Abstract
Controlled nucleocytoplasmic trafficking is an important feature for fine-tuning signaling pathways in eukaryotic organisms. Nuclear pore complexes (NPCs) composed of nucleoporin proteins (Nups) are essential for the exchange of macromolecules across the nuclear envelope. A recent genetic screen in our laboratory identified a partial loss-of-function mutation in Arabidopsis MOS7/Nup88 that causes defects in basal immunity, Resistance (R) protein-mediated defense and systemic acquired resistance. In Drosophila and mammalian cells, exportin-mediated nuclear export of activated Rel/NFκB transcription factors is enhanced in nup88 mutants resulting in immune response failure. Consistent with Nup88 promoting nuclear retention of NFκB, our functional analyses revealed that MOS7/Nup88 is required for appropriate nuclear accumulation of the autoactivated R protein snc1, as well as the key immune regulators EDS1 and NPR1. These results suggest that controlling the nuclear concentrations of specific immune regulators is fundamental for defining defense outputs.
Key words: plant immunity, nucleoporins, nucleocytoplasmic trafficking, snc1, mos
Eukaryotes have evolved elaborate immune systems allowing them to discriminate between self and non-self. In animals and plants, innate immune responses of individual cells constitute a major barrier to pathogen infection. There are two levels of innate immunity in the plant kingdom. The first, termed PAMP-triggered immunity (PTI), is mediated by cell surface-resident Pattern Recognition Receptors (PRRs) that sense conserved pathogen associated molecular patterns (PAMPs). The second, pathogen-specific branch of the immune system, known as effector-triggered immunity (ETI), recognizes and responds to isolate-specific microbial effector molecules or their actions on host molecular targets.1,2 Pathogen effectors are often secreted into host cells during infection to increase virulence, in part by suppressing PTI. Recognition of specific effectors is conferred by R proteins. Most characterized R proteins are intracellular and have conserved Nucleotide-Binding and Leucine-Rich-Repeat (NB-LRR) domains that are also found in animal NOD (Nucleotide-Binding/Oligomerization Domain)-LRR immune receptors.
The role of the NPC and nucleocytoplasmic trafficking machinery in plant innate immunity was first revealed in our Arabidopsis genetic screen aimed at identifying components contributing to auto-immune responses mediated by the deregulated Toll-Interleukin-1 Receptor (TIR)-type NB-LRR R gene snc1 (suppressor of npr1-1, constitutive 1).3,4 Autoactivation of snc1 is caused by a point mutation resulting in an E552K change in the linker region between the NB and LRR domains.3 As a consequence, snc1 mutant plants are dwarf, accumulate high levels of the defense hormone salicylic acid (SA) and exhibit enhanced disease resistance to virulent pathogens.5,3 Intriguingly, certain mutations in the same region of human immune NOD-LRR receptor NOD2 also result in constitutive activation and are associated with a chronic inflammatory disorder known as Crohn's disease.6
The snc1 suppressor screen was designed to identify modifier of snc1 (mos) mutants that resemble wild-type morphology and abolish constitutive pathogen resistance in snc1.7 We previously reported the isolation of MOS3,4 encoding the homolog of vertebrate Nup96 implicated in immunity-related mRNA export in mice and the importin α MOS6.8,9 MOS3 and MOS6 point to a requirement for mRNA export and nuclear localization signal (NLS)-mediated protein nuclear import pathways in plant immunity.
A defining feature of eukaryotic cells is the physical separation of the nucleoplasm from the cytoplasm by the double lipid bilayer of the nuclear envelope (NE). This separation necessitates a gateway and an elaborate trafficking machinery to facilitate the coordinated exchange of information between the two compartments. At the same time, this compartmentalization provides eukaryotic cells with a potent means of spatial and temporal control of signaling events. For example, restraining nuclear factor-kappaB (NFκB) transcription factors (TFs) outside the nucleus through association with inhibitory IτB proteins in the cytoplasm represents a major regulatory step in animal innate immunity. In uninduced cells IκBα/NFκB complexes shuttle between the nucleus and the cytoplasm but steady-state localization appears to be almost exclusively cytosolic due to dominant NES-dependent nuclear export over nuclear localization signal (NLS)-dependent nuclear import.10,11 Inducing stimuli such as ligand recognition of certain PRRs, trigger proteasome-mediated IκB degradation that alters the dynamic balance between IκBα/NFκB cytosolic and nuclear localization signals to favor nuclear accumulation of released NFκB dimers and transcription of target genes.12
Recent data in Drosophila and mammalian cells suggest an additional mechanism controlling the nuclear accumulation of NFκB and Rel-like TFs at the level of nucleocytoplasmic transport that is dependent on the dynamic inhibition of NFκB nuclear export rates by the nuclear pore complex protein Nup88 (Xylourgidis, et al. 2006). Nuclear pore complexes (NPCs) form numerous perforations in the NE and are composed of multiple copies of ∼30 distinct Nups. Nups are modularly assembled in distinct subcomplexes of defined composition and arranged radially around a central channel that serves as the sole conduit and dynamic barrier for the selective bidirectional exchange of molecular cargoes to and from the nucleus. Translocation typically depends on the recognition of NLS and/or NES motifs on the cargo by nuclear transport receptors (NTRs) of the karyopherin family that facilitate nuclear import (importins) or export (exportins).
In Drosophila, the exportin CRM1 mediates nuclear export of Dorsal and Dif, members of the Rel protein family which includes NFκB. Activation of the innate immune response in Drosophila is dependent on the nuclear activity of Dorsal and Dif that accumulate in the nucleus upon degradation of the IκB homolog Cactus (Fig. 1A). Notably, members only (mbo) mutants, encoding the Drosophila homolog of vertebrate Nup88, fail to accumulate Dorsal and Dif in the nucleus or activate an effective immune response upon bacterial infection.13 Nup88 is localized on the cytoplasmic side of the NPC where it associates with Nup214 (Fig. 1A). Since the Nup88/Nup214 complex appears to sequester CRM1 at the NE, the cytoplasmic localization of Dorsal and Dif in mbo mutants likely results from excess CRM1 cargo export activity. This suggests that Nup88 acts as an attenuator of CRM1-mediated nuclear protein export to modulate the expression of NFκB/Rel target genes, thereby controlling the relative strength and duration of innate immune responses at the level of the NPC (Fig. 1A).14,15 Consistent with Nup88 promoting nuclear retention of Rel proteins, depletion of Nup88 in mammalian cells prevents nuclear accumulation of NFκB and inhibits NFκB-dependent target gene expression whereas enhanced expression of Nup88 in malignant melanoma cells might contribute to constitutive NFκB activation.16
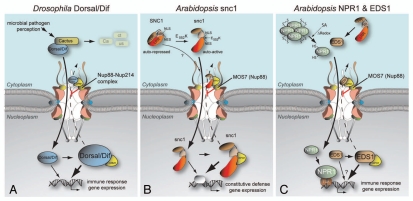
Figure 1.
Nup88 promotes nuclear retention of animal and plant immune regulators. (A) The Drosophila homolog of vertebrate Nup88, Members only (Mbo), is required for nuclear accumulation of the Rel-type transcription factors Dorsal and Dif. Toll receptor signaling upon microbial infection releases the NFκB homologs Dorsal/Dif from the inhibitory IκB homolog Cactus, allowing Dorsal/Dif nuclear translocation. During this immune response, Mbo/Nup88 attenuates nuclear export of Dorsal/Dif by sequestering the Dorsal/Dif-loaded export receptor CRM1 at the nuclear rim. This attenuation in nuclear export results in nuclear accumulation of Dorsal/Dif and efficient defense gene expression. In contrast, mbo mutant animals show enhanced nuclear export of Dorsal/Dif and fail to activate an immune response (Roth et al. 2003; Uv et al. 2000; Xylourgidis et al. 2006).14,13,15 (B) In Arabidopsis, an E552K change in SNC1 renders this TIR-NB-LRR immune receptor constitutively active without a pathogen stimulus.3 Autoactive snc1 localizes to the cytoplasm and the nucleus and possibly shuttles between the two compartments via NLS/Importin-mediated nuclear import and NES/XPO1-mediated nuclear export. Autoimmunity of snc1 requires functional MOS7 (Nup88) since a partial loss-of-function mutation in mos7-1 results in XPO1-mediated nuclear leakage of snc1 and suppression of all known snc1 auto-immune phenotypes. This is consistent with MOS7 promoting nuclear retention and accumulation of autoactive snc1 as a critical process in the constitutive activation of immune responses.17 (C) MOS7 (Nup88) is also required for proper nuclear accumulation of the plant-specific defense regulators NPR1 and EDS1. NPR1 translocates to nuclei of uninduced tissues at a low rate.24 Induction by the plant defense hormone SA and subsequent changes in the cellular redox state promote NPR1 monomerization via thioredoxin-mediated reduction of intermolecular disulfide bonds and nuclear accumulation of NPR1 monomers, a process required for efficient expression of NPR1-regulated defense genes and induction of systemic immunity.19 EDS1 is a key component of immunity triggered by snc1 and other intracellular TIR-NB-LRR immune receptors.25,5 Nucleocytoplasmic EDS1 forms several distinct defense regulatory complexes in the cytoplasm and the nucleus (not shown) and is capable of XPO1-mediated nuclear export.26,27 Altered nuclear translocation rates of NPR1 and EDS1 in mos7-1 might affect NPR1 and EDS1 protein stability that becomes sensed and equilibrated across the NE over time.
We recently identified and functionally characterized mos7-1, a partial loss-of-function mutation in the Arabidopsis homolog of vertebrate and Drosophila Nup88, which fully suppresses all known autoimmune phenotypes of snc1.17 mos7-1 single mutant plants exhibit defects not only in basal defense against virulent pathogens and ETI conditioned by several NB-LRR R proteins of both the TIR- and Coiled-Coil (CC)-type, but also in systemic acquired resistance (SAR). SAR represents a long-lasting and broad spectrum disease resistance that is induced throughout the plant after a local immune response and accumulation of SA.18 SAR induction is mediated by the transcriptional coactivator Nonexpressor of Pathogenesis-Related genes 1 (NPR1). In uninduced tissues NPR1 is retained partially in the cytoplasm as a homo-oligomeric complex formed through intermolecular disulfide bonds (Fig. 1C). In response to pathogen attack, a change in the cellular redox potential leads to thioredoxin-mediated reduction of disulfide bonds allowing nuclear accumulation of NPR1 monomers, possibly due to the exposure of normally obscured NLSs (Mou, et al. 2003; Tada, et a.19,20 Inside the nucleus, NPR1 interacts with members of the TGA family of bZIP TFs to regulate downstream Pathogenesis-Related (PR) gene expression (Fig. 1C).21,22,23
Since mos7-1 mutant plants are impaired in SAR and the nuclear release of NPR1 is reminiscent of NFκB signaling in animal immunity, we investigated the contribution of MOS7 to NPR1 nuclear accumulation. We found that significantly lower amounts of a translational NPR1-GFP fusion expressed under control of the native NPR1 promoter accumulated in nuclei of mos7-1 than in wild type transgenic plants before and after SAR induction.17 As NPR1 nuclear accumulation is essential for SAR, NPR1 may not be able to attain sufficient threshold abundance in the nucleus for activation of SAR in mos7-1. Our cellular fractionation further suggests that a small portion of the cellular NPR1 pool is present in nuclei of uninduced tissues. Recent work by Spoel, et al. shows that, indeed, NPR1 monomer continuously translocates to the nucleus in uninduced tissues at a low rate. Here NPR1 amounts are kept low via proteasome-mediated degradation to prevent target gene transcription in uninduced cells.24
As mos7-1 suppresses snc1 fully whereas npr1 does not, we reasoned that MOS7 must regulate nuclear traffic of further components in snc1-triggered resistance besides increasing nuclear NPR1 levels to induce SAR. To gain further mechanistic insight to the function of MOS7 in innate immunity, we investigated the effect of mos7-1 on the subcellular distribution of another nucleocytoplasmic immune regulator, Enhanced Disease Susceptibility1 (EDS1), which is an indispensable component of snc1 auto-immune responses and able to pass through NPCs via the Arabidopsis CRM1 homolog XPOI.26,27,28,5 As with NPR1, EDS1 total protein was reduced in mos7-1 mutant plants compared to wild type.17 Reduced EDS1 and NPR1 accumulation was not observed at the transcript level indicating a major effect of mos7-1 on protein synthesis or stability. In mos7-1, the ratio of EDS1 distribution in the cytosol and nucleus was not strongly affected, but overall lower accumulation of EDS1 resulted in very low levels being detected in nuclei of mos7-1. This likely contributes to the ability of mos7-1 to suppress snc1 because interfering with nuclear accumulation of EDS1 impairs resistance mediated by another TIR-type NB-LRR receptor, RPS4.27 Moreover, pathogen activation of RPS4 resistance triggers an early increase in the nuclear EDS1 pool that directs EDS1-dependent changes in defense-related gene expression. However, such perturbations of nuclear EDS1 levels apparently become sensed and equilibrated with the EDS1 cytoplasmic pool which is also required for full resistance.27 Therefore, reduced nuclear retention of EDS1 in mos7-1 might perturb proper coordination of EDS1 pools across the NE and thereby alter EDS1 protein stability. This would lower the amount in the cytosolic pool available for nuclear import and result in the observed proportional depletion of EDS1 in both nuclear and cytoplasmic compartments in mos7-1 (Fig. 1C).17
Two EDS1-dependent immune receptors, Arabidopsis RPS4 and tobacco N, have been shown to partially localize to and function inside the plant nucleus to trigger immune responses upon activation by their cognate pathogen effector.29,30 The specific effector detected by wild-type SNC1 in nature is unknown, but the protein contains a predicted NLS and two predicted NES motifs suggesting it might serve as karyopherin cargo substrate for bidirectional transport through NPCs (Fig. 1B).17 Since mos7-1 was isolated as a genetic suppressor of snc1 and MOS7 is required for the expression of all known snc1 phenotypes, we investigated whether the constitutively active immune receptor snc1 shows a MOS7-dependent cellular distribution. A functional, snc1-GFP fusion protein expressed under the native SNC1 promoter localizes to the cytoplasm and the nucleus of transgenic plants.17 While total amounts of snc1-GFP in mos7-1 and wild type are similar, the ratio of cytoplasmic to nuclear localized snc1-GFP is significantly increased in mos7-1, suggesting that resistance conferred by this auto-activated R protein depends on attaining a sufficient concentration in the nucleus (Fig. 1B). In support of this hypothesis, enhancing nuclear export of snc1-GFP through translational fusion to an additional NES reduces its autoimmunity, a similar effect as observed in mos7-1.17 In summary, these results suggest a function of MOS7 in promoting the nuclear retention and thus accumulation of auto-active snc1 as an important step in defense activation.
We previously proposed a model in which nuclear activation of some nucleocytoplasmic NB-LRR receptors is a consequence of enhanced nuclear import and/or decreased nuclear export of the activated R protein, resulting in efficient defense gene expression after the nuclear R protein concentration reaches a certain threshold. Effector-mediated activation could either expose an obscured NLS via a conformational change or recruit additional interacting proteins to alter nuclear shuttling or retention. This model is based on our finding from the mos screen that defects in the NPC or nucleocytoplasmic transport machinery alter resistance responses, and upsetting the ratio of cytoplasmic-to nuclear-localized R protein pools or their downstream regulators causes an immune deficiency in mos7-1 (Cheng, et al. 2009; Palma, et al. 2008; Wiermer, et al. 2007).17,31,32 Based on previous work showing direct association between the CC-NB-LRR R protein MLA and plant-specific WRKY transcription factors, which suggests a direct link between certain R protein activation and transcriptional reprogramming,33 we speculate that snc1 may be able to target components of the transcriptional machinery inside the nucleus. Whether nuclear accumulation of wild type SNC1 is required for defense activation and what kind of proteins snc1/SNC1 interacts with inside nuclei awaits future investigation.
One question that remains is how specific MOS7 functions are to plant immune responses, since perturbations in a conserved housekeeping machinery such as the NPC would be expected to negatively impact a number of signaling pathways. The role of MOS7 in multiple branches of plant immunity, such as basal, systemic acquired and NB-LRR R protein-triggered resistance, suggests it may be more broadly required for modulating the transit of additional, yet unknown, factors during innate immunity. A role for MOS7 as a rather general attenuator of XPO1/CRM1-mediated export is supported by the fact that a chimeric nucleocytoplasmic shuttle protein28 shows enhanced NES-dependent nuclear export in mos7-1 compared with wild type after transient transfection into Arabidopsis mesophyll protoplasts.17 Moreover, null mutations in MOS7 are lethal, consistent with a lethality phenotype of null mbo/nup88 mutations in Drosophila,13 indicating that wild type MOS7/Mbo is necessary for cargo-bound XPOI/CRM1-translocation of unknown proteins required for proper growth and development.
On the other hand, the phenotype of mos7-1 mutants appears to be surprisingly selective. mos7-1 plants do not show obvious pleiotropic defects in development, salt tolerance or plant hormone responses.17 Also, the subcellular localization and cellular abundance of several nuclear, nucleocytoplasmic or cytoplasmic proteins is unaffected in mos7-1, suggesting that the defects of mos7-1 in nuclear retention of snc1, NPR1 and EDS1 are rather specific.17 It is possible that the four amino acid deletion in mos7-1 causes a slight change in protein conformation so that it affects the nuclear pore structure in a way that most acutely influences export of immunity-related nucleocyplasmic regulators. One intriguing piece of data we recently obtained indicates that overexpression not only of wild type MOS7, but also mos7-1, can revert snc1 mos7-1 back to snc1 phenotypes (Cheng Y and Li X, unpublished). This suggests a more complex model in which MOS7 may also contribute to the regulation of numbers of pores on the nuclear envelope. Thus, overexpression of mos7-1 may help to revert the defects of mos7-1 by increasing the frequency of pores on the envelope. At present this idea is purely speculative and future structural research on plant Nups with cell biology tools will enable us to test these hypotheses more directly.
It is conceivable that MOS7 modulates the transit of certain XPO1/CRM1-cargo complexes upon activation of immune responses. This suggests that nuclear retention either depends on the conformation of a given cargo-NTR complex which determines its binding sites within and thus transport route through the NPC or that retention depends on additional pathway-specific signals that might alter the properties of MOS7 to control NTR-cargo translocation. Such mechanisms dovetail with recent evidence that NTR conformation can be influenced by its specific cargo substrate34 and that nuclear transport rates for a given cargo are regulated by altering Nup properties through posttranslational modifications.35 Multiple signaling pathways through the NPC could thus be controlled by distinct Nups and functionally independent routes through the NPC might be directed by providing different binding sites for cargo-bound NTRs within the NPC.
In conclusion, the studies on Nup88 in Drosophila, mammals and Arabidopsis support a conserved function of Nup88 in modulating host immunity (Fig. 1). While the overall mechanisms of nucleocytoplasmic transport and the structural organization of the NPC are conserved among eukaryotes, some components of the NPC and transport mechanisms seem to be unique to plants.36,37 Research on Nups in plant species is an emerging field. The genetic resources available in Arabidopsis provide huge potential for revealing the biological functions of plant Nups, many of which await characterization.
Acknowledgements
We thank Jacqueline Monaghan for careful reading of the manuscript. We are grateful for a postdoctoral fellowship from the Alexander von Humboldt Foundation to M.W., Le Fonds Québécois de la Recherche sur la Nature et les Technologies to H.G., a Natural Sciences and Engineering Research Council of Canada (NSERC) post-graduate scholarship to Y.T.C., and funds from NSERC, the Michael Smith Laboratories (MSL) and the Canadian Foundation for Innovation (CFI) to X.L. J.E.P. acknowledges support of DFG SFB 670 and an IMPRS fellowship to A.V.G.
Extra View to: Cheng YT, Germain H, Wiermer M, Bi D, García AV, Wirthmueller L, et al. Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell. 2009;21:2503–2516. doi: 10.1105/tpc.108.064519.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/12109
References
- 1.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Lukasik E, Takken FL. STANDing strong, resistance proteins instigators of plant defence. Curr Opin Plant Biol. 2009;12:427–436. doi: 10.1016/j.pbi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Goritschnig S, Dong XN, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15:2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Li X. A Putative Nucleoporin 96 Is Required for Both Basal Defense and Constitutive Resistance Responses Mediated by suppressor of npr1-1, constitutive 1. Plant Cell. 2005;17:1306–1316. doi: 10.1105/tpc.104.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Clarke JD, Zhang YL, Dong XN. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe Interact. 2001;14:1131–1139. doi: 10.1094/MPMI.2001.14.10.1131. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Ma X. Role of Nod2 in the development of Crohn's disease. Microbes Infect. 2009;11:912–918. doi: 10.1016/j.micinf.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaghan J, Germain H, Weihmann T, Li X. Dissecting plant defence signal transduction—modifiers of snc1 in Arabidopsis. Can J Plant Pathol. 2010;32:35–42. [Google Scholar]
- 8.Faria AM, Levay A, Wang Y, Kamphorst AO, Rosa ML, Nussenzveig DR, et al. The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity. 2006;24:295–304. doi: 10.1016/j.immuni.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Palma K, Zhang Y, Li X. An Importin α Homolog, MOS6, Plays an Important Role in Plant Innate Immunity. Curr Biol. 2005;15:1129–1135. doi: 10.1016/j.cub.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NFκB/IκBα complexes. Proc Natl Acad Sci USA. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malek S, Chen Y, Huxford T, Ghosh G. IκBβ, but Not IκBα, Functions as a Classical Cytoplasmic Inhibitor of NFκB Dimers by Masking both NFκB Nuclear Localization Sequences in Resting Cells. J Biol Chem. 2001;276:45225–45235. doi: 10.1074/jbc.M105865200. [DOI] [PubMed] [Google Scholar]
- 12.Hayden MS, Ghosh S. Shared principles in NFκB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Uv AE, Roth P, Xylourgidis N, Wickberg A, Cantera R, Samakovlis C. members only encodes a Drosophila nucleoporin required for Rel protein import and immune response activation. Genes Dev. 2000;14:1945–1957. [PMC free article] [PubMed] [Google Scholar]
- 14.Roth P, Xylourgidis N, Sabri N, Uv A, Fornerod M, Samakovlis C. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol. 2003;163:701–706. doi: 10.1083/jcb.200304046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C. The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFκB activation in Drosophila. J Cell Sci. 2006;119:4409–4419. doi: 10.1242/jcs.03201. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi N, van Kilsdonk JW, Ostendorf B, Smeets R, Bruggeman SW, Alonso A, et al. Tumor marker nucleoporin 88 kDa regulates nucleocytoplasmic transport of NFκB. Biochem Biophys Res Commun. 2008;374:424–430. doi: 10.1016/j.bbrc.2008.06.128. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Germain H, Wiermer M, Bi D, García AV, Wirthmueller L, et al. Nuclear Pore Complex Component MOS7/Nup88 Is Required for Innate Immunity and Nuclear Accumulation of Defense Regulators in Arabidopsis. Plant Cell. 2009;21:2503–2516. doi: 10.1105/tpc.108.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 19.Mou Z, Fan WH, Dong XN. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 20.Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, et al. Plant Immunity Requires Conformational Charges of NPR1 via S-Nitrosylation and Thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Després C, DeLong C, Glaze S, Liu E, Fobert PR. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell. 2000;12:279–290. [PMC free article] [PubMed] [Google Scholar]
- 22.Rochon A, Boyle P, Wignes T, Fobert PR, Després C. The Coactivator Function of Arabidopsis NPR1 Requires the Core of Its BTB/POZ Domain and the Oxidation of C-Terminal Cysteines. Plant Cell. 2006;18:3670–3685. doi: 10.1105/tpc.106.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Fan W, Kinkema M, Li X, Dong X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci USA. 1999;96:6523–6528. doi: 10.1073/pnas.96.11.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X. Proteasome-Mediated Turnover of the Transcription Coactivator NPR1 Plays Dual Roles in Regulating Plant Immunity. Cell. 2009;137:860–872. doi: 10.1016/j.cell.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:10306–10311. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, et al. Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell. 2005;17:2601–2613. doi: 10.1105/tpc.105.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García AV, Blanvillain-Baufumé S, Huibers RP, Wiermer M, Li G, Rietz S, et al. Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. Plos Pathogens. 2010 doi: 10.1371/journal.ppat.1000970. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haasen D, Kohler C, Neuhaus G, Merkle T. Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant Journal. 1999;20:695–705. doi: 10.1046/j.1365-313x.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 29.Burch-Smith TM, Schiff M, Caplan JL, Tsao J, Czymmek K, Dinesh-Kumar SP. A Novel Role for the TIR Domain in Association with Pathogen-Derived Elicitors. PLoS Biol. 2007;5:68. doi: 10.1371/journal.pbio.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirthmueller L, Zhang Y, Jones JD, Parker JE. Nuclear Accumulation of the Arabidopsis Immune Receptor RPS4 Is Necessary for Triggering EDS1-Dependent Defense. Curr Biol. 2007;17:2023–2029. doi: 10.1016/j.cub.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Palma K, Wiermer M, Li X. Marshalling the troops: Intracellular dynamics in plant pathogen defense. Annual Plant Reviews. 2008;34:177–219. [Google Scholar]
- 32.Wiermer M, Palma K, Zhang Y, Li X. Should I stay or should I go? Nucleocytoplasmic trafficking in plant innate immunity. Cell Microbiol. 2007;9:1880–1890. doi: 10.1111/j.1462-5822.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen QH, Saijo Y, Mauch S, Biskup C, Bieri S, Keller B, et al. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 2007;315:1098–1103. doi: 10.1126/science.1136372. [DOI] [PubMed] [Google Scholar]
- 34.Wohlwend D, Strasser A, Dickmanns A, Ficner R. Structural basis for RanGTP independent entry of spliceosomal U snRNPs into the nucleus. J Mol Biol. 2007;374:1129–1138. doi: 10.1016/j.jmb.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 35.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, et al. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16:1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- 36.Lu Q, Tang X, Tian G, Wang F, Liu K, Nguyen V, et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. Plant. 2010;61:259–270. doi: 10.1111/j.1365-313X.2009.04048.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Q, Brkljacic J, Meier I. Two Distinct Interacting Classes of Nuclear Envelope-Associated Coiled-Coil Proteins Are Required for the Tissue-Specific Nuclear Envelope Targeting of Arabidopsis RanGAP. Plant Cell. 2008;20:1639–1651. doi: 10.1105/tpc.108.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]