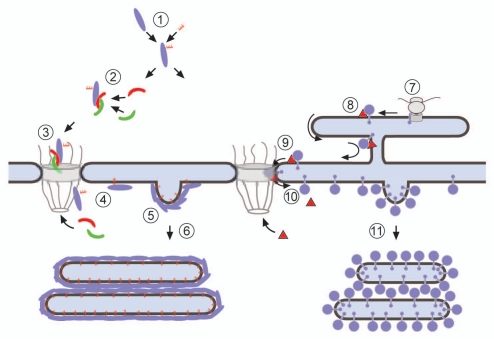
Figure 6.
A model for the formation of intranuclear membrane arrays by expression of lipidated nuclear proteins (left) or by expression of integral INM proteins (right). (1) Newly synthesized proteins are lipidated in the cytosol, (2) they bind to importin α/β (red and green, respectively), (3) the cargo/importin α/β complex passes the central channel of the NPC. (4) At the nucleoplasmic side of the NPC the complex is disassembled by the interaction with RanGTP (RanGTP not shown). (5) Binding of the lipidated proteins to the INM triggers membrane proliferation and vesicle budding of the INM. (6) Through interaction between proteins on the budded vesicle surfaces vesicles zipper up into flattened and stacked arrays. (7) Integral INM proteins that are co- or post-translationally inserted into the ER membrane, (8) are recognized by importin-α-16. The complex reaches the NPC membrane by lateral diffusion. (9) Passage via the membrane of the NPC is mediated by importin-α-16, (10) the import complex disassembles by the interaction with Nup50 (Nup50 not shown). (11) Massive accumulation of the integral membrane protein at the INM will induce membrane proliferation and vesicle budding in a similar way as shown for the lipidated proteins. The right part of the scheme has been modified after Rexach.57