Abstract
AIM: To investigate plasma ghrelin, gastrin and growth hormone secretagogue receptor (GHS-R) expression in advanced gastric cancer (GC) before and after resection.
METHODS: Seventy subjects in whom endoscopy of the upper gastrointestinal tract was performed in the Department of General Surgery at Cracow University during the past decade: (1) 25 patients with GC associated with Helicobacter pylori (H. pylori) infection; (2) 10 patients with GC 4-5 years after (total or subtotal) gastrectomy; (3) 25 healthy H. pylori-negative controls, matched by age and BMI to the above two groups; and (4) 10 GC patients 4-5 years after total gastrectomy. Ghrelin and gastrin plasma concentrations were measured by specific radioimmunoassay under fasting conditions and postprandially at 60 and 90 min after ingestion of a mixed meal. GHS-R expression was examined in biopsy samples from intact healthy mucosa and GC tissue using semi-quantitative reverse transcription-polymerase chain reaction.
RESULTS: In healthy controls, fasting plasma ghrelin levels were significantly elevated and declined markedly at 60 and 90 min after a mixed meal. The concomitant enhanced ghrelin, GHS-R and gastrin expression in GC tissue over that recorded in intact mucosa, and the marked rise in plasma gastrin in these subjects under fasting conditions indicate the role of these hormonal factors in GC formation. Fasting plasma levels and postprandial response of ghrelin and gastrin appear to be inversely correlated in healthy subjects. Feeding in the controls resulted in a significant fall in plasma ghrelin with a subsequent rise in plasma gastrin, but in H. pylori-positive GC patients submitted to total or distal gastrectomy, feeding failed to affect significantly the fall in plasma ghrelin that was recorded in these patients before surgery. Fasting ghrelin concentrations were significantly lower in patients 4-5 years after total gastrectomy compared to those in healthy controls and to these in GC patients before surgery.
CONCLUSION: Elevated plasma gastrin and suppression of fasting ghrelin in patients with GC suggest the existence of a close relationship between these two hormones in gastric carcinogenesis.
Keywords: Ghrelin, Gastrin, Gastric cancer, Gastric resection
INTRODUCTION
Ghrelin, a natural ligand for growth hormone secretagogue receptor (GHS-R), was originally identified in rat gastric mucosa and shown to be expressed by the endocrine ghrelin (Gr)-cells of this mucosa[1,2]. These Gr-cells, represent about 20% of the total population of endocrine cells in human gastric mucosa, and are located in the lower parts of the oxyntic glands in the fundus and body of the stomach. Gastric acid secretion is stimulated physiologically by gastrin and histamine as well as by ghrelin itself[2-6] (Figure 1).
Figure 1.
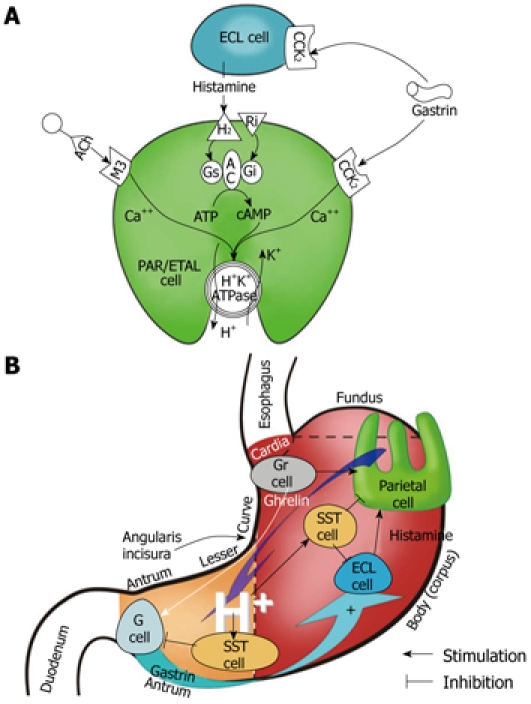
Gastric secretory mechanism at the parietal cell (oxyntic) level (A) and at the level of the whole stomach (B).
Ghrelin is a stimulant of GH secretion, which acts via release of GHS-R in the stomach, but characteristically, it is also the only circulatory gastrointestinal hormone that is known to be secreted by the empty (fasting) stomach, to enhance food intake and maintain energy homeostasis following its central or peripheral administration[6]. Ghrelin is currently considered as the most powerful endogenous orexigenic (appetite-stimulating) hormone, which results in a positive energy balance[4,6,7]. Its action on gastric mucosa might contribute to carcinogenesis, but the role of ghrelin/GHS-R in gastric cancer (GC) pathogenesis[8] awaits explanation, and possible use of ghrelin in severe gastric-originated cachexia remains unknown.
The reduction in the ghrelin levels after gastrectomy by > 60% supports the notion that the gastric mucosa is the main site of ghrelin production[3,4,9]. Gastric cancer modifies the expression of ghrelin in gastric mucosa and this could be attributed, at least in part, to severe atrophic gastritis[10,11], which usually precedes and might lead to gastric carcinogenesis. Mottershead et al[12] have observed a lack of expression of mRNA for ghrelin in GC cells by immunohistochemical and reverse transcription-polymerase chain reaction methods. According to our recent experience, the expression of mRNA for ghrelin in pronounced atrophic gastritis is relatively low, so major changes in plasma ghrelin cannot originate from the Helicobacter pylori (H. pylori)-related atrophic gastritis tissue[12].
The aim of the present study was to analyze the plasma concentrations of the ghrelin-GHS-R complex in patients with advanced GC before and after total or distal gastrectomy. In addition, we analyzed plasma ghrelin concentrations and mucosal gene expression of ghrelin and its receptor, GHR-S, in intact mucosa and in H. pylori-infected GC tissue. Moreover, the analysis of fasting and postprandial plasma ghrelin levels vs plasma gastrin concentrations in patients at 4-5 years after total and distal gastrectomy was assessed to elucidate the possible compensatory extragastric production of these hormones.
MATERIALS AND METHODS
The study included 70 patients, aged 18 and over who, from January 2006 to May 2008, underwent upper gastrointestinal tract endoscopy in a specialized unit of the Department of Surgery, Jagiellonian University Medical College, Cracow.
Exclusion criteria included any of the following: age < 18 years; no consent to participate in the study; a history of diabetes, thyroid disease and neuroendocrine tumors, use of glucocorticoids, progesterone or testosterone, renal and/or liver failure; prior chemotherapy (not applicable to a group of patients 4-5 years after gastrectomy); drugs and/or alcohol addiction; and body mass index (BMI) > 30 kg/m2. Patients included in the research program based on the above criteria were assigned to one of the following groups: (1) 25 patients with GC associated with H. pylori infection; (2) 10 patients with GC 4-5 years after (total or subtotal) gastrectomy; (3) 25 healthy H. pylori-negative controls, matched by age and BMI to the above two groups; and (4) 10 GC patients 4-5 years after total gastrectomy. The basis of this categorization was the initial diagnosis upon admission to our gastrointestinal unit (Table 1). The study was approved and supervised by the Institutional Research Ethical Committee and informed consent was obtained from each participant in this study.
Table 1.
Characteristics of study groups (median and range)
| Patients with GC and H. pylori infection (before operation) | Patients 4-5 yr after total gastrectomy | Controls without pathology in the gastric mucosa and H. pylori infection | |
| n | 25 | 10 | 25 |
| Age (yr) | 64.6 (43-81) | 60.8 (49-69) | 51 (22-67) |
| Male/female | 14/11 | 5/5 | 12/13 |
| BMI (kg/m2) | 23.1 (18.7-29.7) | 22.4 (20.3-24.5) | 23.4 (19.5-30) |
GC: Gastric cancer; H. pylori: Helicobacter pylori; BMI: Body mass index.
All subjects underwent gastroscopy with mucosal biopsy. The study was performed after at least 12 h of fasting. Topical lidocaine (Lignocaine aerosol 10%, Glaxo-Wellcome, UK) was used for local throat anesthesia if there was no history of allergy to it. All gastroscopies were performed by the same highly experienced endoscopists. During each endoscopy procedure, visual assessment was made of the mucosa of the esophagus and gastric cardia, fundus, corpus, pylorus and antrum, and the first portion of the duodenum and fundus of the stomach in inverted gastroscopy. In post-gastrectomy patients, during initial endoscopy, esophago-jejunal anastomosis was evaluated and tissue samples were taken for histological examination.
In patients with GC and in the control group, the rapid urease test was performed at the time of endoscopy. The biopsy samples from the antral mucosa (or in cases of cancer involvement of the distal stomach from any part of the gastric mucosa) were placed on the yellow colored gel that contained urea. The change in color of the indicator contained in the gel from yellow to pink demonstrated the presence of urease in the specimen. In accordance with the attached instructions (Urease Test “GUTplus”, Lencomm Trade International, UK), the results were read at 30 min and 3 h after collection to decide whether or not the mucosa was infected by H. pylori.
In controls during endoscopy, samples from the gastric fundus mucosa, body and antrum were obtained for histopathological examination. In patients with GC, biopsy specimens were also taken from the tumor itself. The morphological form of the cancer was assessed according to the Bormann classification system. Patients with GC underwent surgery; in 10 cases, it was total gastrectomy, and in eight, it was subtotal (distal) gastrectomy.
Venous blood was collected from all patients with an empty stomach (i.e. at least 12 h without eating solid or liquid meals), at 08:00 h, and postprandially at 60 and 90 min after a mixed carbohydrate/protein/fat meal plus 250 mL milk of about 600 Kcal (given at 10:00 h). In the patients with GC, the above-mentioned protocol was repeated twice, that is, before treatment and 10 d after surgery, when the patients started to eat.
Venous blood samples were collected into tubes that contained a 10% aqueous solution of disodium EDTA to prevent clotting (0.05 mL 10% EDTA in 5 mL of blood). The centrifugation was performed within 10 min from of blood collection. The blood was centrifuged for 10 min at 3000 r/min (MPW-340 centrifuge; Precision Mechanics, Warsaw, Poland). Plasma obtained after separation was divided into three portions and frozen (at about -80°C) until quantification analysis of ghrelin, GHS-R and gastrin by radioimmunoassay.
Laboratory tests were performed in the specialized Hormonal Research Laboratory of Isotopic Diagnostics at the Department of Physiology, Jagiellonian University Medical College.
The concentration of ghrelin in the test plasma samples was determined using S-2227RIKU4864 radioimmunoassay kits (Peninsula Laboratories, San Carlos, CA, USA). All measurements were performed in duplicate. Standard curves were prepared by appropriate dilution of 12.8 μL lyophilized peptide. Initial incubation of 100 μL plasma samples and subsequent dilutions of ghrelin standards (1, 2, 4, 8, 16, 32, 64, and 128 pg) was carried out for 24 h at 4°C, after addition of 100 μL highly specific rabbit antibodies for human ghrelin. Later, 100 μL of tracer (125I-ghrelin, 10000-15000 cpm) was added to each sample and incubation was continued for another 24 h at 4°C. Immunoprecipitation by addition of second antibody (goat anti-rabbit IgG) and centrifugation after 90 min incubation at 25°C was performed for the final separation of the free and bound fractions. The concentration of ghrelin in plasma samples was calculated based on the calibration curve that was obtained by “Spline” method, based on measurements of radioactivity (gamma counter) for consecutive concentrations of the standards. Assay sensitivity was 3.0 pg/mL, and the specificity of the antibodies for the labeled human ghrelin was 100%. In accordance with the attached attestation (Peninsula Laboratories, USA) antibodies do not fall (0%) in cross-reaction with motilin, growth hormone releasing factor, orexin, secretin, VIP and galanin.
Assessments of gastrin levels were performed using a commercial kit (GAS-PR RIA; CIS Bio-International, France) following the manufacturer’s recommendations as described before[5,6,13]. The plasma samples (100 μL) were incubated in duplicate at 25°C for 3 h with 100 μL of tracer (125I-gastrin) and 300 μL of anti G-17 antibody. The antibody equally recognized and had affinity to the gastrins G-17 and G-34. Points of the standard curve at concentrations of 11.2, 28.4, 68.4, 255.8, 651.2 pmol/L were prepared from lyophilized synthetic G-17 and incubated as above. Separation of the free from bound fraction was obtained by immunoprecipitation. Final radioactivity in the samples was assayed, and standard curve points were measured in a gamma counter (1574 Clinigamma, Wallac-LKB, Sweden), using the computer program Spline in order to calculate the concentration of gastrin.
Histopathological examination of gastric endoscopic biopsies was performed at the Department of Pathomorphology, Jagiellonian University Medical College. Specimens were fixed in 10% buffered formalin and used for paraffin preparations stained with hematoxylin and eosin, alcian blue and periodic acid-Schiff stain at pH 2.5, and the standard Giemsa method. Fixed specimens were immersed in a series of alcohols and xylenes with increasing concentrations, and then embedded in a paraffin block. The paraffin blocks were cut into sections of 3 μm, deparaffinized in a series of xylenes and alcohols with decreasing concentration, and stained by pathological methods. The Sydney classification system was used for the assessment of inflammation and atrophy, and Lauren’s classification system for the evaluation of the type of gastric cancer.
Comparison of average hormone concentrations was performed using the general linear model, and more specifically, analysis of covariance. If a factor was statistically significant (at α = 0.05), the post-hoc Tukey test (according to unequinumerous groups variant) was used to compare all possible pairs of means resulting from categorization according to factor.
RESULTS
In fasting and 60 and 90 min postprandial plasma samples from healthy controls, ghrelin levels decreased significantly at 60 and 90 min after food intake (Table 2, Figure 2). In contrast, plasma gastrin, which showed the lowest values under fasting conditions, almost doubled at 60 and 90 min after ingestion of a mixed meal (Table 3, Figure 2), so an inverse relationship was seen between fasting and postprandial ghrelin and gastrin concentrations in healthy subjects. Neither group of GC patients, just before surgery or 4-5 years after gastrectomy, showed this clear inverse relation between fasting and postprandial plasma ghrelin and gastrin (Table 2).
Table 2.
Plasma ghrelin concentrations (median and range) recorded under fasting conditions and postprandially (at 60 and 90 min after a meal) in gastric cancer patients before surgery, 4-5 yr after gastrectomy, and healthy controls (95% CI) (pg/mL)
| Median fasting plasma ghrelin level | Median plasma ghrelin level 60 min postprandial | Median plasma ghrelin level 90 min postprandial | |
| Patients GC (before surgery) | 193.31 (150-220) | 161 (125-220) | 160 (153-248) |
| Patients 4-5 yr after gastrectomy | 1572 (107.1-229.4) | 153.11 (108-217.3) | 142 (98.4-205) |
| Controls | 293 (179-480.4) | 234 (148.3-368.4) | 243.2 (151-392) |
Indicates significant difference between fasting levels in controls and those in gastric cancer (GC) patients before surgery (P = 0.0578);
Indicates significant difference in fasting ghrelin levels between controls and patients operated upon 4-5 years earlier (P = 0.0113).
Figure 2.
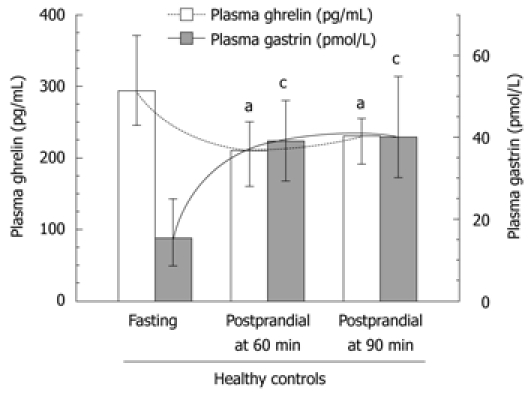
Plasma ghrelin and gastrin concentrations in healthy subjects under fasting and postprandial conditions at 60 and 90 min after a meal. Median values (with range). “a” and “c“ indicates significant (P < 0.05) change compared to fasting ghrelin or gastrin plasma concentrations.
Table 3.
Plasma gastrin concentrations (median and range) in gastric cancer patients before surgery, 4-5 yr after surgery, and in healthy controls, under fasting and postprandial (60 and 90 min after a meal) conditions (95% CI) (pmol/L)
| Median fasting plasma gastrin level | Median plasma gastrin level 60 min postprandial | Median plasma gastrin level 90 min postprandial | |
| Patients with GC (before surgery) | 29 (20–43) | 38 (24.5–54.2) | 37 (26.4–53) |
| 4–5 yr after gastrectomy | 25.4 (14–45.5) | 25.1 (14.5–43.2) | 23 (13.5–39) |
| Controls | 18.312 (8.4–39.5) | 39 (19.1–79) | 40 (20–79) |
Indicates significant difference between control and postprandial value recorded at 60 min after a meal (P = 0.0027);
Indicates significant difference between control and postprandial value recorded at 90 min after a meal (P = 0.0036). GC: Gastric cancer.
Plasma ghrelin originates mainly from the Gr/endocrine/neurocrine cells that are present mainly in the gastric mucosa, and predominantly in the lining of the proximal portion of the stomach. Therefore, we first analyzed the fasting and postprandial plasma ghrelin levels in patients with GC located in the proximal (fundus and corpus) or distal (antrum) stomach. Mean plasma levels of ghrelin (fasting, 60 and 90 min postprandially) in cancer patients before surgery in each of subsequent measurements (fasting, 60 and 90 min after a meal) were significantly lower in those with tumor located in the proximal stomach (fundus or corpus) compared with that in the distal stomach (antrum). As shown in Figure 3 and Table 2, in GC of the proximal stomach, the plasma ghrelin levels were as follows: mean fasting concentration was 193 pg/mL (95% CI: 150-220 pg/mL), and postprandial ghrelin level at 60 min was 161 pg/mL (95% CI: 125-220 pg/mL), and at 90 min, it was 160 pg/mL (95% CI: 153-248 pg/mL). There was no significant difference between fasting and postprandial ghrelin levels recorded in patients with proximal GC, whereas plasma ghrelin levels in patients with distal GC showed a small but significant increase. However, no difference was found between fasting and postprandial ghrelin levels in patients with GC localized in the proximal or distal portion of the stomach (P = 0.3394). As shown in Figure 3, in patients with distal GC, the corresponding plasma ghrelin values in subsequent samples were as follows: fasting, 230 pg/mL (95% CI: 182-252 pg/mL); 60 min postprandially, 201 pg/mL (95% CI: 165-235 pg/mL); and 90 min postprandial, 201 pg/mL (95% CI: 162-225 pg/mL).
Figure 3.
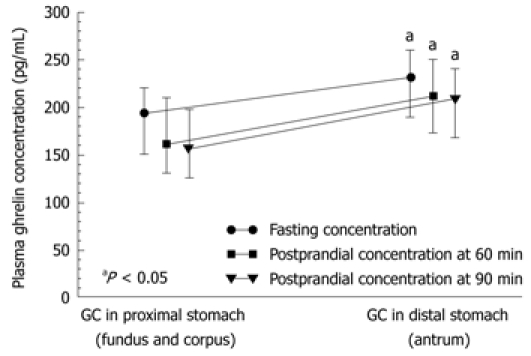
Median values (and range) of fasting and postprandial plasma ghrelin concentrations recorded in patients with gastric cancer localized in the proximal versus distal stomach. “a” indicates significant (aP < 0.05) increase above the ghrelin levels in gastric cancer (GC) localized in proximal stomach.
The mRNA expressions for all three hormones, ghrelin, GHS-R and gastrin, in intact antrum and the gastric corpus and fundic mucosa are shown in Figure 4. Expression of gastrin and GHS-R mRNA was high in the GC tissue, whereas that of ghrelin was low. Expression of GHS-R and gastrin reached a high level in the intact antral mucosa and GC tissue, whereas ghrelin expression was low in GC but high in intact corpus and fundus mucosa (Figure 4).
Figure 4.
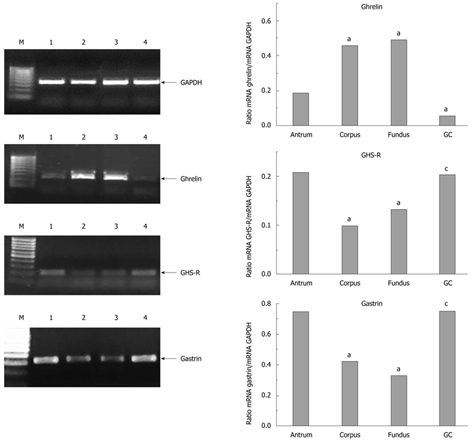
mRNA expression in mucosal and cancer tissue for ghrelin, growth hormone secretagogue receptor and gastrin. “a” indicates significant change in ghrelin, growth hormone secretagogue receptor (GHS-R) and gastrin expression as compared to that in the antral mucosa. “c” indicates significant change in the hormone expression in gastric cancer (GC) tissue compared to that in intact gastric mucosa. M: Marker; 1: Antrum; 2: Corpus; 3: Fundus; 4: GC.
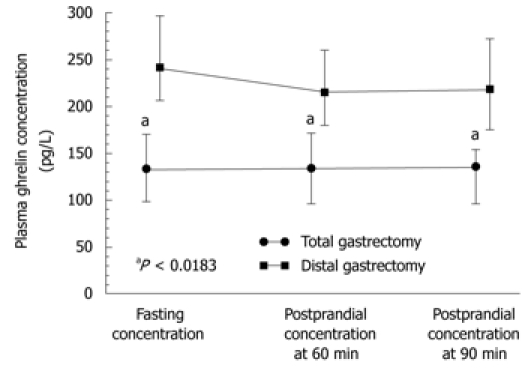
Statistically significant differences in plasma levels of ghrelin under fasting conditions and after meal (at 60 and 90 min postprandially) were found in patients following total gastrectomy [in patients with proximal GC (fundus and body)] compared with those after distal gastrectomy (in patients with distal GC), both under fasting conditions and postprandially (Figure 5). Plasma ghrelin levels were significantly lower in the patients with total gastrectomy compared to those recorded after distal gastrectomy.
Figure 5.
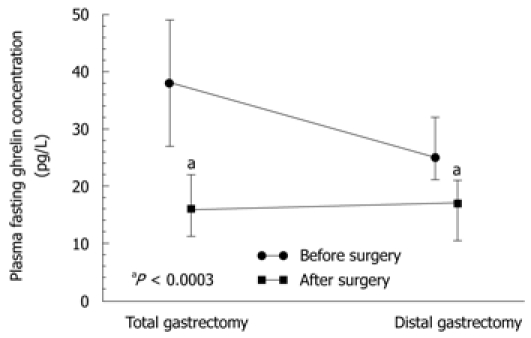
Plasma ghrelin concentrations in total and distal gastrectomy patients. “a” indicates significantly (P < 0.05) lower values recorded after total gastrectomy when compared to those recorded after distal gastrectomy.
The plasma ghrelin levels in patients after total gastrectomy were as follows: fasting, 134 pg/mL (95% CI: 104-174 pg/mL); 60 min postprandial, 134 pg/mL (95% CI: 100.2-178 pg/mL); and 90 min postprandial, 136 pg/mL (95% CI: 100.6-161 pg/mL). In patients after subtotal (distal) gastrectomy, fasting ghrelin was a mean 241 pg/mL (95% CI: 205-300 pg/mL); at 60 min postprandially, it was 215 pg/mL (95% CI: 180-260 pg/mL) (subtotal gastrectomy); and at 90 min postprandial, it was 218 pg/mL (95% CI: 175-270 pg/mL). Plasma ghrelin concentrations under fasting conditions and postprandially at 60 and 90 min in patients following total gastrectomy were significantly lower (P = 0.0183) than those after subtotal gastrectomy (Figure 5).
Plasma gastrin recorded preoperatively under fasting conditions and postprandially (Figure 6) was significantly higher (P < 0.05) than that assessed after distal gastrectomy in patients operated upon for distal GC compared to those who underwent total gastrectomy. Unlike healthy controls, the GC patients who underwent surgery showed no significant difference between fasting and postprandial gastrin levels (at 60 and 90 min) after total or distal gastrectomy (P = 0.4893). It should be mentioned that both fasting and postprandial plasma gastrin levels tended to reach relatively lower values in patients with GC located in the proximal (fundic) portion of the stomach when compared to those recorded in patients with GC located beyond the fundus (Figure 7), but the difference between these levels was not statistically significant.
Figure 6.
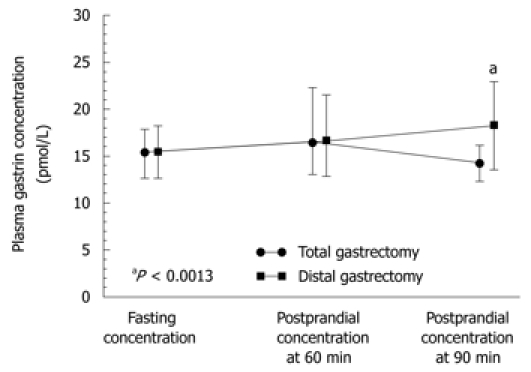
Plasma levels of gastrin under fasting conditions and postprandially (at 60 and 90 min after a meal) in patients after total or distal gastrectomy. “a” indicates significant difference (P < 0.05) compared to fasting level.
Figure 7.
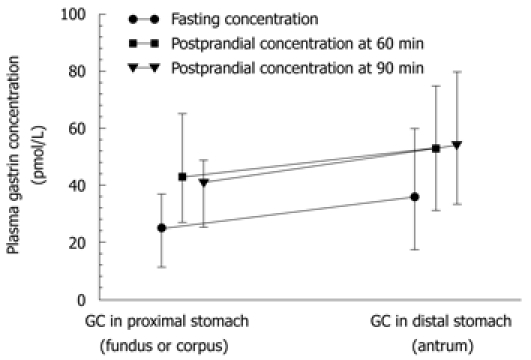
Plasma gastrin levels (median and range) under fasting and postprandial conditions (at 60 and 90 min after a meal) in patients with gastric cancer located in fundus and beyond the fundus (distal stomach).
Plasma gastrin level showed a strong and statistically significant difference between fasting and postprandial conditions in the control subjects (Table 3, Figure 6). The lowest plasma gastrin occurred under fasting conditions, and the highest concentrations were observed at 60 and 90 min after food intake. Basal gastrin level in healthy controls was a mean 18.3 (95% CI: 8.4-39.5) pmol/L and it was almost doubled after 60 and 90 min upon food intake, reaching respectively, 39 pmol/L (95% CI: 19.1-79 pmol/L, P = 0.0027) and 40 pmol/L (95% CI: 20-79 pmol/L P = 0.0036) (Table 3). In GC patients, fasting median plasma gastrin was 29 pmol/L (95% CI: 20-43 pmol/L), which was significantly higher than that observed in healthy controls. However, after food intake, mean plasma gastrin showed a relatively small, though significant increase at 60 min (38 pmol/L; 95% CI: 24.5-54.2 pmol/L) and at 90 min after food intake (37 pmol/L; 95% CI: 26.4-53 pmol/L). These elevations in plasma gastrin after feeding in GC patients were statistically significant only before surgery (Figure 6). At 4-5 years after gastrectomy (combined total and distal gastrectomy), there was no significant change in plasma gastrin after food intake, but median fasting gastrin levels (25.4 pmL/L; 95% CI: 14-45.5 pmol/L) were still significantly higher than those in healthy controls (Table 3).
After distal gastrectomy, fasting plasma gastrin fell significantly from about 25 pmol/L (95% CI: 21-32 pmol/L) before surgery to 15.7 pmol/L (95% CI: 11-21.1 pmol/L) after gastrectomy, and the differences between these concentrations were statistically significant (Figure 8). The decrease of median gastrin concentration after total gastrectomy was relatively stronger than that observed after subtotal gastrectomy (Figure 8).
Figure 8.
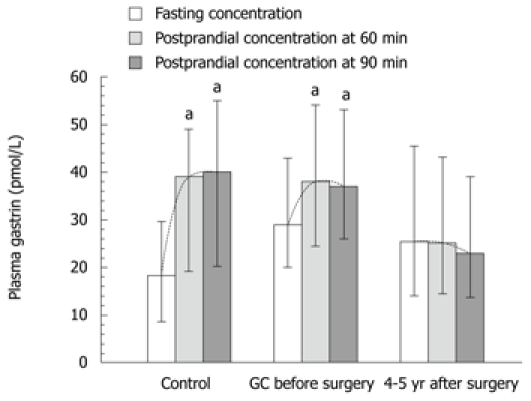
Plasma gastrin levels under fasting and postprandial conditions (at 60 and 90 min after a meal) in healthy controls, gastric cancer patients and patients after 4-5 yr of gastrectomy patients due to gastric cancer. (Medians and range). “a” indicates significant change (P < 0.05) compared to fasting level. GC: Gastric cancer
The decrease in fasting and postprandial plasma gastrin was observed in both gastrectomy groups though postprandial gastrin level was relatively more reduced after total than distal gastrectomy after which small but significant increase in plasma gastrin was observed postprandially in the last 90 min postprandial sample (Figure 9).
Figure 9.
Plasma gastrin concentrations under fasting conditions and postprandially at 60 and 90 min after food intake in gastric cancer patients after total or distal gastrectomy. “a” indicates significant difference (P < 0.05) compared to values recorded before the surgery.
DISCUSSION
The aim of this study was to determine the relationship between plasma ghrelin and gastrin levels under fasting and postprandial conditions in healthy subjects and patients with GC who underwent total and distal gastrectomy. In addition, we analyzed gastric mRNA expression for ghrelin, GHS-R and gastrin in intact gastric mucosa and cancer tissue. It was found that in healthy controls, an inverse relationship existed; such that, when under fasting conditions plasma ghrelin increased, plasma gastrin decreased, and postprandially, when plasma ghrelin was markedly attenuated, plasma gastrin was significantly increased compared to preprandial levels. The reason for this inverse relationship in healthy subjects is not known, but it is likely that, in the fasting stomach, the high level of ghrelin release, that activates gastric motility and stimulates appetite, causes excessive release of gastrin immediately after the entrance of a food bolus into the stomach. The stimulatory synergistic action of ghrelin on gastrin and gastric acid secretion has been observed previously, but mostly in experimental animals[14], and this effect has been attributed to hormonal stimulation of vagal nerves and histamine release from the gastric enterochromaffin-like cells. Our results suggest that increased ghrelin release in the fasting (empty) stomach promotes gastrin secretion immediately after food intake. Our recent but unpublished data with parenteral administration of synthetic ghrelin analog[12] showed that it potently enhances plasma gastrin level, thus providing direct evidence that ghrelin is capable of stimulating gastrin release in humans, but it remains to be elucidated why plasma ghrelin falls immediately after food intake. It is possible that gastric acid stimulated by the combined action of ghrelin and gastrin[13-15] activates local gastric somatostatin release that might cause paracrine inhibition of secretory activity of Gr-cells, which decreases plasma ghrelin level, but this requires further experimental and clinical data.
The major purpose of the present study was to explain the role of ghrelin in gastric carcinogenesis associated with H. pylori infection[16-18], the spread of GC to the proximal or distal portion the stomach, and impairment of the ghrelin-gastrin relationship. The location and extent of tumor infiltration combined with widespread multifocal atrophic gastritis (caused by H. pylori infection) was found to affect ghrelin and gastrin release, and subsequently, to impair the intricate relationship between these two hormones. Indeed, we found that GC patients secrete significantly less ghrelin under fasting conditions, with little tendency of this hormone to decline postprandially, whereas gastrin release greatly increases. The reduction in fasting plasma ghrelin in GC appears to depend upon: (1) the extent and proliferation of cancer in the stomach; and (2) H. pylori-induced atrophic gastritis. As expected from our previous studies, plasma gastrin is enhanced in GC; probably due to its expression and release by cancer cells themselves[17,18]. In fundic GC, the fasting and postprandial ghrelin levels were lower than those in with GC in distal stomach (Figures 3 and 5).
The most marked alteration in ghrelin/gastrin ratio was found in patients who underwent total, and to a lesser extent distal gastrectomy. The inverse relationship between ghrelin and gastrin disappeared and both hormones became markedly altered by total gastrectomy. This agrees with the results of our most recent study[13], which has shown that GC associated with H. pylori infection results in a marked decrease in ghrelin in plasma and cancer tissue. In the present study, we found that plasma levels of ghrelin were lower in patients with GC and in those with previous GC who underwent surgery 4-5 years previously, compared with the control group. Huang et al[19] have shown no correlation between preoperative levels of ghrelin and the proximal or distal location of GC. Similarily, Jeon et al[9] have not found any marked difference in preoperative serum ghrelin concentrations in patients with GC scheduled for gastric resection (total, subtotal and proximal). However, their analysis of ghrelin levels before and after surgery (on day 10) showed a significant difference in plasma ghrelin between patients who underwent total and distal gastrectomy. In the group with total gastrectomy, similar to our study, the decrease in ghrelin after surgery was significantly greater, and reached a maximum of about 70% of the preoperative concentration. This confirms the important role of the stomach, particularly its proximal portion, as the main source of ghrelin secretion[3,8,9].
In our study, the mean ghrelin concentrations tended to increase slightly but significantly after distal but not total gastrectomy. The mean concentrations of plasma ghrelin among GC patients depended on the type of resection, particularly on its extent, and most importantly, upon preservation of the part of the stomach responsible for the production of most ghrelin, that is, the fundus and corpus. In the total resection group, on postoperative day 10, taking into account the plasma ghrelin concentration, it can be concluded that, in some patients, other tissues begin to take over slow production of ghrelin. However, after distal resection, when the fundus of the stomach was preserved, the levels of ghrelin actually increased compared to preoperative values[20].
The plasma level of ghrelin after surgery in the subsequent samples (fasting and postprandial) showed a significant difference between patients who underwent total and subtotal resection. Plasma ghrelin levels at 60 and 90 min postprandially were significantly higher in patients who underwent distal gastric resection, compared to the group treated by total resection. Furthermore, physiological regulation of ghrelin secretion and its plasma level were, however, at least in part preserved; being highest in the fasting state, lowest in the hour after a meal, and intermediate at 90 min after a meal (Figures 3 and 5). Jeon et al[9] have also analyzed plasma levels of ghrelin in patients who underwent various types of gastric resection. However, unlike in our study, patients with early GC or GC infiltrating < 2 cm were examined within the first 7 d after surgery. In patients after resection of the proximal stomach, postoperative ghrelin increased more slowly than in patients after fundus-preserving resection. They concluded that compensatory ghrelin production occurs in the preserved part of the stomach. According to their experience, preservation of the fundus is more important than the distal stomach for ghrelin production after gastrectomy. After complete resection of the stomach, ghrelin levels in serum fell to about 30% of those before surgery. They did not observe compensatory production of ghrelin by other tissues until day 7 after surgery[9]. Also, Takachi et al[21] have demonstrated a significant decrease in the concentration of ghrelin after total gastrectomy, which was up to 12% of preoperative levels (measured on days 3 and 7 after surgery). Very low levels of ghrelin were observed in this group in the long-term follow-up after surgery (mean: 41 mo). By contrast, in patients treated by distal gastrectomy, on postoperative day 3, the hormone levels decreased to 39%, and on day 7, increased back to 56% of preoperative levels. Different results have been presented by Kim et al[22], who found that after distal gastric resection for GC, the number of ghrelin-producing cells failed to increase, which correlated well with low plasma ghrelin levels.
In our study, the concentration of plasma ghrelin in patients who had undergone total gastrectomy 4-5 years previously was significantly lower than that after distal gastrectomy, and lower again when compared to the control group. The fasting levels of ghrelin were also lower compared to those in GC patients before surgery. Plasma ghrelin level in this group of GC patients was at 26%-66% of the reference value in the healthy controls. Thus, in our study group, only partial compensation of ghrelin production by other tissues was observed after removal of the stomach. Hosoda et al[23] have shown that, after complete gastric resection, serum ghrelin concentration tends to normalize gradually as a result of production by other tissues. Plasma ghrelin level in their three patients fell at 30 min after gastrectomy to 50% of the preoperative concentration. At 240 d after gastric resection, plasma ghrelin reached about two-thirds of the preoperative value in two patients and 100% in the other. However, Ariyasu et al[3] have found that plasma ghrelin in patients at 1-8 years after total gastrectomy was comparable with the control group, with a concentration that was about 35% of the preoperative level. Also, the above-mentioned study of Takachi et al[21] has demonstrated very low levels of ghrelin during long-term follow-up after total gastrectomy.
In our study, we analyzed gastrin as well. Basal plasma gastrin was found to be markedly elevated in our patients with GC and coexisting H. pylori infection, as compared to the control group. Patients with GC tended to have higher levels of gastrin (Figures 6-9), which can result from H. pylori infection with accompanying atrophy of the mucous membrane, and increased production of the hormone by the tumor cells themselves[17,18]. We noted, however, significant differences in plasma levels of gastrin before and after surgery only in patients who underwent total gastrectomy.
In summary, a significant decrease to about 70% of preoperative levels of ghrelin was observed in our patients after total gastrectomy, which confirms an important role of the stomach as the principal place of ghrelin expression. After distal resection of the stomach, a compensatory increase in the production of ghrelin in relation to the level before surgery was observed. At 4-5 years after gastrectomy, full compensatory production of ghrelin by other tissues was not demonstrated. Higher basal plasma gastrin levels in patients with GC accompanied by H. pylori infection, compared to the control group, were probably associated with increased production of the hormone by the tumor cells themselves.
COMMENTS
Background
The major purpose of the present study was to explain the role of ghrelin and gastrin in gastric carcinogenesis associated with Helicobacter pylori (H. pylori) infection, the spread of gastric cancer (GC) into the proximal or distal stomach, and the profound impairment of the ghrelin-gastrin relationship.
Research frontiers
The location and extent of tumor infiltration combined with widespread multifocal atrophic gastritis (caused in this study by H. pylori infection) was found to affect ghrelin and gastrin release, and subsequently, to impair the intricate relationship between these two hormones. The authors found that GC patients express significantly less ghrelin under fasting conditions, with little tendency of this hormone to decline postprandially, whereas gastrin release remained strongly increased. The reduction in fasting plasma ghrelin in GC appears to depend upon: (1) extent and proliferation of the cancer in the stomach; and (2) H. pylori-induced atrophic gastritis. As expected from our previous studies, plasma gastrin was markedly enhanced in GC, probably due to its expression and release by cancer cells themselves. In fundic cancer, the fasting and postprandial ghrelin levels were lower than those in healthy controls; probably due to impairment by cancer and accompanying gastritis of the Gr-cells that are present mainly in the mucosal lining of the proximal stomach. In this study, the authors analyzed the plasma concentrations of ghrelin and gastrin in patients with advanced GC before and after total or subtotal gastrectomy. In addition, plasma concentrations of gastrin, ghrelin and gene expression of these hormonal peptides and growth hormone secretagogue receptor (GHS-R), were evaluated in intact mucosa and GC tissue associated with H. pylori infection.
Innovations and breakthroughs
A significant decrease to about 70% of preoperative levels of ghrelin observed in our patients after total gastrectomy confirms a crucial role of the stomach, particularly its proximal portion, as the principal place of ghrelin expression and release. After distal resection of the stomach, a compensatory increase in the production of ghrelin was clearly demonstrated. At 4-5 years after total gastrectomy, full compensatory takeover of ghrelin production by other tissues did not occur possibly due to the absence of the proximal stomach, which is the major source of ghrelin production. Higher basal plasma gastrin levels in patients with GC accompanied by H. pylori infection, compared to the control group, were probably associated with increased production of the hormone by the tumor cells themselves.
Applications
Further study is required: (1) long-term observation of patients after resection of the stomach; (2) measurement of plasma ghrelin to establish whether compensation of hormone production by other tissues can be expected; and (3) consideration of the possible need for substitution of exogenous ghrelin or its active analogs in these patients.
Terminology
Ghrelin is a natural ligand for GHS-R. It was originally identified in rat gastric mucosa and shown to be expressed by endocrine Gr-cells. These ghrelin (Gr)-producing cells, represent about 20% of the total population of endocrine cells in the gastric mucosa. They are located in the lower parts of the oxyntic glands, and are present mostly in the proximal portion of the stomach (fundus and corpus). They are also the major source of hydrochloric acid, intrinsic factor, numerous growth factors, and pepsinogens, and therefore, they are important for digestive processes stimulated by the vagal nerves, gastrin/histamine, as well as by ghrelin itself. Ghrelin is a 28-amino acid peptide that acts as a stimulant for GH secretion by interacting with the receptor GHS-R. Characteristically, it is also the only circulatory gastrointestinal hormone that is known to be secreted by the empty (fasting) stomach to enhance food intake and maintain energy homeostasis following its central or peripheral administration. Ghrelin is currently considered to be the most powerful endogenous orexigenic (appetite stimulating) hormone, which results in a positive energy balance and subsequently in weight gain.
Peer review
This paper reports the results of studies on the relationship of ghrelin to gastrin in GC before and after gastrectomy. Using blood and biopsy specimens obtained from 70 patients who underwent endoscopy of the upper gastrointestinal tract, the authors demonstrated that, in healthy controls, plasma ghrelin reaches higher values under fasting conditions and falls postprandially, while gastrin level is low during fasting and shows a marked increase following food ingestion. Moreover, the decrease in fasting levels of ghrelin was observed in GC patients after total gastrectomy, but the level of plasma gastrin showed an increase. It is concluded, that the elevated plasma gastrin and suppression in fasting ghrelin in patients with GC suggests an intimate relationship between these two hormones in gastric carcinogenesis.
Acknowledgments
We thank Ms Sara Padol, a student at the Medical English School for Foreigners, who corrected the English in this paper and prepared it for publication. The project was supported from the funds of State Committee for Scientific Research for science in the years 2006-2008 as a research project no K/PBP/000012.
Footnotes
Supported by The Ministry of Science and Higher Education in Poland Grant K/PBP/000012 for 2006-2008 and by Jagiellonian University Medical College Research Project for 2006-2009
Peer reviewer: Guida Portela-Gomes, Professor of University of Lisbon, Department of Medicine, Rua Domingos Sequeira-128, Estoril, 2765-525, Portugal
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 3.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Konturek SJ, Brzozowski T, Konturek PC, Schubert ML, Pawlik WW, Padol S, Bayner J. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol. 2008;59 Suppl 2:7–31. [PubMed] [Google Scholar]
- 6.Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004;55:137–154. [PubMed] [Google Scholar]
- 7.De Ambrogi M, Volpe S, Tamanini C. Ghrelin: central and peripheral effects of a novel peptydil hormone. Med Sci Monit. 2003;9:RA217–RA224. [PubMed] [Google Scholar]
- 8.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin’s role on gastrointestinal tract cancer. Surg Oncol. 2010;19:e2–e10. doi: 10.1016/j.suronc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Jeon TY, Lee S, Kim HH, Kim YJ, Son HC, Kim DH, Sim MS. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J Clin Endocrinol Metab. 2004;89:5392–5396. doi: 10.1210/jc.2004-0872. [DOI] [PubMed] [Google Scholar]
- 10.An JY, Choi MG, Noh JH, Sohn TS, Jin DK, Kim S. Clinical significance of ghrelin concentration of plasma and tumor tissue in patients with gastric cancer. J Surg Res. 2007;143:344–349. doi: 10.1016/j.jss.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Checchi S, Montanaro A, Pasqui L, Ciuoli C, Cevenini G, Sestini F, Fioravanti C, Pacini F. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J Clin Endocrinol Metab. 2007;92:4346–4351. doi: 10.1210/jc.2007-0988. [DOI] [PubMed] [Google Scholar]
- 12.Mottershead M, Karteris E, Barclay JY, Suortamo S, Newbold M, Randeva H, Nwokolo CU. Immunohistochemical and quantitative mRNA assessment of ghrelin expression in gastric and oesophageal adenocarcinoma. J Clin Pathol. 2007;60:405–409. doi: 10.1136/jcp.2006.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zub-Pokrowiecka A, Rembiasz K, Konturek SJ, Budzynski A, Konturek PC, Budzynski P. Ghrelin in the gastric mucosa associated with Helicobacter pylori infection. Med Sci Monit. 2010:In press. [PubMed] [Google Scholar]
- 14.Fukumoto K, Nakahara K, Katayama T, Miyazatao M, Kangawa K, Murakami N. Synergistic action of gastrin and ghrelin on gastric acid secretion in rats. Biochem Biophys Res Commun. 2008;374:60–63. doi: 10.1016/j.bbrc.2008.06.114. [DOI] [PubMed] [Google Scholar]
- 15.Yakabi K, Kawashima J, Kato S. Ghrelin and gastric acid secretion. World J Gastroenterol. 2008;14:6334–6338. doi: 10.3748/wjg.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa K, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–1320. doi: 10.1053/j.gastro.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 17.Konturek PC, Konturek SJ, Pierzchalski P, Bielański W, Duda A, Marlicz K, Starzyńska T, Hahn EG. Cancerogenesis in Helicobacter pylori infected stomach--role of growth factors, apoptosis and cyclooxygenases. Med Sci Monit. 2001;7:1092–1107. [PubMed] [Google Scholar]
- 18.Konturek PC, Konturek SJ, Bielanski W, Karczewska E, Pierzchalski P, Duda A, Starzynska T, Marlicz K, Popiela T, Hartwich A, et al. Role of gastrin in gastric cancerogenesis in Helicobacter pylori infected humans. J Physiol Pharmacol. 1999;50:857–873. [PubMed] [Google Scholar]
- 19.Huang Q, Fan YZ, Ge BJ, Zhu Q, Tu ZY. Circulating ghrelin in patients with gastric or colorectal cancer. Dig Dis Sci. 2007;52:803–809. doi: 10.1007/s10620-006-9508-3. [DOI] [PubMed] [Google Scholar]
- 20.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 21.Takachi K, Doki Y, Ishikawa O, Miyashiro I, Sasaki Y, Ohigashi H, Murata K, Nakajima H, Hosoda H, Kangawa K, et al. Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. J Surg Res. 2006;130:1–7. doi: 10.1016/j.jss.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Lee JH, Heo JS, Kwak MJ, Kim SJ, Sohn YB, Kim SE, Song SY, Choe YH, Baek JW, et al. Serum obestatin/ghrelin ratio is altered in patients after distal gastrectomy. Dig Surg. 2009;26:143–148. doi: 10.1159/000207507. [DOI] [PubMed] [Google Scholar]
- 23.Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]


