Abstract
Microtubules form a multifunctional filamentous structure essential for the cell. In interphase, microtubules form networks in the cytoplasm and play pivotal roles in cell polarity and intracellular transport of various biomolecules. In mitosis, microtubules dramatically change their morphology to assemble the mitotic spindle, thereby pulling the chromosomes toward the spindle poles. One long-standing question is how microtubules are reorganized upon mitotic entry. Yeast cells undergo closed mitosis, in which the nuclear envelope persists, whereas higher eukaryotes undergo open mitosis, in which the nuclear envelope breaks down. Microtubule reorganization must be controlled by selective localization of microtubule-assembly factors. Recent findings in fission yeast indicate that several microtubule-associated proteins (MAPs) shuttle between the cytoplasm and the nucleus through regulation by Ran GTPase, the universal organizer of nucleocytoplasmic transport. Furthermore, the synergistic interplay of Ran and cyclin-dependent kinase (CDK) induces the critical spatiotemporal shift of modes in microtubule assembly from cytoplasmic arrays to nuclear spindles. A MAP complex Alp7/TACC-Alp14/TOG undergoes nucleocytoplasmic shuttling in interphase, whereas it is retained in the mitotic nucleus through a decrease of its nuclear export by CDK. Our understanding of how microtubules are reorganized during the cell cycle is beginning to emerge.
Key words: microtubule, cell cycle, nuclear transport, TACC-TOG, Ran, cyclin-dependent kinase (CDK)
Ran: A Universal Regulatory System for Spindle Microtubule Assembly
Microtubules dramatically change their overall configuration during the cell cycle.1,2 In interphase, microtubules form a cytoplasmic array—a mesh-like structure in higher eukaryotes and a cylindrical array of microtubules along the longitudinal cell axis in the fission yeast, Schizosaccharomyces pombe (Fig. 1A). Upon entry into mitosis, the cytoplasmic array is transformed into the bipolar spindle microtubules by the action of many different factors. The γ-tubulin complex is required for microtubule nucleation, whereas a diverse set of microtubule-associated proteins (MAPs), including the kinesin family motor proteins, is required to produce the proper bipolarity and dynamics of spindle microtubules. MAPs also include proteins that stabilize or destabilize the microtubule structure; some of these proteins are regulated by the GTPase, Ran.
Figure 1.
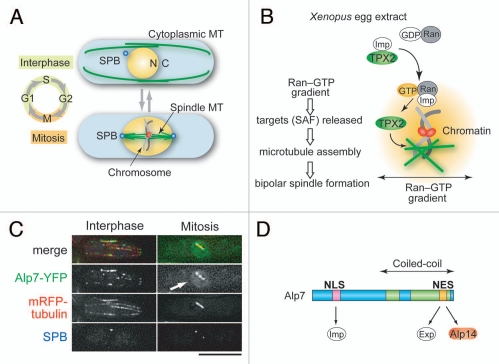
Microtubule reorganization upon mitotic entry. (A) Reorganization of fission yeast microtubule structures during the cell cycle. The cytoplasmic array of interphase microtubules is remodeled into the mitotic nuclear spindle. N, Nucleus; C, Cytoplasm; MT, microtubules; SPB, Spindle Pole Body. (B) In Xenopus egg extract, the targets of Ran for microtubule assembly (called SAF), such as TPX2, are released around chromosomes by GTP-bound Ran, which forms a molecular gradient in the vicinity of the chromatin. Imp, Importin. (C) Localization of Alp7-YFP in interphase and mitosis. The arrow indicates nuclear accumulation of Alp7-YFP. Microtubules were visualized by mRFP-Atb2, whereas SPB was visualized by Cut12-CFP. Bar, 5 µm. (D) Diagram of Alp7/TACC structure. Note that the “Coiled-coil” region represents the conserved “TACC” domain. Exp, Exportin.
Ran influences many cellular processes including nuclear import and export of proteins, the construction and/or integrity of the nuclear envelope, and spindle formation during mitosis. In interphase, GDP-bound Ran resides in the cytoplasm. In the nucleus, GDP on Ran is exchanged for GTP by the action of chromatin-bound RanGEF, the guanine nucleotide exchange factor of Ran, yielding Ran-GTP. The nuclear envelope serves as a nucleocytoplasmic barrier for Ran-GTP. In mitosis of higher eukaryotes, the nuclear envelope breaks down, but a molecular gradient of Ran-GTP forms around the chromatin (Fig. 1B). Addition of chromatin3 or Ran-GTP4,5 into Xenopus egg extract induces microtubule formation in the absence of centrosomes, indicating that Ran possesses an intrinsic activity to promote spindle formation (Fig. 1B). Ran targets several proteins that have spindle formation activity, such as TPX2 and NuMA (collectively called the spindle assembly factor, SAF), and bipolar spindle assembly is induced by releasing these targets at the appropriate sites in the cell (reviewed by Karsenti and Vernos,6 Zheng,7 Kalab and Heald,8 and Clarke and Zhang,9 for instance).
Over the last decade, many studies in somatic cells have established that spindle microtubule formation can be induced both from the centrosomes and around the chromosomes. In fission yeast, it is believed that nucleation of spindle microtubules is exclusively dependent upon the spindle pole body (SPB), a centrosome-equivalent structure, and there is no evidence that microtubules are nucleated from chromosomes. Despite these structural differences, the Ran machinery is evolutionarily conserved as a common scheme to promote spindle microtubule assembly. In fission yeast, molecules involved in Ran regulation, such as RanGEF/RCC1, RanGAP (the GTPase activating protein) and Ran binding proteins (RanBPs) are conserved, and spindle formation is defective in the mutants of Spi1/Ran or Pim1/RanGEF.10–13 This indicates that the Ran-dependent microtubule formation system is conserved in fission yeast, although orthologs of vertebrate Ran target SAFs (e.g., TPX2 and NuMA) are absent in yeast.
Alp7/TACC is a Critical Target of Ran in Fission Yeast
Because the nuclear envelope does not break down during mitosis in fission yeast, it is plausible that some key factors for microtubule regulation must be transported into the nucleus to promote nuclear spindle formation. Thus, the pim1/RanGEF mutant phenotype might be caused by the failure to transport Ran targets into the nucleus. We have investigated Alp7 as a potential Ran target. Alp7 (also called Mia1) is an ortholog of the transforming acidic coiled-coil (TACC) protein conserved among eukaryotes.14 In all organisms examined, TACC forms a complex with another conserved MAP, the tumor-overexpressed gene (TOG) protein (the Xenopus ortholog is XMAP215).15,16 This microtubule-associated protein complex, TACC-TOG, localizes to microtubules and centrosomes. Fission yeast Alp7/TACC also forms a complex with Alp14/TOG and localizes to microtubules, SPBs and kinetochores.14,17,18
Both alp7 and alp14 mutants show fragile spindle formation upon entry into mitosis, which results in frequent chromosome missegregation.14,17,19 This phenotype is reminiscent of that of the pim1 mutant. Alp7 accumulates in the nucleus during mitosis but not in interphase (Fig. 1C) and contains a canonical classical nuclear localization signal (NLS) that is responsible for nuclear entry of the Alp7-Alp14 complex (Fig. 1D). Importantly, artificial nuclear targeting of Alp7 by fusion of part of the Importin α protein substantially overcomes the spindle defects of the temperature-sensitive pim1-F201S mutant; in particular, the amount of microtubules formed around nuclear SPBs is restored to the wild-type level.20 Thus, the Alp7-Alp14 complex is a critical target of Ran GTPase for spindle formation in fission yeast. Surprisingly, TACC-TOG has never been identified as a Ran target in other organisms. It should be noted, however, that human TOG interacts with HURP, a target of Ran.21,22 In addition, TPX2, another target of Ran, binds to and activates Aurora A kinase,23 which in turn phosphorylates and activates TACC.24,25 In fission yeast, therefore, it is possible that Ran directly targets Alp7-TACC/Alp14-TOG because the intermediating factors in humans, such as Aurora A and HURP, do not exist in fission yeast.
Alp7 Undergoes Nucleocytoplasmic Shuttling in Interphase
Alp7-Alp14 localizes to the nucleus only during mitosis, whereas this complex remains in the cytoplasm during interphase (Fig. 1C).14,20 Thus, determining how the cell cycle-specific localization of this MAP complex is achieved will help elucidate the mechanisms underlying microtubule remodeling upon mitotic entry. The most straightforward scenario is that Alp7-Alp14 enters the nucleus upon the onset of mitosis. However, it turns out that this complex actually enters the nucleus even during interphase but is immediately exported out of the nucleus by its intrinsic nuclear export signal (NES) activity (Fig. 2).26–28
Figure 2.
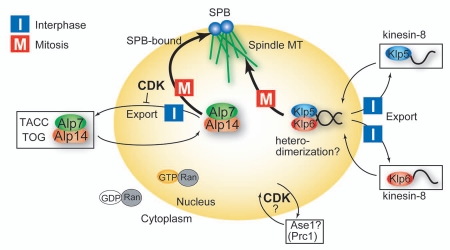
Nucleocytoplasmic regulation of microtubule-associated proteins. Model illustrating how microtubule-associated proteins in fission yeast are regulated in a spatiotemporal manner. Alp7/TACC-Alp14/TOG and Klp5-Klp6 (kinesin-8s) are targets of Ran in fission yeast and undergo nucleocytoplasmic shuttling during the cell cycle. Alp7-Alp14 is retained in the mitotic nucleus through inhibition of nuclear export by the cyclin-dependent kinase 1 (CDK1). See text for Klp5-Klp6 and Ase1. ‘I’ represents the destination of the protein in interphase, whereas ‘M’ is the destination in mitosis.
As discussed above, Alp7/TACC-Alp14/TOG promotes assembly of the mitotic spindle. This complex is also essential to establish cytoplasmic microtubules in interphase, as shown by the alp14 mutant that exhibits fragile and fragmented cytoplasmic microtubules.17 Indeed, artificial targeting of the Alp7-Alp14 complex to the nucleus in interphase leads to crumbling of cytoplasmic microtubules reminiscent of that observed in the alp14 mutant.20,26 Thus, nucleocytoplasmic shuttling of this complex during interphase is crucial for interphase microtubule organization.
Nuclear Export of Alp7 may be Negatively Regulated in Mitosis
The continuous nucleocytoplasmic shuttling of the Alp7-Alp14 complex suggests two possible strategies for how this complex preferentially localizes to the nucleus only during mitosis: (1) acceleration of nuclear import during mitosis, and/or (2) the inhibition of nuclear export during mitosis. It is experimentally difficult to discriminate these possibilities, but our results suggest the second possibility.26 We have found that, during interphase, Alp7-Alp14 enters the nucleus in an NLS-dependent manner and is exported to the cytoplasm in an NES-dependent manner. Upon entry into mitosis, the complex is retained in the nucleus to execute mitotic function, but NLS activity is not specifically potentiated. This suggests that the NLS is not regulated but instead that NES activity may be under cell cycle regulation.
The dynamic shuttling of Alp7-Alp14 thus raises the issue of why Alp7-Alp14 enters the nucleus in interphase at all. We are currently unable to address this issue. The constitutive shuttling of the Alp7-Alp14 complex might transport some materials from the nucleus to the cytoplasm that are important for formation and maintenance of cytoplasmic microtubules. This possibility might be tested if it is possible to express two kinds of engineered Alp7, one constitutively nuclear and another constitutively cytoplasmic. This would tell us the meaning of Alp7-Alp14 shuttling itself. Alternatively, maintaining a certain amount of the Alp7-Alp14 complex in the nucleus by constant influx during interphase might facilitate more rapid mitotic spindle formation upon mitotic entry. The gradual accumulation of the Alp7-Alp14 complex in the nucleus might transform the molecular environment of the nucleus, thereby specifying the timing of mitotic entry at G2/M transition.
Cyclin-Dependent Kinase (CDK) is Required for Nuclear Accumulation of Alp7
The cell cycle-dependent localization of Alp7-Alp14 is established by Cdc2, the fission yeast CDK1. When the expression of B-type cyclin Cdc13 is artificially shut off in meiosis, Alp7 does not accumulate in the nucleus.26 One might argue that this is simply due to the failure to enter meiotic M phase in the absence of Cdc13, rather than support for the requirement of Cdc2/Cdc13 for Alp7 nuclear accumulation per se. To distinguish between these possibilities, we used the cdc2-as mutant, in which the kinase activity is lost in the presence of an ATP analog (1NM-PP1).29 This strain enabled us to inactivate Cdc2 activity even after mitotic entry so that we could overcome the technical burden we encountered in the cdc13 shut-off strain described above or in other temperature-sensitive cdc2 mutants.
The ATP analog was added to prometaphase cells, which are identified by the separated SPBs and also the nuclear accumulation of Alp7. Intriguingly, nuclear Alp7 dispersed immediately (≤2 min) after addition of the ATP analog,26 indicating that CDK1 activity is continuously required for the retention of Alp7 in the mitotic nucleus rather than only for its nuclear entry upon mitotic onset. Because Alp7 with a deletion of the NES-containing C-terminal part was constitutively localized in the nucleus,26 we hypothesize that CDK1 prevents the export of Alp7-Alp14 from the nucleus via the NES during mitosis (Fig. 2). Notably, Alp7 with a mutant NES was also unable to properly bind to Alp14 (Fig. 1D).26,27 This suggests that Alp14 and Crm1/Exportin-1 (an export factor which recognizes NES) compete for the C-terminal domain of Alp7 and that formation of the Alp7-Alp14 complex in the nucleus might inhibit association with Crm1, resulting in nuclear retention of the complex.
Notably, it was impossible to induce ectopic spindle formation even if the Alp7-Alp14 complex was artificially targeted to the nucleus in interphase. This suggests that other factors are also required for mitotic spindle formation including SPB positioning. In fission yeast, SPBs are located in the cytoplasm in interphase and are inserted into the nuclear envelope upon mitotic entry.30–32 This allows microtubule nucleation inside the nucleus as well as association of Alp7-Alp14 with the SPB. In addition, localization of α/β-tubulin dimers in the mitotic nucleus is essential for nuclear spindle formation. It is not known, however, if the subcellular localization of tubulin dimers is regulated spatially and temporally.
Functional Separation of the Alp7/TACC and the Alp14/TOG Subunit
TACC-TOG is a highly conserved MAP complex. TACC is required for the localization of TOG at centrosomes or spindles in Drosophila melanogaster,33,34 Caenorhabditis elegans35–37 and human.38 What is the molecular function of the Alp7/TACC subunit in fission yeast? Alp7/TACC could be a regulatory subunit for the spatiotemporal localization of the complex, whereas Alp14/TOG could be a catalytic subunit to execute microtubule/tubulin regulation. In fact, it was recently shown that TOG serves as a microtubule polymerase.39 Thus, one might propose that Alp7/TACC is merely a nuclear import factor for Alp14, as the intrinsic NLS of Alp7 is responsible for the nuclear accumulation of Alp14/TOG.26 This does not appear to be the case, however, because fusion of the SV40-NLS to Alp14 (Alp14-NLS) does not suppress the mitotic defects of alp7Δ cells, although Alp14-NLS is capable of localizing to the nucleus independently of Alp7 (our unpublished results). This implies that Alp7 is more than just a nuclear import factor for Alp14. It will be intriguing to investigate whether Alp7 is a specific recruitment factor for Alp14 to localize to SPBs as well as a nuclear import factor.
Two Kinesin-8 Proteins, Klp5 and Klp6, are also Targets of Ran
Although forced localization of Alp7 to the nucleus suppresses the microtubule defects in the pim1-F201S mutant with regard to microtubules assembled from the SPB, the recovery was not perfect; microtubules frequently failed to form proper bipolar spindles. This indicates that Ran targets other than Alp7/TACC may help establish bipolar spindle formation. In fact, Klp5 and Klp6, two members of the conserved kinesin-8 family that form a heterodimer,40,41 are also targeted by Ran. Both Klp5 and Klp6 individually shuttle between the nucleus and the cytoplasm during the cell cycle, and their nuclear localization is dependent upon their intrinsic NLSs.42 Because these two kinesins are interdependent for mitotic nuclear localization, lacking the intrinsic NLSs would cause failure to assemble the heterodimer in the nucleus. Heterodimerization of Klp5 and Klp6 is essential to regulate microtubule dynamics; in each mutant the frequency of both catastrophe and rescue of microtubules is reduced, resulting in less dynamic microtubules than in wild type.42 Therefore, the phenotype of the pim1-F201S mutant defective in nuclear transport should partially include that of the klp5/6 mutant, such as the reduction of microtubule dynamics. It is possible that the residual spindle defects that override the forced nuclear Alp7-Alp14 localization may stem from the failure of Klp5-Klp6 heterodimers to accumulate in the nucleus. It is thus of interest to investigate how Klp5 and Klp6 accumulate in the nucleus during mitosis. Mitotic phosphorylation of Klp5 or Klp6 might allow their stable association, which in turn may inhibit nuclear exclusion by the nuclear export factor Crm1/Exportin-1.
Ase1: Another Putative Target of Ran?
A recent study indicated that the microtubule-bundling factor Ase1 (Prc1 in human) might also enter the nucleus in a CDK1-dependent fashion.43 Ase1 is required for the establishment of stable microtubule-interdigitating regions of microtubules. Ase1 localizes to the overlapping zone of the cytoplasmic microtubule bundles and also to that of the spindle microtubule in anaphase.44,45 ase1Δ cells often exhibit a collapse of anaphase spindles due to the disorganized midzone, indicating that Ase1 functions as a structural pedestal for elongation of anti-parallel microtubule bundles. Intriguingly, phosphorylation of Ase1 by CDK1 is essential for its localization to the nuclear spindle midzone in anaphase.43 Thus, the CDK1-dependent translocation of Ase1 is critical for spindle elongation in the upcoming anaphase, rather than for the spindle formation in early mitosis (from prophase to metaphase) in which Alp7 is involved. It has not been reported if Ase1 harbors an NLS recognizable by the Importin-Ran system; it would be of interest to see if the pim1 mutant exhibits spindle collapse in anaphase and if it is explained by a lack of nuclear Ase1.
Open and Closed Mitoses
Ran has many targets required for spindle microtubule assembly, as summarized in Clarke and Zhang9 and in Kalab and Heald.8 How can we explain such diversity of targets for spindle formation? We speculate that it could be related to the size and complexity of the mitotic spindle, as well as to the persistence of the yeast nuclear envelope during mitosis. The size of metaphase spindles varies between 10 and 30 µm in higher eukaryotes and is ∼2 µm in fission yeast. In higher eukaryotes, Ran-GTP generates a molecular gradient around the chromatin. The diameter of the Ran-GTP gradient that has the potential to promote spindle microtubule assembly in organisms that carry out open mitosis is comparable to the diameter of a fission yeast nucleus. Thus, it may be impossible to form a Ran-GTP gradient inside the yeast nucleus; therefore, Alp7/TACC, which localizes to both the SPB and microtubules, is an ideal candidate as a target of Ran to allow effective spindle formation from the SPB in the yeast nucleus. We thus envision that the Ran-dependent spindle-formation system has evolved from yeast, an organism with closed mitosis, and has since developed and diverged along with the increase in size and complexity of the mitotic spindle in open-mitosis organisms. This might be one of the reasons why spindle assembly in higher organisms is regulated by the universal nuclear transport factor, Ran, even though the nuclear envelope disappears and nuclear transport no longer operates during mitosis.
Acknowledgements
We thank Masayuki Yamamoto for support and the Cytology Team in his lab for discussion. We also thank Minoru Yoshida for collaboration. This work is supported by Cancer Research UK (T.T.) and by a Grant-in-Aid for Young Scientists (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.S.).
Extra View to: Sato M, Okada N, Kakui Y, Yamamoto M, Yoshida M, Toda T. Nucleocytoplasmic transport of Alp7/TACC organizes spatiotemporal microtubule formation in fission yeast. EMBO Rep. 2009;10:1161–1167. doi: 10.1038/embor.2009.158.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/11443
References
- 1.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 2.Hayles J, Nurse P. A journey into space. Nat Rev Mol Cell Biol. 2001;2:647–656. doi: 10.1038/35089520. [DOI] [PubMed] [Google Scholar]
- 3.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 4.Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- 5.Ohba T, Nakamura M, Nishitani H, Nishimoto T. Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science. 1999;284:1356–1358. doi: 10.1126/science.284.5418.1356. [DOI] [PubMed] [Google Scholar]
- 6.Karsenti E, Vernos I. The mitotic spindle: a self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y. G protein control of microtubule assembly. Annu Rev Cell Dev Biol. 2004;20:867–894. doi: 10.1146/annurev.cellbio.20.012103.094648. [DOI] [PubMed] [Google Scholar]
- 8.Kalab P, Heald R. The RanGTP gradient—a GPS for the mitotic spindle. J Cell Sci. 2008;121:1577–1586. doi: 10.1242/jcs.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol. 2008;9:464–477. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- 10.Sazer S, Nurse P. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO J. 1994;13:606–615. doi: 10.1002/j.1460-2075.1994.tb06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleig U, Salus SS, Karig I, Sazer S. The fission yeast ran GTPase is required for microtubule integrity. J Cell Biol. 2000;151:1101–1111. doi: 10.1083/jcb.151.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto T, Beach D. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell. 1991;66:347–360. doi: 10.1016/0092-8674(91)90624-8. [DOI] [PubMed] [Google Scholar]
- 13.Salus SS, Demeter J, Sazer S. The Ran GTPase system in fission yeast affects microtubules and cytokinesis in cells that are competent for nucleocytoplasmic protein transport. Mol Cell Biol. 2002;22:8491–8505. doi: 10.1128/MCB.22.24.8491-8505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, Vardy L, Garcia MA, Koonrugsa N, Toda T. Interdependency of fission yeast Alp14/TOG and coiled coil protein Alp7 in microtubule localization and bipolar spindle formation. Mol Biol Cell. 2004;15:1609–1622. doi: 10.1091/mbc.E03-11-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gergely F. Centrosomal TACCtics. Bioessays. 2002;24:915–925. doi: 10.1002/bies.10162. [DOI] [PubMed] [Google Scholar]
- 16.Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–388. doi: 10.1016/j.tcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Garcia MA, Vardy L, Koonrugsa N, Toda T. Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 2001;20:3389–3401. doi: 10.1093/emboj/20.13.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakaseko Y, Goshima G, Morishita J, Yanagida M. M phase-specific kinetochore proteins in fission yeast: microtubule-associating Dis1 and Mtc1 display rapid separation and segregation during anaphase. Curr Biol. 2001;11:537–549. doi: 10.1016/s0960-9822(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 19.Oliferenko S, Balasubramanian MK. Astral microtubules monitor metaphase spindle alignment in fission yeast. Nat Cell Biol. 2002;4:816–820. doi: 10.1038/ncb861. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Toda T. Alp7/TACC is a crucial target in Ran-GTPase-dependent spindle formation in fission yeast. Nature. 2007;447:334–337. doi: 10.1038/nature05773. [DOI] [PubMed] [Google Scholar]
- 21.Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW. HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol. 2006;16:743–754. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Sillje HH, Nagel S, Korner R, Nigg EA. HURP is a Ran-importin β-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr Biol. 2006;16:731–742. doi: 10.1016/j.cub.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 23.Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros TP, Kinoshita K, Hyman AA, Raff JW. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peset I, Seiler J, Sardon T, Bejarano LA, Rybina S, Vernos I. Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J Cell Biol. 2005;170:1057–1066. doi: 10.1083/jcb.200504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato M, Okada N, Kakui Y, Yamamoto M, Yoshida M, Toda T. Nucleocytoplasmic transport of Alp7/TACC organizes spatiotemporal microtubule formation in fission yeast. EMBO Rep. 2009;10:1161–1167. doi: 10.1038/embor.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling YC, Vjestica A, Oliferenko S. Nucleocytoplasmic shuttling of the TACC protein Mia1p/Alp7p is required for remodeling of microtubule arrays during the cell cycle. PLoS ONE. 2009;4:6255. doi: 10.1371/journal.pone.0006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuyama A, Arai R, Yashiroda Y, Shirai A, Kamata A, Sekido S, et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- 29.Dischinger S, Krapp A, Xie L, Paulson JR, Simanis V. Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network. J Cell Sci. 2008;121:843–853. doi: 10.1242/jcs.021584. [DOI] [PubMed] [Google Scholar]
- 30.Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallada VA, Tanaka K, Yanagida M, Hagan IM. The S. pombe mitotic regulator Cut12 promotes spindle pole body activation and integration into the nuclear envelope. J Cell Biol. 2009;185:875–888. doi: 10.1083/jcb.200812108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong CS, Sato M, Toda T. Fission yeast Pcp1 links polo kinase-mediated mitotic entry to gamma-tubulin-dependent spindle formation. EMBO J. 2010;29:120–130. doi: 10.1038/emboj.2009.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cullen CF, Ohkura H. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat Cell Biol. 2001;3:637–642. doi: 10.1038/35083025. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- 35.Bellanger JM, Gönczy P. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr Biol. 2003;13:1488–1498. doi: 10.1016/s0960-9822(03)00582-7. [DOI] [PubMed] [Google Scholar]
- 36.Le Bot N, Tsai MC, Andrews RK, Ahringer J. TAC-1, a regulator of microtubule length in the C. elegans embryo. Curr Biol. 2003;13:1499–1505. doi: 10.1016/s0960-9822(03)00577-3. [DOI] [PubMed] [Google Scholar]
- 37.Srayko M, Quintin S, Schwager A, Hyman AA. Caenorhabditis elegans TAC-1 and ZYG-9 form a complex that is essential for long astral and spindle microtubules. Curr Biol. 2003;13:1506–1511. doi: 10.1016/s0960-9822(03)00597-9. [DOI] [PubMed] [Google Scholar]
- 38.Gergely F, Draviam VM, Raff JW. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, et al. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West RR, Malmstrom T, Troxell CL, McIntosh JR. Two related kinesins, klp5+ and klp6+, foster microtubule disassembly and are required for meiosis in fission yeast. Mol Biol Cell. 2001;12:3919–3932. doi: 10.1091/mbc.12.12.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia MA, Koonrugsa N, Toda T. Two kinesin-like Kin I family proteins in fission yeast regulate the establishment of metaphase and the onset of anaphase A. Curr Biol. 2002;12:610–621. doi: 10.1016/s0960-9822(02)00761-3. [DOI] [PubMed] [Google Scholar]
- 42.Unsworth A, Masuda H, Dhut S, Toda T. Fission yeast kinesin-8 Klp5 and Klp6 are interdependent for mitotic nuclear retention and required for proper microtubule dynamics. Mol Biol Cell. 2008;19:5104–5115. doi: 10.1091/mbc.E08-02-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu C, Ward JJ, Loïodice I, Velve-Casquillas G, Nedelec FJ, Tran PT. Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev Cell. 2009;17:257–267. doi: 10.1016/j.devcel.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loïodice I, Staub J, Setty TG, Nguyen NP, Paoletti A, Tran PT. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol Biol Cell. 2005;16:1756–1768. doi: 10.1091/mbc.E04-10-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita A, Sato M, Fujita A, Yamamoto M, Toda T. The roles of fission yeast Ase1 in mitotic cell division, meiotic nuclear oscillation and cytokinesis checkpoint signaling. Mol Biol Cell. 2005;16:1378–1395. doi: 10.1091/mbc.E04-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]