Abstract
AIM: To compare the results for endoscopic ultrasound (EUS)-guided drainage of clear fluid pancreatic pseudocysts with the results for abscess drainage.
METHODS: All patients referred for endoscopic drainage of a fluid collection were prospectively included. The outcome was recorded.
RESULTS: Altogether 26 pseudocysts or abscesses were treated in 25 (6 female) patients. One endoscopist performed the procedures. Non-infected pseudocysts were present in 15 patients and 10 patients had infected fluid collections. The cyst size ranged between 28 cm × 13 cm and 5 cm × 5 cm. The EUS drainage was successful in 94% of the pseudocysts and in 80% of the abscesses (P = 0.04). The complication rate in pseudocysts was 6% and in abscesses was 30% (P = 0.02). Recurrence of a pseudocyst occurred in one patient (4%) after 6 mo; the patient was successfully retreated.
CONCLUSION: EUS-guided drainage of pseudocysts is associated with a higher success rate and a lower complication rate compared with abscess drainage.
Keywords: Pancreas, Endoscopic ultrasound, Drainage, Pseudocyst, Abscess
INTRODUCTION
Endoscopic ultrasound (EUS)-guided drainage of pancreatic pseudocysts and abscesses has become a first line therapy in many centers[1-4]. This is due to the ability of the EUS instrument to assess the wall thickness, identify major vessels and find the closest access to the fluid cavity[5-7]. Moreover, this procedure will create an internal fistula and avoid the inconvenience of an external drainage and the risk for cutaneous fistula. A recent study has shown that percutaneous drainage failed in 36% of patients with normal pancreatic ductal anatomy. In patients with ductal abnormalities such as stricture, ductal cut-off and dilated duct the failure rate was 62%-100%. In those patients with an abnormal duct, cutaneous fistula developed in more than 50%[8].
Surgery may be avoided in cases when successful EUS-guided drainage is performed, with no recurrence of the cyst. However, acute surgery may be needed in some cases of complications such as perforation or major bleeding[9,10].
In spite of these assumed advantages of EUS-guided drainage there are only a few prospective studies assessing the full scale of the advantages and shortcomings of this procedure. Moreover, prospective studies assessing this procedure in comparison to radiological intervention and surgery have not been performed. Furthermore, the results of non-infected pseudocyst drainage have not been prospectively compared with the drainage of abscesses. Kahaleh et al[9] compared EUS-guided drainage and conventional transmural drainage in a prospective study. Long-term success assessed at 6 mo was 84% and 91%, respectively (P = NS). The complication rate was 18% and 19%, respectively (P = NS). The complications in this study by Kahaleh consisted of bleeding in three patients, infection in eight, stent migration into the cyst in three and pneumoperitoneum in five.
The aim of our prospective study was to compare the results for drainage of clear fluid pseudocysts with the results for abscess drainage. This prospective quality analysis may guide us in improving the performance of this procedure.
MATERIALS AND METHODS
All patients referred for EUS-guided drainage of a fluid collection were prospectively included in this study. The data were collected in the period between February 2006 and June 2010. Our center is the tertiary center for pancreatic surgery in the region of west Sweden, with access to EUS as well as interventional radiology.
The fluid collections were pseudocysts with clear fluid in 16 cases. The other fluid collections were 9 pseudocysts with infected fluid and one postoperative abscess. The abscess developed after an operation for a perforated duodenal ulcer. The indications for drainage were abdominal pain in 18 patients, infection in 10, food obstruction in one patient and jaundice in one patient. In some cases there was more than one symptom. All patients underwent a computed tomography (CT) scan before the procedure to assess accessibility of the pseudocyst from the stomach or duodenum and to assess an eventual communication between the cyst and the pancreatic duct. Hemoglobin, leucocytes, thrombocytes, CRP and INR were controlled before the procedure. The patients received verbal and written information about the procedure. The procedure was performed either under conscious sedation with pethidine and midazolam or, when possible, under intubation anaesthesia. Intravenous cefotaxime 1 g was administered before the procedure in patients without ongoing antibiotic treatment. After the procedure, therapeutic antibiotics were only given to patients with an infection. The procedures were performed by one endoscopist (RS) with extensive endoscopy experience.
A linear echoendoscope (Pentax EG3830UT and GF-UCT 140, Olympus) was used to find the closest axes to the pseudocyst. Special attention was given to avoid vessels and to find areas of the wall that were not thicker than 1 cm.
Different procedures were used to enter and stent the cavity: (1) The Giovannini Needle Wire (Wilson-Cook Medical GI Endoscopy) stenting system was used to place a stent in one step[10]. This was mainly used in clear fluid pseudocysts to place one stent; (2) A 19-guage needle (Wilson-Cook Medical GI Endoscopy) was also used to access the pseudocyst and to place a 0.035-inch guide wire into the cyst before the opening was dilated using a balloon sized between 12 and 18.5 mm. The guide wire was then used to stent the cyst. This procedure was used to place more than one stent in infected cavities; and (3) A cystotome (Wilson-Cook Medical GI Endoscopy) was also used to enter the cyst and establish an opening. This procedure was also used to place more than one stent in infected cavities.
Both cut and coagulation current were used. Coagulation current and endocut (ERBE®) current were more often used in the procedures which took place during the later part of the study period in order to avoid bleeding.
Technical success of the EUS intervention was defined as the ability to improve clinical outcome of the pseudocyst or abscess without the need for surgical interventions. A complication was defined as an adverse event that led to a longer hospital stay and/or to emergency surgery. The ethics committee of the University of Göteborg had approved the study.
Statistical analysis
The primary endpoint was between-group differences in success rate and complication rate. McNemar’s exact test was utilized to assess the difference between the results for pseudocysts and abscesses.
RESULTS
One patient with alcohol-related pancreatitis did not attend at the endoscopy department and was therefore excluded. All of the other 25 out of 26 referred patients were included in the study. Altogether 26 pseudocysts or abscesses were treated in 25 patients.
The mean follow up time of all patients was 20 mo, range 2-45 mo.
The EUS drainage was successful in 94% of the pseudocysts and in 80% of the abscesses (P = 0.04).
Table 1 shows the results for the patients with pseudocysts; Table 2 shows the results for abscesses; and Table 3 shows some technical aspects, etiologies and locations of the cysts.
Table 1.
Results for patients with pseudocysts
| Patient age and gender | Etiology | Cyst size (cm) | No. of stents | Complication | Follow-up (mo) | Final result |
| (52)M | Idiopathic | 13 × 10 | 1 (10F) | No | 45 | Total regression |
| (66)M | Malignancy | 6 × 7 | 1 (10F) | No | 12 | Total regression |
| (61)M | Alcohol | 15 × 13 | 3 (7F) | No | 41 | Total regression |
| (76)M | Alcohol | 5 × 5 | 1 (10F) | No | 34 | Total regression |
| (47)M | Alcohol | 7 × 5 | 3 (7F) | No | 33 | Total regression |
| (61)M | Alcohol | 10 × 10 | 2 (10F) | No | 32 | Total regression |
| (43)M | Alcohol | 12 × 10 | 1 (10F) + aspiration | No | 23 | Total regression |
| (36)M | Idiopathic | 28 × 13 | 1 (10F) | No | 22 | Total regression |
| (24)M | Trauma | 5 × 4 | 0 | No | 14 | Spontaneous regression |
| (60)M | Idiopathic | 12 × 11 | 1 (10F) | No | 8 | Total regression |
| (60)M | Idiopathic | 13 × 9 | 1 (10F) | Pneumo-peritoneum | 8 | Regression, operation |
| (6)M | Medication related | 10 × 8 | 0, aspiration | No | 8 | Regression to 3 cm × 4 cm after 7 mo, no symptoms |
| (22)M | Idiopathic | 15 × 12 | 0, aspiration | No | 6 | Total regression |
| (43)M | Idiopathic | 20 × 10 | 1 (10F) + aspiration | No | 6 | Regression, no symptoms |
| (29)F | Gallstone | 8 × 5 | 1 (10F) | No | 3 | Regression, EUS control planned |
| (48)F | Idiopathic | 11 × 14 | 1 (10F) | No | 2 | Regression, EUS control planned |
EUS: Endoscopic ultrasound.
Table 2.
Results for patients with abscesses
| Patient age and gender | Etiology | Cyst size (cm) | No. of stents | Complication | Follow-up (mo) | Final result |
| (76)F | Gallstone | 7 × 7 | 3 (7F) | No | 37 | Total regression |
| (32)M | Alcohol | 5 × 4 | Aspiration | No | 36 | Total regression |
| (57)M | Alcohol | 5 × 5 | 1 (10F) | Bleeding | 32 | Operation |
| (68)F | Gallstone | 18 × 13 | 5 (7F) + 1 naso-cystic | No | 30 | Total regression |
| (51)M | Hyperlipidemia | 8 × 5 | 2 (7F) | Pneumoperitoneum | 25 | Regression, pneumo-peritoneum during a second procedure, operation |
| (53)F | Alcohol | 7 × 5 | Aspiration | No | 18 | Total regression |
| (64)M | Gallstone | 23 × 10 | 1 (10F), 3 (7F), 1 naso-cystic | No | 15 | Total regression |
| (63)F | Postoperative abscess | 6 × 5 | 1 (7F), 1 naso-cystic | Pneumomediastinum | 10 | Conservative treatment, total regression |
| (39)M | Systemic lupus erythematosus | 12 × 7 | Aspiration | No | 3 | Regression, death due to renal insufficiency |
| (67)M | Idiopathic | 20 × 6 | 1 (10F), 1 (7F) | No | 5 | Total regression |
Table 3.
Some technical aspects of the drainage, etiology and location of the cysts
| Drainage technique | No. of procedures | Anesthesia | Etiology | Type of pancreatitis | Location |
| Giovannini Needle Wire1 in 16 cases | One in 19 patients | Sedation2 in 19 patients | Alcohol (11) | Chronic pancreatitis in 14 patients | Cauda in 12 patients |
| 19-guage needle1 in 11 cases | Two in 6 patients | Intubation anesthesia in 7 patients | Idiopathic (6) | Acute pancreatitis in 12 patients | Corpus in 11 patients |
| Cystotome1 in 1 case | Eight in one patient | Gallstone (3) | Caput in 3 patients | ||
| 1 hyperlipidemia | |||||
| 1 trauma | |||||
| 1 postoperative | |||||
| 1 SLE | |||||
| 1 medication | |||||
| 1 malignant |
Cook Endoscopy, Winston-Salem, NC;
Pethidine + midazolam. SLE: Systemic lupus erythematosus.
Complications and recurrence
The complication rate after treatment of pseudocysts was 6% and after treatment of abscesses was 30% (P = 0.02). Overall, 3 patients (11.5% of the cases) needed surgery due to a procedure-related complication.
One of the patients developed a major bleed from the gastroepiploic artery that was not possible to control conservatively. In this case the Giovannini Needle Wire with cut current was used to enter the cyst according to the manufacturer’s recommendations. The patient was operated upon immediately and received a cystoenteric anastomosis with a good final outcome.
In two patients perforation occurred in abscesses stented from the gastric fundus. The dilatation technique was used in one of them and a combination of diathermia and dilatation in the other. The first patient underwent surgical resection of the cyst with a good result. The second patient with a postoperative abscess was treated conservatively with total regression, and could be discharged in good health without further interventions.
A perforation after a technically successful stenting of the cyst occurred in one patient with a non-infected pseudocyst. This patient developed abdominal pain and a leak of both fluid and air was present on a CT-scan. The patient underwent surgery after two days with a resection of the cyst with a good outcome. The surgeon observed that the stent was placed in a proper position; however, the pseudocyst was not attached to the gastric wall.
Two stents migrated during the procedure into the cyst and were retrieved endoscopically.
No procedure-related mortality was observed. One patient died of a non-procedure-related terminal renal insufficiency 3 mo after the aspiration of a pseudocyst.
Recurrence of a pseudocyst occurred in one patient (4%) after 6 mo (Pseudocyst No. 3 in Table 1). The patient underwent a repeat successful EUS drainage with a new follow up time of 29 mo (Pseudocyst no. 6 in Table 1).
Complicated cases
Nine patients had multiple pseudocysts. Two cases were complicated by gastric varices in the stomach. Despite this, it was possible to stent these patients. In the youngest patient (6 years) the pseudocyst was close to the heart; therefore, the cyst was aspirated and not stented. In two patients the distance between the cyst wall and the duodenum and stomach was 3 cm and 2 cm, respectively. Aspiration was therefore performed without stenting. In one patient the cyst was only accessible via the esophagus and was therefore not stented to avoid fistula formation to the esophagus.
Other procedures and aspects
Flushing of the abscess (Figures 1, 2, 3) and necrosectomy (Figure 4) were performed in 10 patients. Patients with large amounts of necrotic material received a naso-cystic drain after a mechanical necrosectomy with a foreign body forceps. The naso-cystic drain was left in place for between a couple of days and three weeks. During this period the drain was flushed with 250-500 mL saline once or twice daily.
Figure 1.
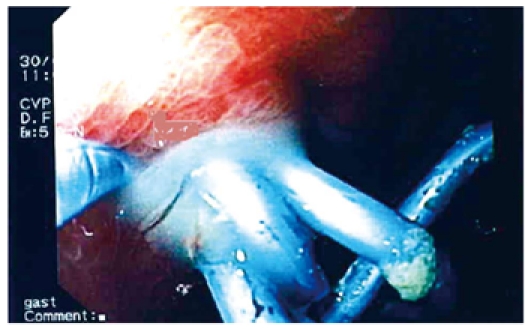
Pus drained from an infected pseudocyst.
Figure 2.
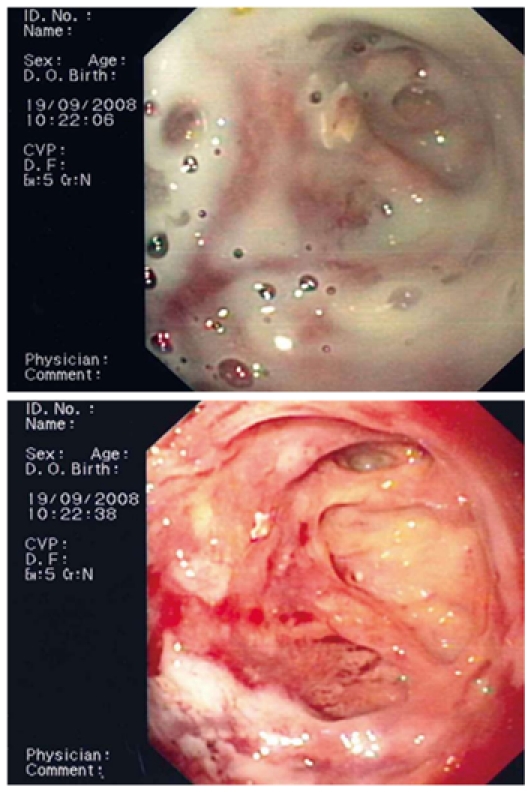
An infected pseudocyst before and after flushing.
Figure 3.
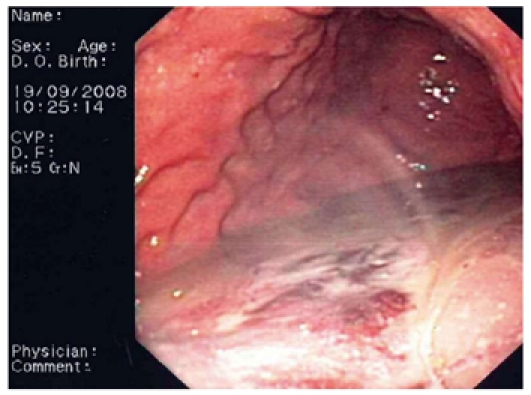
Pus accumulating in the stomach.
Figure 4.
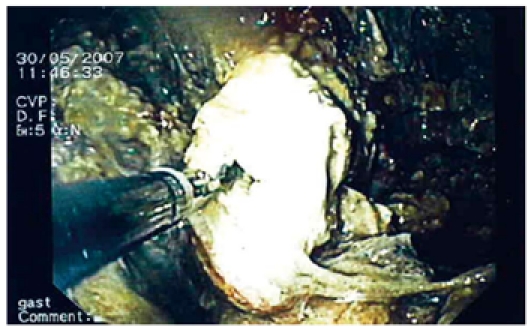
Necrosectomy in a pseudocyst.
In six patients the stents were placed in the gastric fundus; in 12 patients in the gastric corpus; in one patient in the duodenum; and in one patient in the antrum.
The stents were withdrawn after 3 mo in four patients, in three other patients the stents were withdrawn after 4, 5 and 13 mo. In the other patients (13 patients) the stents have not been removed yet. Fluoroscopy was not utilized in 15 (58%) patients during the procedure.
Three patients underwent percutaneous drainage procedures before they were referred for the EUS-guided drainage. One patient received a percutaneous drainage after the EUS-guided aspiration without stenting because of the large distance (3 cm) between the duodenal wall and the cyst.
CT scan showed a communication between the cyst and pancreatic duct in three patients. These three patients underwent an endoscopic retrograde cholangiopancreatography (ERCP) to stent the pancreatic duct. The procedures were, however, not successful and the patients subsequently underwent an EUS-guided drainage.
In one patient a pseudocyst was clinically suspected and the appearance on the CT scan as well as the EUS findings supported the benign diagnosis. Accordingly, a drainage procedure was performed during the EUS session. The patient was stented to the duodenum and had a total regression of the cyst with symptom improvement. However, the patient developed jaundice after 5 mo and was then found to have a pancreatic cystadenocarcinoma.
DISCUSSION
This is the first prospective study to show that the success rate of EUS-guided drainage of pseudocysts is high compared with that of the drainage of abscesses. Moreover, the complication rate was lower for pseudocysts compared with abscesses.
The present study provides novel knowledge about the success rate of EUS-guided drainage of clear fluid pseudocysts and abscesses. The data indicate that the drainage of clear fluid pseudocysts is a more straightforward procedure whereas the success rate for abscesses is lower. This could be partially explained by the fact that patients with abscesses are sicker and more vulnerable and are therefore more prone to develop complications. Another contributing factor is that an abscess with necrosis is usually less clearly demarcated compared with a clear fluid pseudocyst. This may make the procedure more difficult. Moreover, the wall of an abscess with excessive inflammation may rupture more easily compared with the wall of a less inflamed pseudocyst. The data support a more cautious approach to abscesses compared with pseudocysts. Abscesses should be drained only when conservative therapy has failed and enough time has passed for the formation of a well demarcated abscess wall.
The results presented in this work on pseudocyst drainage are comparable to previously reported data. A retrospective study by Cahen et al[11] showed a success rate of 97% and a complication rate of 34%. Kahaleh et al[9] published prospective data comparing EUS-guided drainage and conventional endoscopic drainage showing a long-term success rate of 84% and complication rate of 19% for EUS-guided drainage. There was no difference between EUS-guided drainage and conventional drainage. However, a recent prospective study[12] has also compared the results of EUS-guided drainage and conventional endoscopic drainage, showing a clear advantage for EUS-guided drainage with a success rate of 100% and only 33% for conventional endoscopic drainage. The complication rate in the EUS group was 4% and 20% in the conventional group. The differences in success rate and complication rates between different series are probably due to different patient populations and heterogeneous interventions[13].
EUS-guided drainage has been advocated as the first line treatment for pseudocysts[12]. Moreover, there is an enthusiasm about the possibilities of EUS-guided procedures. However, our knowledge from large prospective studies is still limited. There is probably a publication bias for data coming from large and experienced centers with good results. Therefore, there is a need for large prospective multicenter studies to assess the full scale of advantages and complications of EUS-guided drainage.
Our results are comparable to previously published data. However, we do have to further improve the success rate and reduce the complication rate. Due to limited resources intubation anesthesia was possible only in a minority of our patients. This type of anesthesia should be adopted for the majority of patients whenever possible in order to provide stable conditions during the drainage procedure. However, the importance of anesthesia has not yet been addressed in a study. Available techniques also need further improvement and there are many issues that need to be addressed in multicenter studies. Whether to use a dilatation technique or diathermia to enter the cyst is such an issue. The type of current to use and the optimal site in the stomach in which to place the stents are other unanswered questions.
In our study we observed perforations in two abscesses stented from the gastric fundus. This area is rather thin and should be handled carefully during the procedure. The gastric cardia should be avoided because it is close to the diaphragm. In one patient we did not stent the cyst because it was only accessible from the esophagus. A stent to the esophagus may result in a permanent fistula[8].
We experienced one perforation in a patient with a pseudocyst that was not attached to the gastric wall. This complication has been discussed for children but has not been published in adults so far. During the EUS procedure it can be difficult to know if the cyst is firmly attached to the gastric wall. However, a movement of the cyst under the gastric wall indicates that the cyst is not attached to the wall.
One of our patients developed a major bleed after the use of cutting current to enter a cyst from the stomach. After this complication more coagulation current and endocut (ERBE®) current was used to enter the cyst, rather than pure cut current, according to the manufacturer’s recommendations. Moreover, the entrance to the cyst was performed slowly to avoid fast and large movements of the needle wire.
No procedure-related mortality was observed in this study. Mortality rate varies in different series between 1/29 in a patient who underwent conventional endoscopic drainage[12] to 1% in retrospective material[11]. We found a recurrence rate of 4%, which is comparable with 5% reported by Cahen et al[11].
Primary surgery may be an alternative treatment for pseudocysts and abscesses. There are no prospective studies comparing surgery with EUS-guided drainage. One study has addressed the results and complications of necrosectomy by open surgery or minimally invasive surgery. The study showed no difference between the two approaches and a mortality rate of 28% in these severely ill patients[14]. Recent results from a large study by Seifert et al[15] indicate that initial endoscopic necrosectomy in the early phase of pancreatic necrosis is more risky and less successful and should be considered only if all other options have failed. Another study showed that delayed surgery correlated positively with reduced mortality[16].
The length of time a stent should be left in place is still a matter for debate. In general, endosonographers tend to expand the time until the stent is withdrawn to avoid recurrence of the cyst. It has been recommended to leave the stent in place in selected patients[13]. This is another issue that needs further studies to assess the optimal time for leaving the stent in place.
One patient was found to have a pancreatic adenocarcinoma and not a pseudocyst; this emphasizes the need for a close follow-up of the patients.
We have routine access to fluoroscopy during the drainage procedure. However, when the Giovannini Needle Wire was utilized there was no need for fluoroscopy in many cases. This is in agreement with a recent report showing that EUS-guided drainage was possible without access to fluoroscopy since not all endoscopy centers have fluoroscopy in the EUS room[17].
In conclusion, this prospective study shows for the first time that the results of EUS-guided drainage are more favorable in pseudocysts compared with abscesses. This knowledge should guide the choice and timing of therapy in patients with fluid collection. Large and preferably multicenter studies are needed to further expand our knowledge regarding different aspects of EUS-guided drainage, including the optimal technical procedure.
COMMENTS
Background
Inflammation in the pancreas may cause the development of fluid collection in the abdomen. This fluid collection may become infected. These fluid collections may be treated by surgery or endoscopic ultrasound. Endoscopic ultrasound is an endoscope equipped with a camera and ultrasound probe. This instrument gives a detailed visualization of the organs around the gut. These organs then become accessible for sampling and treatment. Using endoscopic ultrasound guidance it is possible to create a path between the fluid collection and the stomach to drain the fluid.
Research frontiers
Endoscopic ultrasound guidance is increasingly used to treat fluid collections instead of surgery. However, no study has compared the results of this treatment between infected and non-infected fluid collections.
Innovations and breakthroughs
This study shows that the results of endoscopic ultrasound treatment for non-infected fluid collections is very good and significantly better then the results for infected fluid collections.
Applications
Treatment using endoscopic ultrasound guidance should be applied in symptomatic patients with non-infected fluid collection. In patients with infected fluid collection the treatment should be applied when other non-surgical treatments have failed and not early in the course of the disease.
Peer review
Very interesting paper dealing with an alternative approach to one of the most frequent complications of chronic pancreatitis. Statistically sound (McNemar test was a fantastic choice), this study is very convincing.
Footnotes
Supported by The Health and Medical Care Executive Board of the Västra Götaland Region, Sweden
Peer reviewer: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
References
- 1.Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223–227. doi: 10.1016/j.gie.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Mönkemüller KE, Baron TH, Morgan DE. Transmural drainage of pancreatic fluid collections without electrocautery using the Seldinger technique. Gastrointest Endosc. 1998;48:195–200. doi: 10.1016/s0016-5107(98)70164-6. [DOI] [PubMed] [Google Scholar]
- 3.Kalaitzakis E, Panos M, Sadik R, Aabakken L, Koumi A, Meenan J. Clinicians’ attitudes towards endoscopic ultrasound: a survey of four European countries. Scand J Gastroenterol. 2009;44:100–107. doi: 10.1080/00365520802495545. [DOI] [PubMed] [Google Scholar]
- 4.Seewald S, Ang TL, Teng KC, Soehendra N. EUS-guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21 Suppl 1:S61–S65. doi: 10.1111/j.1443-1661.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 5.Vilmann P, Hancke S, Pless T, Schell-Hincke JD, Henriksen FW. One-step endosonography-guided drainage of a pancreatic pseudocyst: a new technique of stent delivery through the echo endoscope. Endoscopy. 1998;30:730–733. doi: 10.1055/s-2007-1001399. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–477. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 7.Seifert H, Faust D, Schmitt T, Dietrich C, Caspary W, Wehrmann T. Transmural drainage of cystic peripancreatic lesions with a new large-channel echo endoscope. Endoscopy. 2001;33:1022–1026. doi: 10.1055/s-2001-18927. [DOI] [PubMed] [Google Scholar]
- 8.Nealon WH, Bhutani M, Riall TS, Raju G, Ozkan O, Neilan R. A unifying concept: pancreatic ductal anatomy both predicts and determines the major complications resulting from pancreatitis. J Am Coll Surg. 2009;208:790–799; discussion 799-801. doi: 10.1016/j.jamcollsurg.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 10.Krüger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–416. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 12.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Baron TH. Endoscopic drainage of pancreatic pseudocysts. J Gastrointest Surg. 2008;12:369–372. doi: 10.1007/s11605-007-0334-5. [DOI] [PubMed] [Google Scholar]
- 14.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study) Gut. 2009;58:1260–1266. doi: 10.1136/gut.2008.163733. [DOI] [PubMed] [Google Scholar]
- 16.Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, Nieuwenhuijs VB, Gooszen HG. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194–1201. doi: 10.1001/archsurg.142.12.1194. [DOI] [PubMed] [Google Scholar]
- 17.Ayub K, Patterson D, Irani S, Schembre D, Gluck M, Brandabur J, Jiranek G, Ross A, Lin O, Kozarek R. Endoscopic ultrasound directed pseudocyst drainage without the use of fluoroscopy: a case series. Gastrointest Endosc. 2009;69:S234. [Google Scholar]


