Abstract
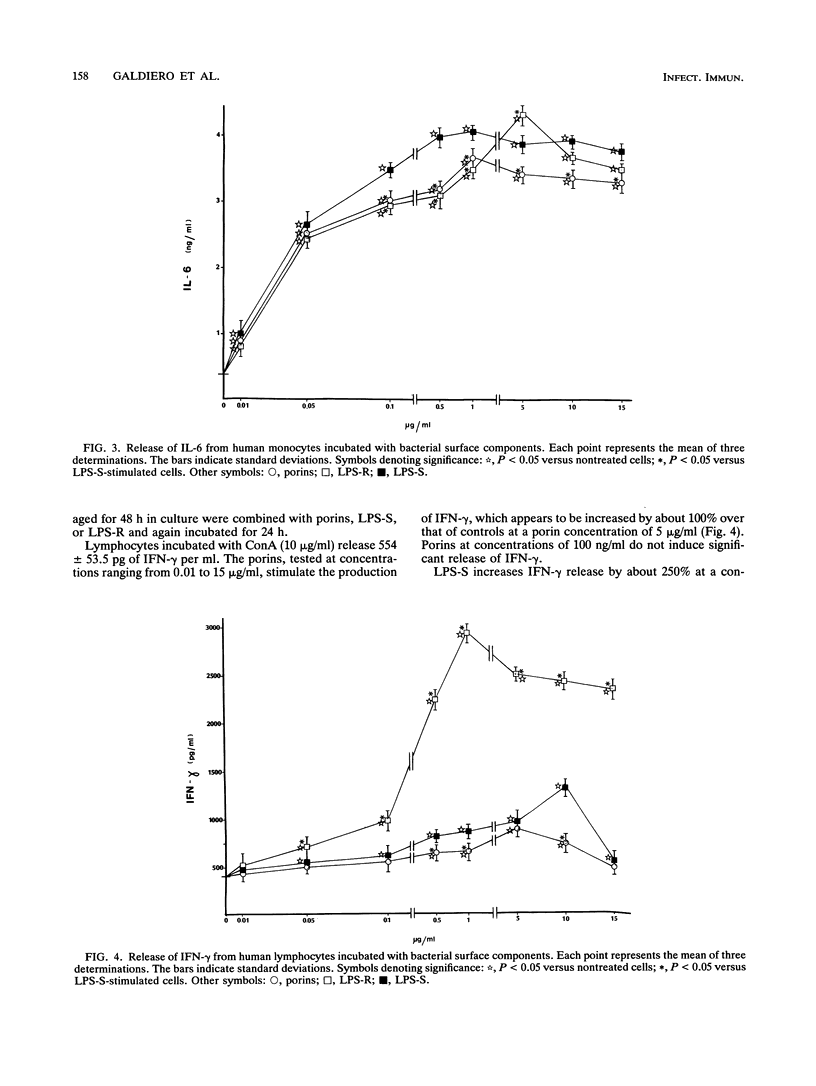
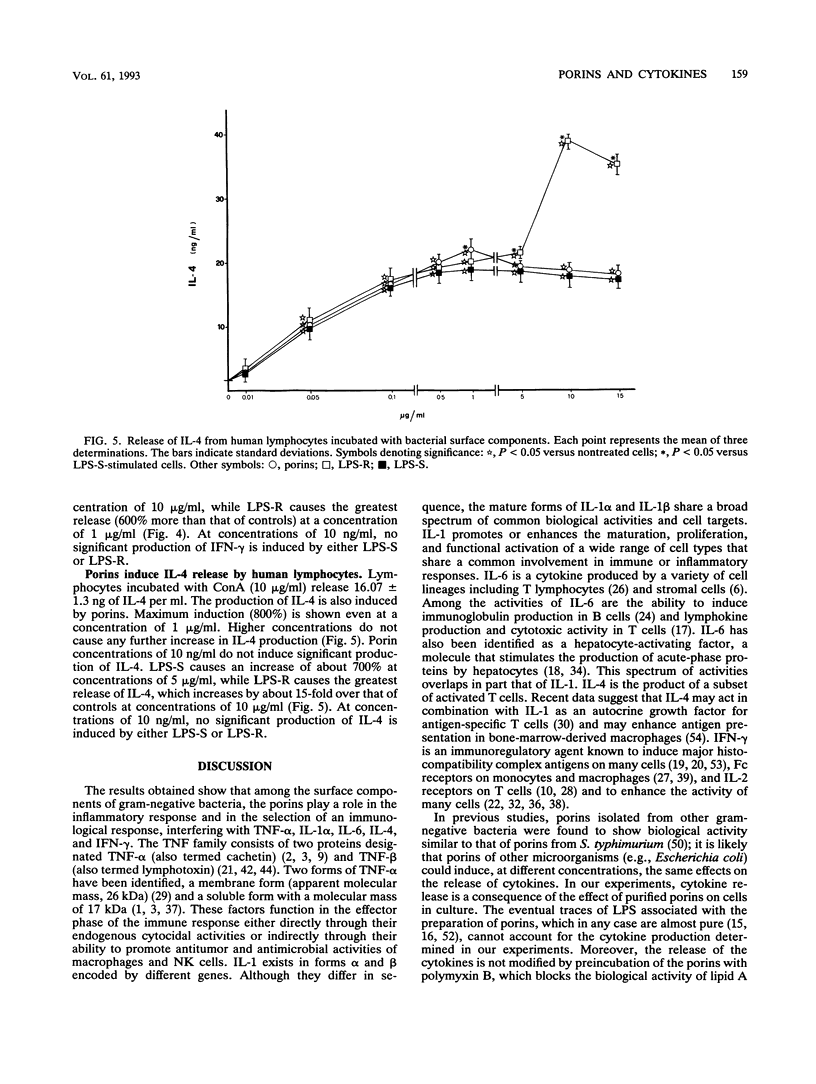
Salmonella typhimurium SH5014 porins induce the release of tumor necrosis factor alpha (TNF-alpha), interleukin 1 alpha (IL-1 alpha), and IL-6 by human monocytes and of gamma interferon (IFN-gamma) and IL-4 by human lymphocytes. Porins at 1 microgram/ml induce the greatest release of TNF-alpha, IL-1 alpha, and IL-6 by monocytes and of IL-4 by lymphocytes, while porins at 5 micrograms/ml induce the greatest release of IFN-gamma by lymphocytes. The R form of lipopolysaccharide (LPS-R) induces the greatest release of TNF-alpha and IL-1 alpha by monocytes when used at a low concentration (1 microgram/ml). At higher concentrations (5 and 10 micrograms/ml, respectively), LPS-R induces the maximal release of IL-6 from monocytes and the maximal release of IL-4 from lymphocytes. The S form of LPS (LPS-S) induces the greatest release of TNF-alpha, IL-1 alpha, and IL-6 by monocytes and that of IL-4 by lymphocytes when used at a concentration of 1 microgram/ml. After stimulation with LPS-S, the largest quantity of TNF-alpha and IL-1 alpha released was less than that obtained after stimulation with LPS-R at the same concentration, while the quantity of IL-6 released was found to be slightly higher than that obtained after stimulation with porins or LPS-R. LPS-S (1 microgram/ml) induces IFN-gamma release from lymphocytes in notably smaller quantities than that obtained with LPS-R and slightly larger quantities than that obtained with porins. The preparation of porins used was found to be contaminated with 10 pg of LPS per 10 micrograms of porins, a quantity which was found to have no biological effect; furthermore, porin preparations with the addition of polymyxin B gave the same results.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986 Feb 1;136(3):963–970. [PubMed] [Google Scholar]
- Di Donato A., Draetta G. F., Illiano G., Tufano M. A., Sommese L., Galdiero F. Do porins inhibit the macrophage phagocyting activity by stimulating the adenylate cyclase? J Cyclic Nucleotide Protein Phosphor Res. 1986;11(2):87–97. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988 Jan-Feb;10(1):168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galdiero F., Gorga F., Bentivoglio C., Mancuso R., Galdiero E., Tufano M. A. The action of LPS porins and peptidoglycan fragments on human spermatozoa. Infection. 1988;16(6):349–353. doi: 10.1007/BF01644545. [DOI] [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Galdiero M., Masiello S., Di Rosa M. Inflammatory effects of Salmonella typhimurium porins. Infect Immun. 1990 Oct;58(10):3183–3186. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Sommese L., Folgore A., Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984 Nov;46(2):559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard T. L., Dyer D. R., Zoon K. C., zur Nedden D., Siegel J. P. Modulation of class I and class II histocompatibility antigens on human T cell lines by IFN-gamma. J Immunol. 1988 May 15;140(10):3450–3455. [PubMed] [Google Scholar]
- Giacomini P., Tecce R., Gambari R., Sacchi A., Fisher P. B., Natali P. G. Recombinant human IFN-gamma, but not IFN-alpha or IFN-beta, enhances MHC- and non-MHC-encoded glycoproteins by a protein synthesis-dependent mechanism. J Immunol. 1988 May 1;140(9):3073–3081. [PubMed] [Google Scholar]
- Granger G. A., Williams T. W. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature. 1968 Jun 29;218(5148):1253–1254. doi: 10.1038/2181253a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Homma J. Y. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Z Allg Mikrobiol. 1968;8(3):227–248. doi: 10.1002/jobm.3630080310. [DOI] [PubMed] [Google Scholar]
- Horii Y., Muraguchi A., Suematsu S., Matsuda T., Yoshizaki K., Hirano T., Kishimoto T. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J Immunol. 1988 Sep 1;141(5):1529–1535. [PubMed] [Google Scholar]
- Itoh K., Inoue M., Kataoka S., Kumagai K. Differential effect of interferon expression of IgG- and IgM-Fc receptors on human lymphocytes. J Immunol. 1980 Jun;124(6):2589–2595. [PubMed] [Google Scholar]
- Johnson H. M., Farrar W. L. The role of a gamma interferon-like lymphokine in the activation of T cells for expression of interleukin 2 receptors. Cell Immunol. 1983 Jan;75(1):154–159. doi: 10.1016/0008-8749(83)90314-3. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kupper T., Horowitz M., Lee F., Robb R., Flood P. M. Autocrine growth of T cells independent of interleukin 2: identification of interleukin 4 (IL 4, BSF-1) as an autocrine growth factor for a cloned antigen-specific helper T cell. J Immunol. 1987 Jun 15;138(12):4280–4287. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Lymphokine-mediated activation of human monocytes: neutralization by monoclonal antibody to interferon-gamma. Cell Immunol. 1984 Apr 15;85(1):278–283. doi: 10.1016/0008-8749(84)90299-5. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Galanos C. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect Immun. 1990 Apr;58(4):935–937. doi: 10.1128/iai.58.4.935-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijsten M. W., de Groot E. R., ten Duis H. J., Klasen H. J., Hack C. E., Aarden L. A. Serum levels of interleukin-6 and acute phase responses. Lancet. 1987 Oct 17;2(8564):921–921. doi: 10.1016/s0140-6736(87)91413-9. [DOI] [PubMed] [Google Scholar]
- Omata Y., Sethi K. K., Brandis H. Analysis of the roles of immune interferon (IFN-gamma) and colony-stimulating factor(s) in the induction of macrophage anti-toxoplasma activity. Immunobiology. 1984 Mar;166(2):146–156. doi: 10.1016/s0171-2985(84)80033-9. [DOI] [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Petroni K. C., Shen L., Guyre P. M. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J Immunol. 1988 May 15;140(10):3467–3472. [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- Rifkind D., Palmer J. D. Neutralization of endotoxin toxicity in chick embryos by antibiotics. J Bacteriol. 1966 Oct;92(4):815–819. doi: 10.1128/jb.92.4.815-819.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968 Dec 1;128(6):1267–1279. doi: 10.1084/jem.128.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahdev I., O'Reilly R., Hoffman M. K. Correlation between interleukin-1 production and engraftment of transplanted bone marrow stem cells in patients with lethal immunodeficiencies. Blood. 1989 May 1;73(6):1712–1719. [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988 Feb;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tufano M. A., Berlingieri M. T., Sommese L., Galdiero F. Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica. 1984 Oct;7(4):353–366. [PubMed] [Google Scholar]
- Tufano M. A., Capasso G., Anastasio P., Giordano D. R., Giordano D., Galdiero E., Sommese L., De Santo N. G. Clearance studies on the renal action of porins extracted from Salmonella typhimurium. Int J Pediatr Nephrol. 1987 Oct-Dec;8(4):193–198. [PubMed] [Google Scholar]
- Tufano M. A., Ianniello R., Galdiero M., De Martino L., Galdiero F. Effect of Salmonella typhimurium porins on biological activities of human polymorphonuclear leukocytes. Microb Pathog. 1989 Nov;7(5):337–346. doi: 10.1016/0882-4010(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Tufano M. A., Sommese L., Capasso C., Folgore A., Scafa F., Galdiero E. Comparative study of some biological activities of porins extracted from various microorganisms. Microbiologica. 1986 Oct;9(4):431–442. [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., Harris A. W., Schrader J. W. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984 Jan;14(1):52–56. doi: 10.1002/eji.1830140110. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]


