Abstract
Endoplasmic reticulum and nuclear envelope rearrangements after mitosis are often studied in the reconstitution system based on Xenopus egg extract. In our recent work we partially replaced the membrane vesicles in the reconstitution mix with protein-free liposomes to explore the relative contributions of cytosolic and transmembrane proteins. Here we discuss our finding that cytosolic proteins mediate fusion between membranes lacking functional transmembrane proteins and the role of membrane fusion in endoplasmic reticulum and nuclear envelope reorganization. Cytosol-dependent liposome fusion has allowed us to restore, without adding transmembrane nucleoporins, functionality of nuclear pores, their spatial distribution and chromatin decondensation in nuclei formed at insufficient amounts of membrane material and characterized by only partial decondensation of chromatin and lack of nuclear transport. Both the mechanisms and the biological implications of the discovered coupling between spatial distribution of nuclear pores, chromatin decondensation and nuclear transport are discussed.
Key words: nuclear envelope, endoplasmic reticulum, nuclear pore complex, membrane fusion, Xenopus egg extract, liposome
Introduction
Cell division is associated with changes in the morphology and function of intracellular membrane structures, including the endocytic system, the Golgi apparatus and the endoplasmic reticulum (ER). In species with an open mitosis, the nuclear envelope (NE) breaks up early in mitosis and then starts to reassemble in anaphase. NE reassembly involves formation of a double membrane around segregated chromosomes, insertion of multiprotein nuclear pore complexes (NPCs) responsible for selective nucleocytoplasmic transport, followed by further NE expansion and chromatin decondensation. Reassembly of the ER/NE system has been reconstituted in vitro using fractionated Xenopus leavis egg extract and sperm chromatin and requires both transmembrane and cytosolic proteins.1,2 Our work on further simplifying this experimental system by using liposomes has, surprisingly, demonstrated that cytosolic proteins are sufficient to mediate fusion between membranes lacking functional transmembrane proteins and revealed an unexpected dependence of nuclear transport on NE expansion.3 In this short review we discuss how cytosol-dependent fusion may fit into the current models of ER and NE reorganization; and, more generally, what is the place of membrane fusion in this reorganization and whether proteins lacking transmembrane domains can merge membranes. We also discuss the biological relevance of interplay between the growth of NE membrane area, NPC distribution and chromatin decondensation.
Membrane Fusion in ER Dynamics
It has long been thought that disassembly of NE proceeds by its fragmentation into vesicles and at the end of mitosis these vesicles fuse on the surface of segregated chromosomes. This model has been mainly supported by data from an in vitro reconstitution system based on fractionated Xenopus leavis egg extract. In this in vitro system, ER fragments fuse with each other in the presence of interphase cytosol and sperm chromatin to form both an ER-like network and round-shaped nuclei pierced with NPCs and capable of active nuclear transport, DNA replication and NE breakdown.2 However, studies in living cells suggest that at the onset of mitosis, the NE retracts into the ER, which remains continuous throughout mitosis (reviewed in ref. 4). NE reassembly at the end of mitosis proceeds through the merging of the tubular ER network on the chromatin surface. The tips of ER tubules bind to the chromatin, and then flatten and expand at the chromatin surface to yield a sealed nuclear membrane. This process, in contrast to fusion between membrane vesicles in ER reconstitution, is not sensitive to the non-hydrolysable nucleotide triphosphate analogues ATPγS and GTPγS, suggesting that NE assembly relies only on intrinsic dynamics and the integrity of the ER network rather than vesicle fusion.4,5 This reasoning is based on the assumption that ATP- and GTP-hydrolysis are involved in the actual fusion event. This is not a trivial assumption, since GTP- and ATP-hydrolysis dependent stages may follow rather than precede membrane rearrangements,6 as in the case of SNARE-dependent fusion, where ATP hydrolysis “recharges” SNARE machinery after fusion.7
The ER is a very dynamic membrane structure that undergoes significant rearrangements during interphase, such as the branching of the ER tubules, the sliding of the ER junctions along the tubules, the closure of polygonal rings of the ER network and the fusion of the tubules to reform ER rings.8 Presumably, these specific membrane rearrangements are regulated and linked to ER functional activity. ER structure is largely maintained by proteins of the DP1/Yop1p and reticulon families, which shape the ER membrane into tubules9,10 and by atlastin GTPases, which implement fusion between tubules.11,12 Loss of atlastin function causes ER fragmentation, indicating that fusion is indispensable for maintaining ER integrity.12 Although the structure of mitotic ER is still under debate,13,14 it is clear that ER/NE membranes undergo significant morphological changes during the cell cycle. A recent study14 shows that during mitosis, ER/NE membranes are organized into extended cisternae with only a small fraction of tubules. The cell cycle-specific transition from cisternae-like structures to the branched tubular network of the interphase ER involves formation of polygonal ER rings. Topologically, this transformation is similar to increasing the number of holes in a pretzel and cannot be carried out without breaking and fusing the membranes. Indeed, imaging of living C. elegans embryos has suggested a massive fusion between ER membranes during this transition.15 While the specific role of fusion between ER membranes in maintenance and rearrangement of the ER network throughout the cell cycle remains to be clarified, recent studies have provided important insights into the mechanisms of these fusion processes.
Transmembrane and Cytosolic Factors Involved in ER Fusion
Homotypic fusion of ER membranes during in vitro formation of the ER network and its maintenance in a living cell requires transmembrane protein dynamin-like GTPase atlastins.11,12 Fusion between ER vesicles and between proteoliposomes with reconstituted atlastin involves GTP-hydrolysis and formation of trans-oligomeric complexes by atlastin present in both fusing membranes. This organization of fusion machinery (with the same protein fusogens on both membranes) reminds the homotypic fusion machinery suggested for mitochondrial fusion16 and for cell fusion during development of C. elegans.17 In contrast, in viral fusion protein fusogens are present only in one of the membranes and in SNARE-dependent intracellular fusion opposing membranes carry different protein fusogens.
While transmembrane proteins atlastins can drive ER fusion on their own, we have recently reported that Xenopus egg interphase cytosol contains additional fusogenic protein factors involved in the ER/NE reconstitution.3,18 We have shown that membrane vesicles, rendered fusion-incompetent by trypsin or NEM treatments, and liposomes fuse in the presence of the interphase cytosol, as established using a lipid mixing assay. Liposomes, incubated in cytosol and purified on a sucrose gradient, carry tightly-attached cytosolic proteins and fuse with each other. Importantly, liposomes formed from phosphatidylcholine that were used in these experiments do not support spontaneous fusion.19 Like fusion between native membrane vesicles, cytosol-mediated fusion of membranes lacking functional transmembrane proteins depends on GTP-hydrolysis and the cell cycle stage of the cytosol preparation.
Not surprisingly, fusion between liposomes yields neither the ER network nor functional nuclei. However, in the presence of native membrane vesicles, liposomes and fusion-incompetent membrane vesicles do participate in the ER and NE assembly and partially replace the native vesicles.3 Lowering the concentration of native membrane vesicles decreases the size of assembled nuclei.20 We found that these small nuclei, while completely enclosed by envelope, have only partially decondensed chromatin and support neither nuclear transport nor DNA replication. Addition of liposomes transforms these undeveloped nuclei into nuclei with rescued nuclear transport, DNA replication, chromatin decondensation and partially recovered size. We demonstrated using different approaches that the rescue of nuclei formation involves cytosol-dependent fusion of liposomes with native membrane vesicles and that up to two thirds of membrane material in those nuclei originated from liposomes.
Fusion of liposomes with native membrane vesicles (and between liposomes) was not supported by mitotic cytosol,3 suggesting that cytosol-mediated fusion is cell cycle regulated. It also depends on the presence of GTP and ATP and is inhibited by an excess of αSNAP, the NSF-adapter that blocks SNAREs indicating the possible involvement of SNARE proteins. SNARE machinery has been also implicated in ER and NE reassembly in ref. 21, where antibodies against NSF, NSF depletion and an excess of exogenous αSNAP were found to inhibit fusion between membrane vesicles in the Xenopus extract reconstitution system. Most SNAREs are transmembrane proteins but some of the SNAREs lack transmembrane domains and shuttle between cytosolic and membrane-bound forms.22,23 Taken together, our data suggest that interphase cytosol contains fusogenic proteins that can be tightly associated with membranes but do not have transmembrane domains and can replace impaired or, in the case of liposomes, missing fusogenic proteins with intact copies of the same proteins.
Can Proteins Lacking Transmembrane Domain Mediate Fusion?
The best characterized viral, intracellular and developmental fusogens are transmembrane proteins. Moreover, for some of these proteins deletion or modifications of their transmembrane domain is shown to inhibit fusion (reviewed in ref. 24). However, there are also reports suggesting that a transmembrane domain is not a pre-requisite for a protein fusogen. For some proteins clearly involved in fusion (e.g., influenza hemagglutinin and synaptotagmin), large polypeptide fragments lacking transmembrane domains have been reported to initiate fusion.25,26 Moreover, nuclear membrane fusion in yeasts27,28 and fusion in autophagy29 have been proposed to be mediated by peripheral proteins. Another peripheral protein, annexin, has been implicated in the fusion stage of membrane repair30 partitions between the cytosol and nuclear envelope and this redistribution can be cell cycle dependent.31,32
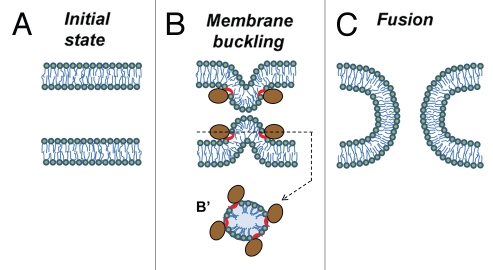
Accumulating evidence indicates that fusion proteins drive lipid rearrangements by generating the membrane elastic stresses that are released by fusion.33,34 For instance, proteins may bring two bilayers into immediate contact by buckling one or both of them (Fig. 1). The protein-depleted spherical tops of the bulges have very high curvature and, as a result, are primed for fusion. Clearly, peripheral proteins are quite capable of bending membranes.35 Indeed, peripheral proteins such as dynamin, BAR domain proteins and small G-proteins do generate elastic stresses that drive membrane fission; a process that, similarly to fusion, involves the local breaking of continuity in membranes followed by their reassembly in a new way.
Figure 1.
Mechanism by which peripheral proteins might mediate fusion. (A) Opposing lipid bilayers of biological membranes before fusion. Lipid molecules are depicted as rings (polar groups) with two tails (acyl chains). (B) Fusion proteins (brown-colored shapes with red-colored amphiphilic regions) encircle future fusion site. Shallow insertion of amphiphilic protein regions spreads polar heads of the surrounding lipids. Buckling of the membrane(s) brings membrane bilayers into immediate contact and generates bending stresses at the bulge top(s). (B′) Cross section view from the top. (C) Elastic stresses are released by membrane fusion.
Based on these considerations, we conclude that transmembrane domain is not a prerequisite for the potential fusogen. Therefore, peripheral membrane proteins shuttling in a regulated manner between cytosol and intracellular membranes can serve as fusogens. It is natural to suggest that diversity of fusion reactions in ER/NE reassembly requires more than one type of fusion machinery including atlastins, SNAREs and yet unidentified cytosolic proteins. An unexpected tolerance of nucleus assembly to concentrations of NE- and ER-specific transmembrane proteins3 emphasizes relatively promiscuous character of the cytosol-dependent fusion. One may suggest that utilization of transmembrane vs. cytosolic fusion proteins may depend on relative importance of specificity vs. breadth for a particular kind of fusion reaction.
Interplay between NE Expansion, NPC Formation and Chromatin Decondensation
Our work has revealed an unexpected link between lateral distribution of NPCs, nuclear transport and chromatin decondensation at early stages of NE assembly.3 We restricted NE growth and chromatin decondensation by removing yet-unbound membrane vesicles, or in an alternative approach, by lowering the initial concentrations of membrane vesicles in Xenopus nucleus reconstitution mix. Resulting undeveloped nuclei were characterized by membrane enclosure of only partially decondensed chromatin, smaller size and absence of nuclear transport. Surprisingly, these nuclei have the same average surface density of NPCs as control ones, as measured with Ab414 antibody. This antibody reacts with a number of “late” nucleoporins36 and, thus, is commonly used to identify assembled NPCs. Since even condensed chromatin domains are readily accessible to large macromolecules,37 it is unlikely that partial decondensation of chromatin within undeveloped nuclei restricts macromolecule entry into the nuclei by steric hindrance. This raises the question of why NPCs in undeveloped nuclei are already recognizable by the Ab414 antibody but yet functionally-incompetent. In spite of similar average density of NPCs, their distribution along NE in control and undeveloped nuclei was strikingly different: even for control nuclei and clustered for undeveloped ones.
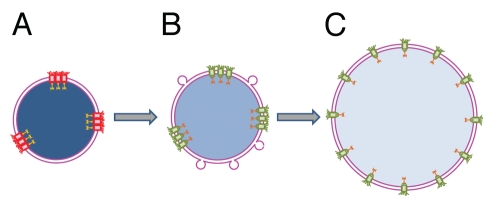
As mentioned above, fusion of liposomes into undeveloped nuclei in the presence of cytosol yields larger nuclei with fully decondensed chromatin and active nuclear transport. This transformation correlates with the restoration of the even distribution of NPCs and a modest increase in their number. How can the amount of membrane material available for nucleus assembly affect NPC distribution? At early stages of NE assembly, sites of NPC formation are marked by binding of ELYS protein to AT-rich regions of chromatin.38,39 We suggest that a shortage of membrane area in undeveloped nuclei mechanically restricts chromatin decondensation and uneven distribution of NPC seeding sites at the surface of partially decondensed chromatin results in uneven distribution of NPC (Fig. 2).
Figure 2.
Proposed mechanism of nuclear transport rescue by cytosol-dependent liposome fusion. (A) Shortage of membrane material mechanically restricts chromatin decondensation leading to formation of small undeveloped nuclei enclosed by double membrane NE. Uneven distribution of fully assembled NPCs following uneven distribution of NPC anchoring sites at lamina and chromatin results in functional incompetence of closely spaced pores. (B) Addition of membrane material by cytosol-dependent fusion of liposomes allows additional chromatin decondensation, increases the distances between NPC anchors, and thus, the pores and restores active nuclear transport. (C) Further NE growth is accompanied by full chromatin decondensation and restoration of an even NPC distribution. Functional and non-functional NPCs are shown in green and red, respectively. Degree of chromatin decondensed is depicted by color saturation with less saturated blue color representing more decondensed chromatin.
Since the external diameter of a nuclear pore in the Xenopus egg nucleus is ∼ 0.12–0.15 µm and there are ∼50 NPCs/µm2,40 the density of NPCs at the surface of Xenopus egg nuclei is very high and 60 to 80% of the total surface area of NE is under NPCs. It is likely that clustered NPCs in undeveloped nuclei, which are placed even closer than pores evenly distributed in control nuclei,- become dysfunctional. Fusion of liposomes provides additional membrane area and stimulates further chromatin remodeling and spreading. This leads to an increase in the distances between clustered NPCs, thus restoring NPC distribution and functionality. These events are followed by full DNA decondensation, assembly of additional NPCs and nuclear growth. We suppose that in the case of unrestricted membrane availability, NPCs assembly goes along with an increase in the NE area per pore and chromatin de-condensation, yielding an even pore distribution.
Concluding Remarks
To place our recent findings that cytosolic proteins lacking transmembrane domains fuse membranes and that cytosol-mediated fusion can be involved in ER and NE reassembly3,18 into the context of ER and NE dynamics in living cells, a number of outstanding questions have to be addressed. To start with, the identity of the cytosolic protein fusogens present in Xenopus egg extract remains to be established. What is the molecular mechanism of fusion mediated by these fusogens? How is the job of fusing intracellular membranes in ER maintenance and in post-mitotic rearrangements of ER and NE distributed between transmembrane and cytosolic fusogens?
Regardless of the mechanism of cytosol-dependent fusion and its role in vivo, this fusion allowed us to reveal the existence of a feedback that couples NE growth and chromatin decondensation with the spatial distribution of NPCs and nuclear transport. It is tempting to suggest that NPC clustering observed in some pathological conditions41 leads to local inhibition of nuclear transport and disruption of local chromatin organization and gene regulation.
Acknowledgements
We thank Sergei Pourmal for critical reading of the manuscript. This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (L.V.C.).
Abbreviations
- ER
endoplasmic reticulum
- NE
nuclear envelope
- NPC
nuclear pore complex
- Nup
nucleoporin
Extra View to: Rafikova ER, Melikov K, Ramos C, Dye L, Chernomordik LV. Transmembrane protein-free membranes fuse into xenopus nuclear envelope and promote assembly of functional pores. J Biol Chem. 2009;284:29847–29859. doi: 10.1074/jbc.M109.044453.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/13514
References
- 1.Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan MA, Mills AD, Sleeman AM, Laskey RA, Blow JJ. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988;106:1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafikova ER, Melikov K, Ramos C, Dye L, Chernomordik LV. Transmembrane protein-free membranes fuse into xenopus nuclear envelope and promote assembly of functional pores. J Biol Chem. 2009;284:29847–29859. doi: 10.1074/jbc.M109.044453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetzer MW, Wente SR. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev Cell. 2009;17:606–616. doi: 10.1016/j.devcel.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]
- 6.Larijani B, Poccia DL. Nuclear envelope formation: mind the gaps. Annu Rev Biophys. 2009;38:107–124. doi: 10.1146/annurev.biophys.050708.133625. [DOI] [PubMed] [Google Scholar]
- 7.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Chen LB. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- 9.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, et al. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–561. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–983. doi: 10.1038/nature08280. [DOI] [PubMed] [Google Scholar]
- 13.Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179:895–909. doi: 10.1083/jcb.200705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Ladinsky MS, Kirchhausen T. Cisternal organization of the endoplasmic reticulum during mitosis. Mol Biol Cell. 2009;20:3471–3480. doi: 10.1091/mbc.E09-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poteryaev D, Squirrell JM, Campbell JM, White JG, Spang A. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol Biol Cell. 2005;16:2139–2153. doi: 10.1091/mbc.E04-08-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 17.Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, et al. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramos C, Rafikova ER, Melikov K, Chernomordik LV. Transmembrane proteins are not required for early stages of nuclear envelope assembly. Biochem J. 2006;400:393–400. doi: 10.1042/BJ20061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 20.Wilson KL, Newport J. A trypsin-sensitive receptor on membrane vesicles is required for nuclear envelope formation in vitro. J Cell Biol. 1988;107:57–68. doi: 10.1083/jcb.107.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- 22.Vogel K, Cabaniols JP, Roche PA. Targeting of SNAP-25 to membranes is mediated by its association with the target SNARE syntaxin. J Biol Chem. 2000;275:2959–2965. doi: 10.1074/jbc.275.4.2959. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Linder ME. Differential palmitoylation of the endosomal SNAREs syntaxin 7 and syntaxin 8. J Lipid Res. 2009;50:398–404. doi: 10.1194/jlr.M800360-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langosch D, Hofmann M, Ungermann C. The role of transmembrane domains in membrane fusion. Cell Mol Life Sci. 2007;64:850–864. doi: 10.1007/s00018-007-6439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leikina E, LeDuc DL, Macosko JC, Epand R, Shin YK, Chernomordik LV. The 1-127 HA2 construct of influenza virus hemagglutinin induces cell-cell hemifusion. Biochemistry. 2001;40:8378–8386. doi: 10.1021/bi010466+. [DOI] [PubMed] [Google Scholar]
- 26.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 27.Melloy P, Shen S, White E, Rose MD. Distinct roles for key karyogamy proteins during yeast nuclear fusion. Mol Biol Cell. 2009;20:3773–3782. doi: 10.1091/mbc.E09-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen S, Tobery CE, Rose MD. Prm3p is a pheromone-induced peripheral nuclear envelope protein required for yeast nuclear fusion. Mol Biol Cell. 2009;20:2438–2450. doi: 10.1091/mbc.E08-10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 30.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 31.Tomas A, Moss SE. Calcium- and cell cycle-dependent association of annexin 11 with the nuclear envelope. J Biol Chem. 2003;278:20210–20216. doi: 10.1074/jbc.M212669200. [DOI] [PubMed] [Google Scholar]
- 32.Skrahina T, Piljic A, Schultz C. Heterogeneity and timing of translocation and membrane-mediated assembly of different annexins. Exp Cell Res. 2008;314:1039–1047. doi: 10.1016/j.yexcr.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon HT, Kozlov MM, Martens S. Membrane curvature in synaptic vesicle fusion and beyond. Cell. 2010;140:601–605. doi: 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 36.Davis LI, Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci USA. 1987;84:7552–7556. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verschure PJ, van der Kraan I, Manders EM, Hoogstraten D, Houtsmuller AB, van Driel R. Condensed chromatin domains in the mammalian nucleus are accessible to large macromolecules. EMBO Rep. 2003;4:861–866. doi: 10.1038/sj.embor.embor922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasala BA, Ramos C, Harel A, Forbes DJ. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell. 2008;19:3982–3996. doi: 10.1091/mbc.E08-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rotem A, Gruber R, Shorer H, Shaulov L, Klein E, Harel A. Importin beta regulates the seeding of chromatin with initiation sites for nuclear pore assembly. Mol Biol Cell. 2009;20:4031–4042. doi: 10.1091/mbc.E09-02-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maul GG, Price JW, Lieberman MW. Formation and distribution of nuclear pore complexes in interphase. J Cell Biol. 1971;51:405–418. doi: 10.1083/jcb.51.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]