Abstract
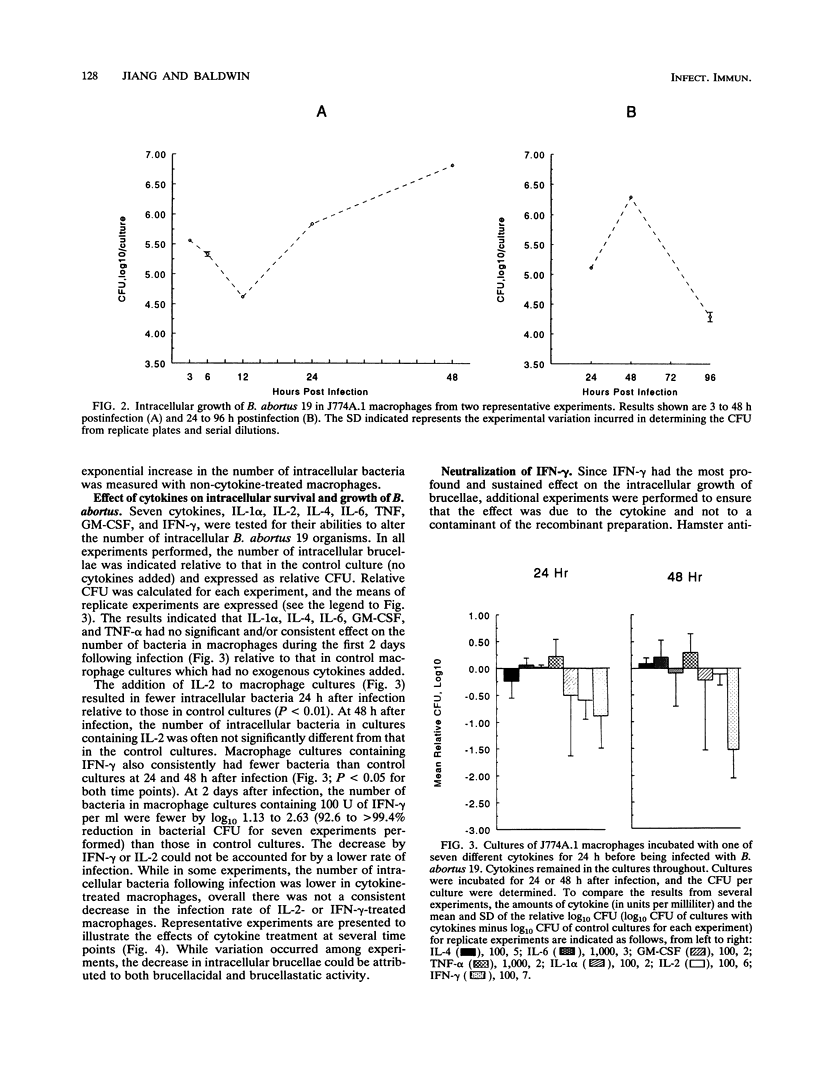
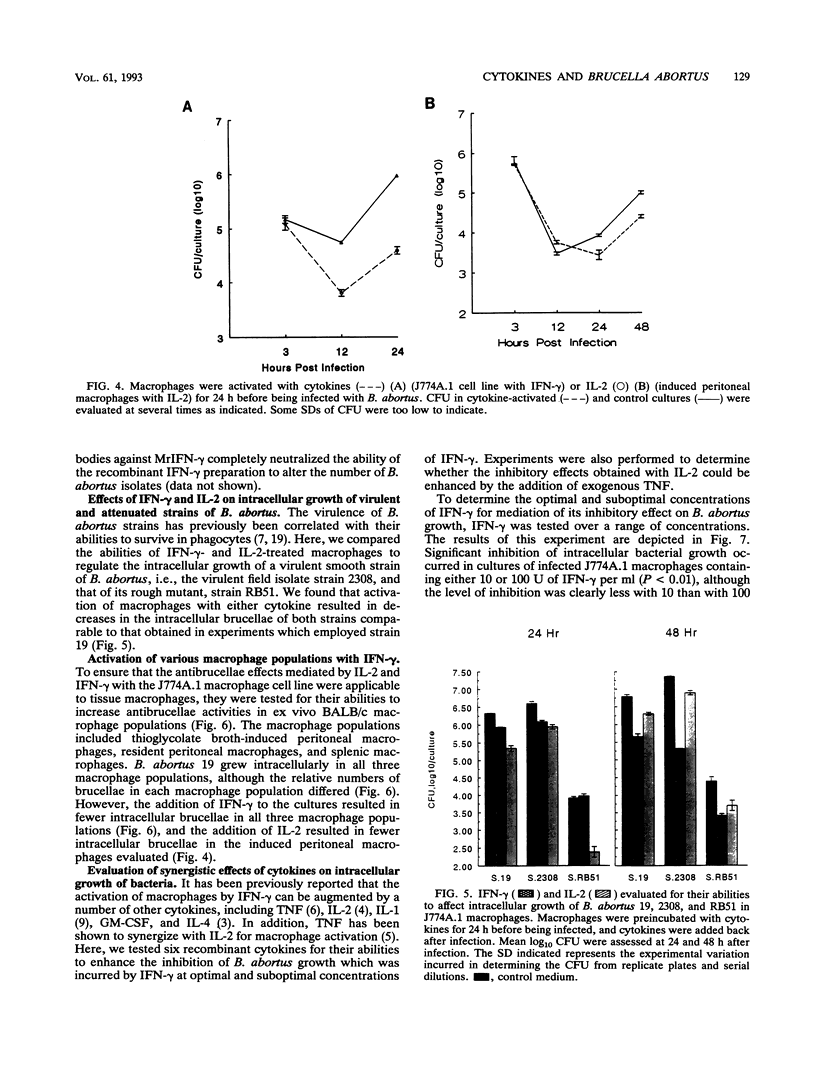
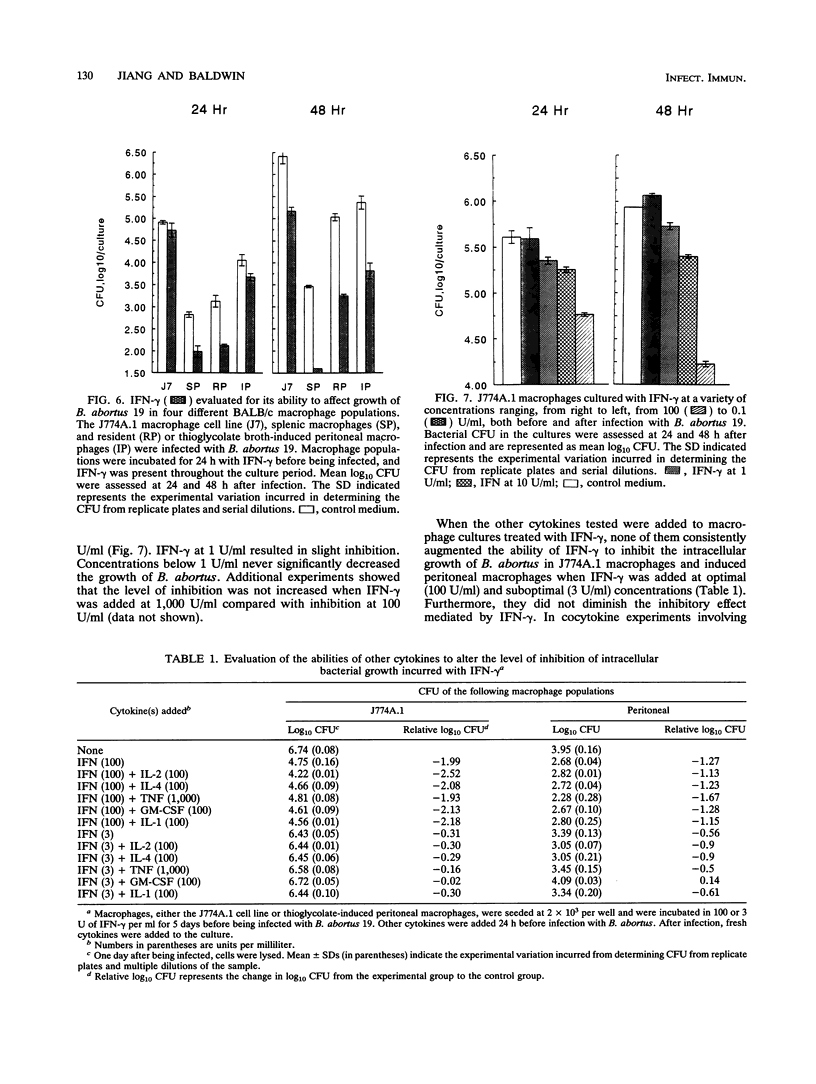
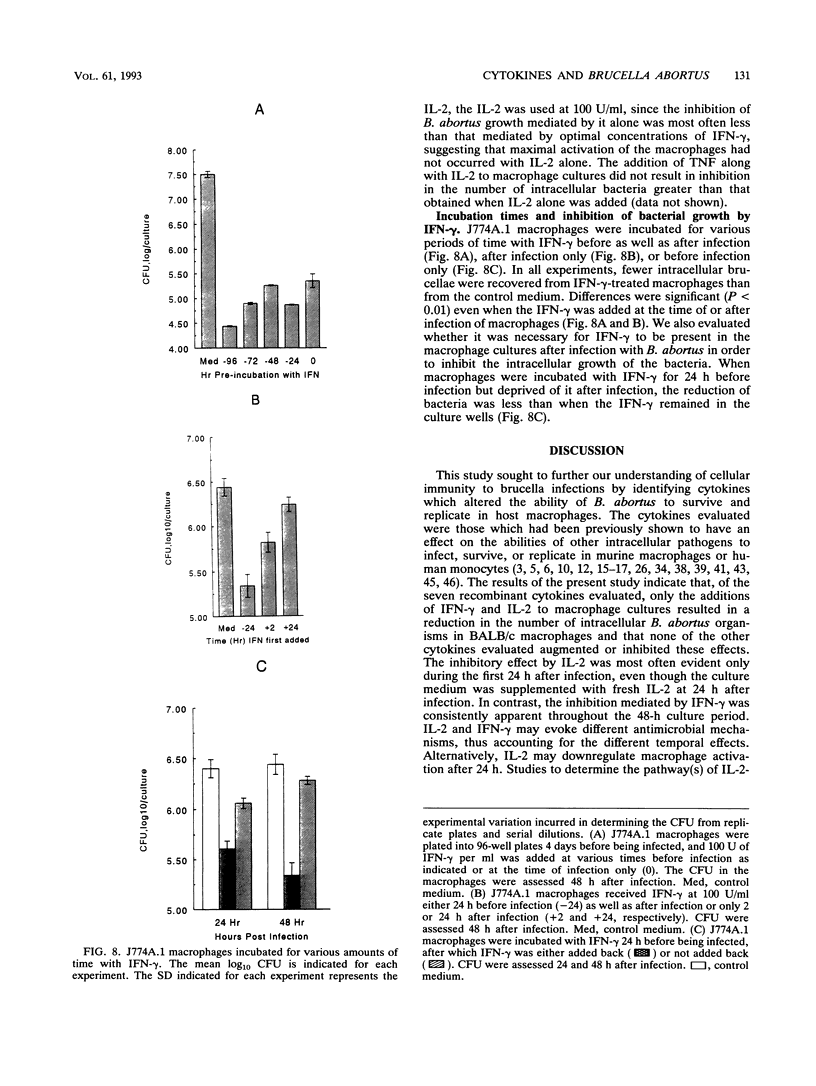
Interleukin 1 alpha (IL-1 alpha), IL-2, IL-4, IL-6, gamma interferon (IFN-gamma), tumor necrosis factor alpha (TNF-alpha), and granulocyte macrophage colony-stimulating factor (GM-CSF) were tested for their abilities to alter the growth of Brucella abortus in BALB/c J774A.1 murine macrophages. IL-1 alpha, IL-4, IL-6, tumor necrosis factor alpha, and granulocyte macrophage-colony-stimulating factor had no consistent or significant effect on the growth of the avirulent B. abortus strain 19. In contrast, the addition of either IFN-gamma or IL-2 at 100 U/ml to the macrophage cultures resulted in a significant reduction in the number of intracellular bacteria that was not attributable to decreased infection rates. With IL-2, the reduction was most often apparent only during the first 24 h after infection, while inhibition with IFN-gamma was apparent throughout the culture period of 48 h. The addition of either IL-2 or IFN-gamma to macrophage cultures also resulted in reduced intracellular CFU of the virulent B. abortus strain 2308 and the attenuated rough mutant B. abortus strain RB51. Inhibition of intracellular growth was not augmented by combinations of cytokines. Additional studies with IFN-gamma and IL-2 indicated that they could mediate the inhibition of intracellular growth of B. abortus in resident and thioglycolate broth-induced BALB/c peritoneal macrophages and in splenic macrophages. IFN-gamma also inhibited bacterial growth when added after infection of the macrophages, although the magnitude of the antibrucellae effects was less than that when it was added before infection. Furthermore, the maximal inhibitory effect was sustained only when IFN-gamma remained in the cultures after infection of the macrophages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya L. N., Elzer P. H., Rowe G. E., Enright F. M., Winter A. J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989 Nov 15;143(10):3330–3337. [PubMed] [Google Scholar]
- Araya L. N., Winter A. J. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect Immun. 1990 Jan;58(1):254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Meltzer M. S., Nacy C. A. IL-2. A cofactor for induction of activated macrophage resistance to infection. J Immunol. 1990 Aug 1;145(3):831–839. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Carlin J. M., Ozaki Y., Byrne G. I., Brown R. R., Borden E. C. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989 Jun 15;45(6):535–541. doi: 10.1007/BF01990503. [DOI] [PubMed] [Google Scholar]
- Chen L., Suzuki Y., Wheelock E. F. Interferon-gamma synergizes with tumor necrosis factor and with interleukin 1 and requires the presence of both monokines to induce antitumor cytotoxic activity in macrophages. J Immunol. 1987 Dec 15;139(12):4096–4101. [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990 Sep;71(1):139–141. [PMC free article] [PubMed] [Google Scholar]
- Denis M. Growth of Mycobacterium avium in human monocytes: identification of cytokines which reduce and enhance intracellular microbial growth. Eur J Immunol. 1991 Feb;21(2):391–395. doi: 10.1002/eji.1830210221. [DOI] [PubMed] [Google Scholar]
- Denis M. Modulation of Mycobacterium lepraemurium growth in murine macrophages: beneficial effect of tumor necrosis factor alpha and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1991 Feb;59(2):705–707. doi: 10.1128/iai.59.2.705-707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detilleux P. G., Deyoe B. L., Cheville N. F. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect Immun. 1990 Jul;58(7):2320–2328. doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988 Jun;56(6):1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Halling S. M., Detilleux P. G., Tatum F. M., Judge B. A., Mayfield J. E. Deletion of the BCSP31 gene of Brucella abortus by replacement. Infect Immun. 1991 Nov;59(11):3863–3868. doi: 10.1128/iai.59.11.3863-3868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon B. G., Adams L. G., Frey M. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am J Vet Res. 1988 Jul;49(7):1092–1097. [PubMed] [Google Scholar]
- Heidenreich S., Weyers M., Gong J. H., Sprenger H., Nain M., Gemsa D. Potentiation of lymphokine-induced macrophage activation by tumor necrosis factor-alpha. J Immunol. 1988 Mar 1;140(5):1511–1518. [PubMed] [Google Scholar]
- Ho M., Cheers C. Resistance and susceptibility of mice to bacterial infection. IV. Genetic and cellular basis of resistance to chronic infection with Brucella abortus. J Infect Dis. 1982 Sep;146(3):381–387. doi: 10.1093/infdis/146.3.381. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Winter A. J. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992 Jul;60(7):3011–3014. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Keist R., Frei K. Lymphokines and bacteria, that induce tumoricidal activity, trigger a different secretory response in macrophages. Eur J Immunol. 1990 Mar;20(3):695–698. doi: 10.1002/eji.1830200334. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- McGee Z. A., Gorby G. L., Updike W. S. The use of neutrophils, macrophages and organ cultures to assess the penetration of human cells by antimicrobials. Prog Drug Res. 1989;33:83–92. doi: 10.1007/978-3-0348-9146-2_4. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Borowiak D., Mayer H. Brucella lipopolysaccharides and polysaccharides. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):102–105. doi: 10.1016/0769-2609(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Moriyón I., Gamazo C., Díaz R. Properties of the outer membrane of Brucella. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):89–91. doi: 10.1016/0769-2609(87)90082-2. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P. The epidemiology of bovine brucellosis. Adv Vet Sci Comp Med. 1980;24:69–98. [PubMed] [Google Scholar]
- Passwell J. H., Shor R., Shoham J. The enhancing effect of interferon-beta and -gamma on the killing of Leishmania tropica major in human mononuclear phagocytes in vitro. J Immunol. 1986 Apr 15;136(8):3062–3066. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Schreiber R. D., Connelly P., Tilney L. G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989 Dec 1;170(6):2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollag H., Degré M., Sonnenfeld G. Effects of interferon-alpha/beta and interferon-gamma preparations on phagocytosis by mouse peritoneal macrophages. Scand J Immunol. 1984 Aug;20(2):149–155. doi: 10.1111/j.1365-3083.1984.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Roop R. M., 2nd, Bagchi T., Boyle S., Buhrman D., Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991 Jul;28(2):171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Hunt D. A., Smith P. D., Wahl L. M., Katona I. M. Monocyte interleukin 2 receptor gene expression and interleukin 2 augmentation of microbicidal activity. J Immunol. 1987 Aug 15;139(4):1342–1347. [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. J. Human brucellosis. Rev Infect Dis. 1983 Sep-Oct;5(5):821–842. doi: 10.1093/clinids/5.5.821. [DOI] [PubMed] [Google Scholar]
- Zhan Y. F., Stanley E. R., Cheers C. Prophylaxis or treatment of experimental brucellosis with interleukin-1. Infect Immun. 1991 May;59(5):1790–1794. doi: 10.1128/iai.59.5.1790-1794.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]