM severity, which is likely to increase with disease duration; for DM duration, we created a binary variable using the look-back period, with 1 designating individuals diagnosed with DM 3+ years before entry in the sample and 0 for other sample persons. Persons with insulin-dependent DM were identified using the five-digit ICD-9-CM DM diagnoses “250.01” and “250.03.” Individuals with 2+ claims with such diagnoses were considered to be insulin dependent.
We accounted for renal, ocular, cardiovascular, and cerebrovascular system function, and other body systems frequently affected by DM. Covariates for each system were (1) renal—DM with renal manifestations (ICD-9-CM code: 250.4), proteinuria/nephrotic syndrome (791.0, 581.8), chronic renal failure,3 and end-stage renal disease4; (2) ocular—background diabetic retinopathy (362.01), proliferative diabetic retinopathy (362.02), and diabetic macular edema (362.07, 362.53, 362.83); (3) cardiovascular—coronary artery disease (410, 411, 413, 414), with separate variables for diagnosis in an outpatient or inpatient setting, and congestive heart failure5; (4) cerebrovascular—carotid bruit (785.9; CPT-4: 76536), occlusion or stenosis of cerebral artery (433–434), transient ischemic attack (435), and stroke (430–432, 436).
Other DM-associated conditions were hypertension (401), lipidemia (272.0–272.4), and obesity (278.0). Strict adherence to American Diabetes Association guidelines for all three of these conditions is part of optimal DM control (American Diabetes Association 2002, 2005, 2006, 2008b, 2009). Persons diagnosed with hypertension or lipidemia are more likely to have been receiving medications for these diagnoses. We included a binary variable for arthritis because it may affect use of the lower extremities. We also included a binary variable for Alzheimer's disease or other dementia (ADOD: 331.0, 290.x, 310.1, 331.2, 438.0) because ADOD may affect an individual's ability to control his/her DM and investments in care (Sloan et al. 2003).
The Charlson index (Charlson et al. 1987), a widely used comorbidity measure, was constructed from data from the calendar year before diagnosis of the sample complication being studied. We excluded diagnoses of DM and DM complications from the Charlson index because we included separate covariates for these.
We included binary variables representing the quartile ranking of Medicare payments in the previous year, measured by services performed by nonstudy health professionals (those not included in the podiatrist, LEC specialist, or other health professional groups). The omitted reference group was the lowest payment quartile.
Accounting for Endogeneity of Receipt of Podiatric and Medical Care
Rationale
A problem with observational data is that the intervention of interest, here receipt of particular types of personal health care services, is plausibly endogenous to outcomes. Endogeneity may occur when procedures are performed in response to clinical problems that are not recorded in the claims data. Although we included various covariates for health, some dimensions of health affecting receipt of services were plausibly observable to providers and patients but were not captured by our data.
Approach
To deal with endogeneity, we included variables to account for omitted heterogeneity. These variables were sets of residuals from a multinomial logit analysis of choice among the five mutually exclusive provider-type categories (Shea et al. 2007; Terza, Basu, and Rathouz 2008;). Main covariates in the multinomial logit regression were measures of minimum distance to other health professionals, and relative minimum distances to podiatrists and LEC specialists (distance to the nearest podiatrist or LEC specialist minus distance to nearest other health professional). Other explanatory variables were listed above under “Other explanatory variables.”
Next, we used the residuals from the multinomial logit analysis to construct four explanatory variables, one for each of the residuals for four visit-type categories, other health professional being the omitted reference group.
Data on distances to the nearest health professional came from Medicare 5 percent claims. The database contained information on the beneficiary's and the provider's zip code. Because we lacked more precise location information, we measured distance in miles (air distance) between the center of the beneficiary's zip code of residence and the zip code in which the provider's office was located. Each DM care provider in the claims data was considered to be an alternative for each beneficiary. Thus, even if a particular beneficiary obtained care from a provider who was not the nearest from his or her place of residence, we only considered the nearest provider in the calculations of minimum distance. Individuals living in a zip code with an other health professional were considered to have 0 miles to the nearest other health professional. For all others, we used SAS 9.2 software (SAS; Cary, NC, USA) to calculate the distance to the nearest other health professional. SAS 9.2 evaluated the distance between the centroids of two zip codes. Our program then saved this distance and calculated distance between the individual and another zip code with a study health professional. With each iteration, SAS kept the shorter of the distances. We expected minimum distances to be negatively related to visits, but not to affect disease progression. We found minimum distances to be highly correlated with receipt of visits of various types (not shown).
Statistical Analysis
A Cox proportional hazards model was used to analyze time to amputation. The analysis was performed both with and without the variables for the residuals as covariates. A log likelihood test revealed whether the covariates for the residuals were statistically significant when considered as a group.
RESULTS
There were 189,598 individuals in the combined sample, 84.2 percent of whom were white, 11.3 percent black, and 4.5 percent other race (Table 1). Nearly two fifths of individuals were male; mean age was 77.7 years. Compared with the DM with no reported LEC, our sample was older, more female, much more likely to have seen “other health professionals,” and had higher rates of comorbidities. Mean distances to the nearest health professional, podiatrist, and LEC specialist did not differ by stage by more than 0.3 miles. A higher proportion of individuals never diagnosed with DM were white compared with our sample.
Adjusting for censoring, 6 percent of the combined sample underwent an amputation of the lower extremity during the study period (Figure S1). Individuals classified in the other health professional group were slightly more likely to receive an LEC amputation. Individuals diagnosed with diabetes LEC experienced high rates of mortality. Approximately half of sample individuals died during the 6-year follow-up.
Considering amputation rates at any time before death, persons entering the analysis at Stage 1 were least likely (2.3 percent, Table 2) and at Stage 4 were most likely (14.4 percent) to have an amputation. As with amputations, death within the 6-year follow-up period increased monotonically by stage—from 44.4 percent for Stage 1 to 64.2 percent for Stage 4.
Table 2.
Descriptive Statistic Means
| Combined Sample | DM No LEC† | Non DM† | Stage 1‡ | Stage 2‡ | Stage 3‡ | Stage 4‡ | |
|---|---|---|---|---|---|---|---|
| Outcome variables | |||||||
| Underwent lower extremity amputation | 0.059 | 0.001*** | 0.004*** | 0.023*** | 0.080*** | 0.085*** | 0.144 |
| Total died (regardless of amputation) | 0.499 | 0.379*** | 0.337*** | 0.444*** | 0.571*** | 0.614*** | 0.642 |
| Explanatory variables | |||||||
| Receipt of care in the year before stage diagnosis | |||||||
| Saw an other health professional (HP) only | 0.151 | 0.293*** | 0.181*** | 0.180*** | 0.127*** | 0.080*** | 0.080 |
| Saw a podiatrist | 0.221 | 0.062*** | 0.089*** | 0.041*** | 0.059*** | 0.130*** | 0.088 |
| Saw a podiatrist and other HP | 0.909 | 0.889*** | 0.863*** | 0.901 | 0.915 | 0.914 | 0.919 |
| Saw a lower extremity clinician (LEC) specialist | 0.061 | 0.026*** | 0.031*** | 0.592*** | 0.514*** | 0.334*** | 0.400 |
| Saw an LEC specialist and other HP | 0.822 | 0.805* | 0.740*** | 0.827*** | 0.822* | 0.812 | 0.822 |
| Saw a podiatrist and LEC specialist | 0.525 | 0.527*** | 0.444*** | 0.138*** | 0.264*** | 0.436*** | 0.411 |
| Saw a podiatrist and LEC specialist and other HP | 0.898 | 0.869*** | 0.826*** | 0.893*** | 0.906 | 0.916 | 0.912 |
| Did not see any study HP | 0.042 | 0.092*** | 0.254*** | 0.050*** | 0.036*** | 0.020 | 0.021 |
| Severity of diabetes mellitus (DM) | |||||||
| Diagnosed with DM 3 year prior | 0.687 | 0.005*** | NA | 0.675*** | 0.729*** | 0.753*** | 0.811 |
| Insulin dependent | 0.259 | 0.075*** | NA | 0.215*** | 0.288*** | 0.299*** | 0.452 |
| Renal comorbidities | |||||||
| DM with renal manifestations | 0.007 | 0.002*** | NA | 0.005*** | 0.008*** | 0.008*** | 0.015 |
| Proteinuria/nephrotic syndrome | 0.024 | 0.009*** | 0.004*** | 0.022*** | 0.027*** | 0.028*** | 0.033 |
| Chronic renal failure | 0.083 | 0.037*** | 0.017*** | 0.063*** | 0.109*** | 0.127*** | 0.142 |
| End-stage renal disease | 0.017 | 0.005*** | 0.002*** | 0.011*** | 0.019*** | 0.030*** | 0.041 |
| Ocular comorbidities | |||||||
| Background diabetic retinopathy | 0.119 | 0.033*** | NA | 0.098*** | 0.127*** | 0.141*** | 0.214 |
| Proliferative diabetic retinopathy | 0.031 | 0.008*** | NA | 0.022*** | 0.034*** | 0.039*** | 0.063 |
| Diabetic macular edema | 0.040 | 0.017*** | NA | 0.034*** | 0.043*** | 0.047*** | 0.062 |
| Cardiovascular comorbidities | |||||||
| Coronary artery disease—inpatient | 0.252 | 0.136*** | 0.090*** | 0.215*** | 0.318*** | 0.297*** | 0.351 |
| Coronary artery disease—outpatient | 0.511 | 0.381*** | 0.251*** | 0.474*** | 0.565*** | 0.577*** | 0.594 |
| Congestive heart failure | 0.353 | 0.195*** | 0.128*** | 0.298*** | 0.458*** | 0.449*** | 0.475 |
| Cerebrovascular comorbidities | |||||||
| Carotid bruit | 0.064 | 0.033*** | 0.031*** | 0.059*** | 0.070*** | 0.075*** | 0.083 |
| Occlusion/stenosis of cerebral artery | 0.190 | 0.102*** | 0.079*** | 0.169*** | 0.205*** | 0.232*** | 0.262 |
| Transient ischemic attack | 0.127 | 0.067*** | 0.061*** | 0.112*** | 0.141*** | 0.156** | 0.165 |
| Stroke | 0.165 | 0.088*** | 0.066*** | 0.137*** | 0.181*** | 0.223*** | 0.241 |
| Other comorbidities | |||||||
| Hypertension | 0.813 | 0.704*** | 0.485*** | 0.803*** | 0.832*** | 0.841** | 0.849 |
| Lipidemia | 0.495 | 0.453*** | 0.276*** | 0.511*** | 0.481*** | 0.494*** | 0.469 |
| Obesity | 0.046 | 0.017*** | 0.006*** | 0.041*** | 0.066*** | 0.060 | 0.060 |
| Arthritis | 0.320 | 0.179*** | 0.202*** | 0.296*** | 0.372*** | 0.396*** | 0.344 |
| Charlson index | 1.581 | 1.196*** | 0.904*** | 1.358*** | 1.833*** | 2.060 | 2.069 |
| Alzheimer's and other dementia | 0.080 | 0.053*** | 0.055*** | 0.080*** | 0.114*** | 0.169*** | 0.155*** |
| Demographic characteristics | |||||||
| Black | 0.113 | 0.108*** | 0.061*** | 0.109*** | 0.087*** | 0.122*** | 0.141 |
| Other race | 0.045 | 0.062*** | 0.036*** | 0.046 | 0.043* | 0.043* | 0.046 |
| Male | 0.396 | 0.476*** | 0.402*** | 0.406 | 0.389*** | 0.365*** | 0.408 |
| Baseline age | 77.662 | 75.718*** | 77.068*** | 76.86*** | 78.13 | 79.10*** | 78.05 |
| Distance to HPs | |||||||
| Distance to nearest other HP (miles) | 0.854 | 0.880* | 0.967*** | 0.913 | 0.927 | 0.682 | 0.723 |
| Relative distance to nearest podiatrist (miles) | −4.202 | −4.358*** | −4.477*** | −4.490 | −4.556 | −3.220 | −3.598 |
| Relative distance to nearest LEC specialist (miles) | −1.638 | −1.617 | −1.615 | −1.673 | −1.706 | −1.619 | −1.590 |
| Observations | 189,598 | 110,330 | 698,909 | 117,879 | 31,582 | 31,199 | 55,068 |
T-tests compared with combined sample.
T-tests compared with Stage 4 sample.
p<0.001
p<0.01
p<0.05.
There were also systematic differences by group in the mix of health professionals seen. Stage 1 persons were most likely to have only seen an other health professional and an LEC specialist only, and least likely to have seen a combination of a podiatrist and LEC specialist. By contrast, Stage 3 and Stage 4 persons were more likely to have seen a podiatrist and an LEC specialist and least likely to have not seen a study health care provider.
Persons in Stage 4 had more severe diabetes, measured by duration of diabetes, insulin dependence, and DM complications. Over four fifths of Stage 4 individuals had been diagnosed with DM 3 years prior, while nearly half of them had insulin-dependent diabetes. Corresponding rates for those in Stage 1 were 68 percent and 22 percent. Patterns by stage for other comorbidities are mixed. A higher percentage of persons at Stage 4 were black than for the other stages.
Sample persons lived less than a mile from the nearest other (study) health professional. Persons at Stages 1 and 2 had higher relative distance to the nearest podiatrist than did those at Stages 3 and 4, the mean differences ranging from slightly under 1 mile to about 1.5 miles. Relative distance to the nearest LEC specialist did not differ by more than 0.2 miles for all four stages.
Based on the results of log likelihood ratio tests, the null hypothesis of exogeneity of receipt of care was accepted for Stage 2, and hence covariates for the residuals were excluded in the results shown in Table 2. By contrast, for Stages 1, 3, and 4, the null hypothesis of exogeneity of care was rejected, and therefore results with the covariates for the residuals in the results are presented.
Adjusting for Medicare expenditures from care received from nonstudy health professionals, overall care, measured by the hazard of the first LEC amputation during the 6-year follow-up period, was productive for persons at all stages. For Stage 1, the hazard ratio for “saw a podiatrist” implies that persons diagnosed with DM were more than twice as likely to have an LEC amputation during follow-up (hazard ratio [HR]=2.20; 95 percent confidence interval [CI]: 1.15, 4.22), while individuals who “saw a podiatrist and LEC specialist” were 47 percent as likely to have an LEC amputation (HR: 0.47; 95 percent CI: 0.27, 0.81) (Table 3).
Table 3.
Hazard Ratios of Undergoing Amputation from Cox Proportional Hazards Models (with 95% Confidence Intervals in Parentheses)
| Stage 1* | Stage 2 | Stage 3* | Stage 4* | |
|---|---|---|---|---|
| Receipt of care in the year before stage diagnosis | ||||
| Saw a podiatrist | 2.20 | 0.85 | 0.44 | 0.36 |
| (1.15, 4.22) | (0.70, 1.03) | (0.14, 1.42) | (0.17, 0.78) | |
| Saw a lower extremity clinician (LEC) specialist | 0.80 | 0.84 | 2.23 | 1.85 |
| (0.54, 1.18) | (0.74, 0.95) | (0.82, 6.09) | (1.03, 3.33) | |
| Saw a podiatrist and LEC specialist | 0.47 | 0.81 | 0.36 | 0.42 |
| (0.27,0.81) | (0.70,0.93) | (0.14,0.94) | (0.24,0.74) | |
| Did not see any study health professional (HP) | 0.93 | 1.29 | 0.87 | 1.40 |
| (0.49, 1.76) | (1.07, 1.57) | (0.11, 6.91) | (0.46, 4.22) | |
| Severity of diabetes mellitus (DM) | ||||
| Diagnosed with DM 3 year prior | 1.23 | 1.26 | 1.35 | 1.01 |
| (1.13, 1.35) | (1.14, 1.39) | (1.22, 1.50) | (0.95, 1.07) | |
| Insulin dependent | 1.56 | 1.53 | 1.57 | 1.11 |
| (1.43, 1.69) | (1.40, 1.67) | (1.44, 1.71) | (1.06, 1.17) | |
| Renal DM comorbidities | ||||
| DM with renal manifestations | 1.66 | 1.36 | 0.87 | 1.05 |
| (1.25, 2.20) | (1.04, 1.78) | (0.63, 1.21) | (0.91, 1.22) | |
| Proteinuria/nephrotic syndrome | 1.19 | 1.17 | 1.01 | 1.02 |
| (0.97, 1.47) | (0.96, 1.43) | (0.83, 1.23) | (0.91, 1.15) | |
| Chronic renal failure | 1.70 | 1.16 | 1.12 | 1.19 |
| (1.48, 1.96) | (1.01, 1.33) | (0.98, 1.28) | (1.10, 1.28) | |
| End-stage renal disease | 3.40 | 2.87 | 2.39 | 2.10 |
| (2.76, 4.18) | (2.33, 3.52) | (1.99, 2.87) | (1.90, 2.33) | |
| Ocular DM comorbidities | ||||
| Background diabetic retinopathy | 1.84 | 1.65 | 1.65 | 1.25 |
| (1.66, 2.05) | (1.48, 1.84) | (1.48, 1.83) | (1.18, 1.33) | |
| Proliferative diabetic retinopathy | 1.50 | 1.49 | 1.44 | 1.46 |
| (1.27, 1.78) | (1.28, 1.75) | (1.24, 1.67) | (1.35, 1.59) | |
| Diabetic macular edema | 1.14 | 1.03 | 1.07 | 1.05 |
| (0.97, 1.35) | (0.87, 1.22) | (0.91, 1.25) | (0.96, 1.15) | |
| Cardiovascular DM comorbidities | ||||
| Coronary artery disease—inpatient | 1.25 | 1.23 | 1.19 | 1.19 |
| (1.12, 1.39) | (1.11, 1.37) | (1.06, 1.34) | (1.11, 1.26) | |
| Coronary artery disease—outpatient | 0.93 | 0.83 | 0.94 | 0.91 |
| (0.85, 1.03) | (0.76, 0.92) | (0.85, 1.04) | (0.86, 0.97) | |
| Congestive heart failure | 1.48 | 1.26 | 1.19 | 1.28 |
| (1.36, 1.62) | (1.15, 1.38) | (1.08, 1.31) | (1.21, 1.35) | |
| Cerebrovascular DM comorbidities | ||||
| Carotid bruit | 1.09 | 1.05 | 0.94 | 0.94 |
| (0.93, 1.27) | (0.90, 1.22) | (0.81, 1.10) | (0.86, 1.02) | |
| Occlusion/stenosis of cerebral artery | 1.23 | 1.30 | 1.12 | 1.09 |
| (1.10, 1.37) | (1.17, 1.45) | (1.01, 1.24) | (1.03, 1.16) | |
| Transient ischemic attack | 0.90 | 0.92 | 0.93 | 0.91 |
| (0.79, 1.02) | (0.81, 1.04) | (0.83, 1.05) | (0.85, 0.97) | |
| Stroke | 1.59 | 1.30 | 1.22 | 1.21 |
| (1.43, 1.78) | (1.16, 1.45) | (1.10, 1.36) | (1.14, 1.28) | |
| Other comorbidities | ||||
| Hypertension | 0.98 | 1.06 | 1.09 | 0.93 |
| (0.88, 1.08) | (0.95, 1.18) | (0.96, 1.22) | (0.87, 1.00) | |
| Lipidemia | 0.64 | 0.73 | 0.83 | 0.75 |
| (0.59, 0.70) | (0.67, 0.79) | (0.76, 0.90) | (0.71, 0.79) | |
| Obesity | 1.03 | 0.73 | 0.77 | 0.84 |
| (0.84, 1.26) | (0.60, 0.88) | (0.65, 0.92) | (0.75, 0.94) | |
| Arthritis | 0.92 | 0.88 | 0.96 | 0.98 |
| (0.84, 1.01) | (0.80, 0.96) | (0.87, 1.06) | (0.93, 1.04) | |
| Charlson index | 1.01 | 1.00 | 1.05 | 1.03 |
| (0.98, 1.04) | (0.97, 1.02) | (1.02, 1.08) | (1.01, 1.04) | |
| Alzheimer's and other dementia | 1.45 | 1.27 | 1.26 | 1.33 |
| (1.25, 1.69) | (1.11, 1.44) | (1.12, 1.42) | (1.25, 1.43) | |
| Demographic characteristics | ||||
| Black | 2.03 | 1.89 | 1.64 | 1.63 |
| (1.84, 2.23) | (1.69, 2.10) | (1.48, 1.81) | (1.54, 1.73) | |
| Other race | 1.12 | 1.09 | 0.79 | 1.05 |
| (0.94, 1.34) | (0.91, 1.32) | (0.64, 0.97) | (0.95, 1.17) | |
| Male | 1.79 | 1.75 | 1.67 | 1.49 |
| (1.65, 1.95) | (1.61, 1.89) | (1.52, 1.83) | (1.42, 1.56) | |
| Baseline age | 1.01 | 1.00 | 1.01 | 1.02 |
| (1.00, 1.02) | (1.00, 1.01) | (1.00, 1.01) | (1.01, 1.02) | |
| Residuals | ||||
| Residual—saw a podiatrist | 0.51 | 1.41 | 2.03 | |
| (0.26, 1.01) | (0.44, 4.58) | (0.94, 4.39) | ||
| Residual—saw an LEC specialist | 1.13 | 0.46 | 0.54 | |
| (0.76, 1.70) | (0.17, 1.26) | (0.30, 0.97) | ||
| Residual—saw a podiatrist and an LEC specialist | 2.33 | 2.27 | 2.39 | |
| (1.33, 4.09) | (0.87, 5.95) | (1.35, 4.22) | ||
| Residual—did not see a study HP | 1.33 | 1.54 | 0.86 | |
| (0.68, 2.59) | (0.19, 12.49) | (0.28, 2.63) | ||
| Quartile of spending on non study health professionals | ||||
| Second | 0.88 | 0.83 | 0.99 | 0.80 |
| (0.79, 0.97) | (0.74, 0.94) | (0.87, 1.13) | (0.74, 0.86) | |
| Third | 0.90 | 0.86 | 0.85 | 0.82 |
| (0.80, 1.01) | (0.76, 0.97) | (0.74, 0.96) | (0.76, 0.88) | |
| Fourth | 0.95 | 0.88 | 0.88 | 0.87 |
| (0.84, 1.08) | (0.78, 1.01) | (0.77, 1.00) | (0.80, 0.94) | |
Note. Bold type denotes p value < 0.05.
Specification included residuals.
For Stage 2, persons receiving care from an LEC specialist only were 16 percent less likely than those receiving care from an other health professional to have had an LEC amputation during follow-up (HR=0.84; 95 percent CI: 0.74, 0.95); those seeing a podiatrist and an LEC specialist experienced about the same risk of having an amputation (HR=0.81; 95 percent CI: 0.70, 0.93). Persons not seeing any of the study health professionals had a higher risk of an amputation than those who saw an other health professional (HR=1.29; 95 percent CI: 1.07, 1.57). To put these results in perspective, the annual hazard of an amputation during follow-up was 1.3 percent annually.
For Stage 3, hazard ratios tended to be appreciably lower than for Stage 2. In particular, persons receiving care from both a podiatrist and an LEC specialist were 36 percent as likely to have received an amputation during follow-up than were those who only saw an other heath professional (HR=0.36; 95 percent CI 0.14, 0.94). The annual hazard of having been amputated for Stage 3 was 1.4 percent. Seeing a podiatrist only was productive for persons at Stage 3 (HR=0.44; 95 percent CI: 0.14, 1.42), although this result was not statistically significant.
Stage 4 results were similar to Stage 3's. Both seeing a podiatrist only (HR=0.36; 95 percent CI: 0.17, 0.78) and seeing a combination of a podiatrist and an LEC specialist (HR=0.42; 95 percent CI: 0.24, 0.74) reduced the hazard of an LEC amputation. Individuals seeing an LEC specialist only were 85 percent more likely to undergo amputation (HR=1.85; 95 percent CI: 1.03, 3.33). The annual hazard of an amputation for Stage 4 individuals was nearly double that of Stage 3. The hazard ratio for “residual—saw a podiatrist” and “residual—saw a podiatrist and LEC specialist” was slightly higher for Stage 4 than for Stage 3.
Diabetes diagnosis duration, being insulin dependent, having chronic renal failure, end-stage renal disease, diabetic retinopathy, coronary artery disease diagnosed from an inpatient claim, chronic heart failure, occlusion/stenosis of a cerebral artery, stroke, and Alzheimer's or other dementia tended to increase the hazard of an LEC amputation. Blacks, males, and older individuals were more likely to have an LEC amputation. Some of the other diagnoses were associated with a lower hazard of amputation. But for these diagnoses, especially lipidemia, the favorable results may reflect the treatment for the diagnoses, for example, use of statins, rather than the diagnosis itself. Individuals incurring higher Medicare expenditures from health professionals besides podiatrists, LEC specialists, and “other health professionals” were generally less likely to have an amputation.
DISCUSSION
About half of Medicare beneficiaries diagnosed with a LEC of diabetes died during the 6-year follow-up. The hazard of a first amputation of part or all of a foot or leg was far lower than for mortality, but it was appreciably higher for persons who entered the analysis with a Stage 4 diagnosis—osteomyelitis or gangrene—than for persons at less advanced stages.
The main study question was whether care oriented to treatment of lower extremity complications is productive as measured by reduced rates of first lower extremity amputations. The results were most favorable to a pattern of care involving a combination of podiatrists and lower extremity specialists; the latter group included general surgeons, orthopedic surgeons, diagnostic radiologists, and depending on the stage, dermatologists, neurologists, physical medicine, and rehabilitation specialists, physical therapists, infectious disease specialists, and plastic and reconstructive surgeons. That this combination was especially productive in terms of preventing or forestalling LEC amputations was particularly evident after we accounted for endogeneity of LEC care receipt.
Survival should primarily reflect success in patient diabetes control rather than control of LEC in particular. Yet each patient encounter with a health professional potentially contributes to improved general diabetes control. Mortality rates increased with increasing severity of the LEC, with over 64 percent of those with a Stage 4 LEC dying within 6 years of diagnosis.
Previous literature has suggested podiatric care, foot education programs, and multidisciplinary care for individuals with DM-related LECs lead to better LEC outcomes. One study, examining the effect of podiatrist care on callosities, found that the podiatrist group had a lower prevalence and reduced size of calluses compared with individuals only receiving written instructions for foot care (Ronnemaa et al. 1997). Persons under 50 experienced a greater reduction in callosities. Our study expands on these results, demonstrating that podiatric intervention is effective in an elderly cohort.
We found an even stronger association between visits to a podiatrist and an LEC specialist and lower amputation rates. Previous studies examining multidisciplinary disease management programs were limited to single community settings or randomized, controlled trials with shorter follow-up periods than ours (Litzelman et al. 1993; Patout et al. 2000; Lavery, Wunderlich, and Tredwell 2005; Trautner et al. 2007; Canavan et al. 2008; Hedetoft et al. 2009;). These studies documented falling rates of diabetes-related lower extremity amputations after entering community-based podiatric services. Other services provided in these community-based clinics included educational programs (Litzelman et al. 1993; Patout et al. 2000;), access to pedorthists (Patout et al. 2000; Lavery, Wunderlich, and Tredwell 2005;), DM specialists, orthopedic surgeons (Trautner et al. 2007; Hedetoft et al. 2009;), and vascular surgeons (Trautner et al. 2007).
Individuals receiving care from both podiatrists and LEC specialists in the year before all stage diagnoses were much less likely to undergo a lower extremity amputation. Receiving care from multiple specialists may have allowed for a more coordinated care.
Our study has several strengths. The sample is representative of the U.S. elderly population with a DM diagnosis. The follow-up period extended for 6 years. We used a technique to account for the potential endogeneity of receipt of care. We studied the most severe LEC complication, amputation, and accounted for other DM complications in our analysis of the hazard of amputation.
We acknowledge the following limitations. First, we used observational data from Medicare records. Medicare claims data are designed for administrative purposes, not for comparative effectiveness analysis.
Second, many studies have used patient and provider education programs as an intervention measure. Our analysis did not permit this type of comparison. Third, health care provider variables were defined for care received during the year before the diagnosis of an LEC stage. Care patterns may have changed subsequently in ways that our analysis did not capture. Fourth, although we included many covariates and adjusted for endogeneity, we could not completely account for patients' differences in case mix, differences that could have been apparent to both patients and providers but are not observable to researchers.
While randomized controlled trials and other studies have demonstrated the positive impact of educational programs and other interventions on amputation rates in more limited settings, we found that, in a large Medicare sample, coordinated care between podiatrists and LEC specialists substantially reduced amputation rates compared with care only provided by other health professionals, while care provided by podiatrists alone was also highly protective of undergoing amputation in those with severe LECs.
Additional research should be conducted on care coordination and LEC outcomes, in particular whether actual coordinated care improves LEC outcomes. Our analysis just accounted for the presence of Medicare claims from particular types of providers during a year. Specific practice arrangements and financial incentives may improve care coordination and thus health outcomes. More should also be learned about the patient's role in a diabetes diagnosis and his/her role—both positive and normative—in coordinating care for this complex and highly prevalent disease.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was supported in part by a grant from the National Institute on Aging (NIA) (grant no. 2R56AG017473-09). The sponsor had no role in the design and conduct of this study. The authors have no financial interest that might be affected by this study's findings or conclusions.
Disclosures: None.
Disclaimers: None.
NOTES
Because endocrinologists are more involved in DM control than in treating LEC complications, we included them in the “other health professional” category rather than the LEC category.
Cardiologists were not study physician specialists if they were not listed as internists; however, we included measures of heart disease as covariates. We did not include cardiologists because they would most likely not have treated lower extremity complications.
404.12, 404.13, 404.92, 404.93, 403.01, 403.11, 585.xx, 586.xx.
50340, 50360, 50365, V42.0, V56.0, V45.1, V56.8, 39.27, 39.42, 39.43, 39.49, 39.50, 39.53, 39.93, 39.94, 90921, 90935, 90937, 90940, 90989, 90993, 90997, 90999, 93990.
428.0, 428.1, 428.9, 428.2x, 428.3x, 428.4x, 398.91, 402.01, 402.11, 402.91, 404.01, 404.11, 404.91.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Figure S1. Kaplan–Meier Survival Curve—Time to Amputation.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- American Diabetes Association. Standards of Medical Care for Patients with Diabetes Mellitus. Diabetes Care. 2002;25:213–29. doi: 10.2337/diacare.25.1.213. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2005;28:S4–36. [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes-2006. Diabetes Care. 2006;29:S4–42. [PubMed] [Google Scholar]
- American Diabetes Association. Economic Costs of Diabetes in the US in 2007. Diabetes Care. 2008a;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes 2008. Diabetes Care. 2008b;31:S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes-2009. Diabetes Care. 2009;32:S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist J, Larsson J. What Is the Most Effective Way to Reduce Incidence of Amputation in the Diabetic Foot? Diabetes–Metabolism Research and Reviews. 2000;16(suppl 1):S75–83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal Incidence and Prevalence of Adverse Outcomes of Diabetes Mellitus in Elderly Patients. Archives of Internal Medicine. 2007;167(9):21–7. doi: 10.1001/archinte.167.9.921. [DOI] [PubMed] [Google Scholar]
- Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes- and Nondiabetes-Related Lower Extremity Amputation Incidence before and after the Introduction of Better Organized Diabetes Foot Care—Continuous Longitudinal Monitoring Using a Standard Method. Diabetes Care. 2008;31(3):459–63. doi: 10.2337/dc07-1159. [DOI] [PubMed] [Google Scholar]
- Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and Management of Foot Disease in Patients with Diabetes. New England Journal of Medicine. 1994;331(13):854–60. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, Mackenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. Journal of Chronic Diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Stevens LK, Fuller JH, Lee ET, Lu M. Risk Factors, Ethnic Differences and Mortality Associated with Lower-Extremity Gangrene and Amputation in Diabetes. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44:S65–S71. doi: 10.1007/pl00002941. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Engelgau MM, Rust KF, Saydah SH, Byrd-Holt DD, Williams DE, Geiss LS, Gregg EW. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the US Population, National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- Cusick M, Meleth AD, Agron E, Fisher MR, Reed GF, Knatterud GL, et al. Associations of Mortality and Diabetes Complications in Patients with Type 1 and Type 2 Diabetes: Early Treatment Diabetic Retinopathy Study Report no. 27. Diabetes Care. 2005;28(3):617–25. doi: 10.2337/diacare.28.3.617. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Cederholm J, Nilsson P, Gudbjornsdottir S. The Gap between Guidelines and Reality: Type 2 Diabetes in a National Diabetes Register 1996–2003. Diabetic Medicine. 2005;22(10):1420–6. doi: 10.1111/j.1464-5491.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- Faglia E, F Favales, A Morabito. New Ulceration, New Major Amputation, and Survival Rates in Diabetic Subjects Hospitalized for Foot Ulceration from 1990 to 1993: A 6.5-year Follow-up. Diabetes Care. 2001;24(1):78–83. doi: 10.2337/diacare.24.1.78. [DOI] [PubMed] [Google Scholar]
- Gordois A, Scuffham P, A Shearer, A Oglesby, Tobian JA. The Health Care Costs of Diabetic Peripheral Neuropathy in the US. Diabetes Care. 2003;26(6):1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- Hedetoft C, Rasmussen A, Fabrin J, Kolendorf K. Four-Fold Increase in Foot Ulcers in Type 2 Diabetic Subjects without an Increase in Major Amputations by a Multidisciplinary Setting. Diabetes Research and Clinical Practice. 2009;83(3):353–7. doi: 10.1016/j.diabres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Jeffcoate WJ, Harding KG. Diabetic Foot Ulcers. Lancet. 2003;361(9368):1545–51. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic Control from 1988 to 2000 among US Adults Diagnosed with Type 2 Diabetes. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- Lavery LA, Wunderlich RP, Tredwell JL. Disease Management for the Diabetic Foot: Effectiveness of a Diabetic Foot Prevention Program to Reduce Amputations and Hospitalizations. Diabetes Research and Clinical Practice. 2005;70(1):31–7. doi: 10.1016/j.diabres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lee PP, Feldman ZW, Ostermann JO, Brown DS, Sloan FA. Longitudinal Rates of Annual Eye Examinations of Persons with Diabetes and Chronic Eye Diseases. Ophthalmology. 2003;110:1952–9. doi: 10.1016/S0161-6420(03)00817-0. [DOI] [PubMed] [Google Scholar]
- Litzelman DK, Slemenda CW, Langefeld CD, Hays LM, Welch MA, Bild DE, Ford ES, Vinicor F. Reduction of Lower-Extremity Clinical Abnormalities in Patients with Noninsulin-Dependent Diabetes-Mellitus—A Randomized, Controlled Trial. Annals of Internal Medicine. 1993;119(1):36–41. doi: 10.7326/0003-4819-119-1-199307010-00006. [DOI] [PubMed] [Google Scholar]
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive Foot Care in People with Diabetes. Diabetes Care. 1998;21(12):2161–77. doi: 10.2337/diacare.21.12.2161. [DOI] [PubMed] [Google Scholar]
- McClellan WM, Millman L, Presley R, Couzins J, Flanders WD. Improved Diabetes Care by Primary Care Physicians: Results of a Group-Randomized Evaluation of the Medicare Health Care Quality Improvement Program (HCQIP) Journal of Clinical Epidemiology. 2003;56:1210–7. doi: 10.1016/s0895-4356(03)00198-7. [DOI] [PubMed] [Google Scholar]
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The Quality of Health Care Delivered to Adults in the United States. New England Journal of Medicine. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States, 2000. Journal of the American Medical Association. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Patrick AR, Caro J. Estimates of Direct Medical Costs for Microvascular and Macrovascular Complications Resulting from Type 2 Diabetes Mellitus in the United States in 2000. Clinical Therapeutics. 2003;25(3):1017–38. doi: 10.1016/s0149-2918(03)80122-4. [DOI] [PubMed] [Google Scholar]
- Patout CA, Birke JA, Horswell R, Williams D, Cerise FP. Effectiveness of a Comprehensive Diabetes Lower-Extremity Amputation Prevention Program in a Predominantly Low-Income African-American Population. Diabetes Care. 2000;23(9):1339–42. doi: 10.2337/diacare.23.9.1339. [DOI] [PubMed] [Google Scholar]
- Plank J, Haas W, Rakovac I, Gorzer E, Sommer R, Siebenhofer A, Pieber TR. Evaluation of the Impact of Chiropodist Care in the Secondary Prevention of Foot Ulcerations in Diabetic Subjects. Diabetes Care. 2003;26(6):1691–5. doi: 10.2337/diacare.26.6.1691. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Sandhu N, Newton K, Reiber GE, Blough D, Wagner EH, McCullough DK. Incidence, Outcomes, and Cost of Foot Ulcers in Patients with Diabetes. Diabetes Care. 1999;22(3):382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Ledoux WR. Epidemiology of Diabetic Foot Ulcers and Amputations: Evidence for Prevention. In: Williams R, Herman W, Kinmonth AL, Wareham NJ, editors. The Evidence Base for Diabetes Care. West Sussex, England: John Wiley & Sons; 2002. pp. 641–665. [Google Scholar]
- Rith-Najarian S, C Branchaud, O Beaulieu, D Gohdes, G Simonson, R Mazze. Reducing Lower-Extremity Amputations Due to Diabetes: Application of the Staged Diabetes Management Approach in a Primary Care Setting. Journal of Family Practice. 1998;47(2):127–32. [PubMed] [Google Scholar]
- Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The Burden of Mortality Attributable to Diabetes: Realistic Estimates for the Year 2000. Diabetes Care. 2005;28:2130–5. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- Ronnemaa T, Hamalainen H, Toikka T, Liukkonen I. Evaluation of the Impact of Podiatrist Care in the Primary Prevention of Foot Problems in Diabetic Subjects. Diabetes Care. 1997;20(12):1833–7. doi: 10.2337/diacare.20.12.1833. [DOI] [PubMed] [Google Scholar]
- Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KMV. A Diabetes Report Card for the United States: Quality of Care in the 1990s. Annals of Internal Medicine. 2002;136:565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- Shea DG, Terza JV, Stuart BC, Briesacher B. Estimating the Effects of Prescription Drug Coverage for Medicare Beneficiaries. Health Services Research. 2007;42(3):933–49. doi: 10.1111/j.1475-6773.2006.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A, P Scuffham, A Gordois, A Oglesby. Predicted Costs and Outcomes from Reduced Vibration Detection in People with Diabetes in the US. Diabetes Care. 2003;26(8):2305–10. doi: 10.2337/diacare.26.8.2305. [DOI] [PubMed] [Google Scholar]
- Singh N, Armstrong DG, Lipsky BA. Preventing Foot Ulcers in Patients with Diabetes. Journal of the American Medical Association. 2005;293(2):217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- Sloan FA, Bethel MA, Lee PP, Brown DS, Feinglos MN. Adherence to Guidelines and its Effects on Hospitalizations with Complications of Type 2 Diabetes. Review of Diabetic Studies. 2004;1:29–38. doi: 10.1900/RDS.2004.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan FA, Trogdon JG, Curtis LH, Schulman KA. Does the Ownership of the Admitting Hospital Make a Difference? Outcomes and Process of Care of Medicare Beneficiaries Admitted with Acute Myocardial Infarction. Medical Care. 2003;41(10):1193–205. doi: 10.1097/01.MLR.0000088569.50763.15. [DOI] [PubMed] [Google Scholar]
- Terza JV, Basu A, Rathouz PJ. Two-Stage Residual Inclusion Estimation: Addressing Endogeneity in Health Econometric Modeling. Journal of Health Economics. 2008;27(3):531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner C, Haastert B, Mauckner P, Gaetcke LM, Giani G. Reduced Incidence of Lower-Limb Amputations in the Diabetic Population of a German City, 1990–2005. Diabetes Care. 2007;30(10):2633–7. doi: 10.2337/dc07-0876. [DOI] [PubMed] [Google Scholar]
- Williams R, Van Gaal L, Lucioni C. Assessing the Impact of Complications on the Costs of Type II Diabetes. Diabetologia. 2002;45:S13–7. doi: 10.1007/s00125-002-0859-9. [DOI] [PubMed] [Google Scholar]
- Zoorob RJ, Hagen MD. Guidelines on the Care of Diabetic Nephropathy, Retinopathy and Foot Disease. American Family Physician. 1997;56:2021–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


