Abstract
The Current Opioid Misuse Measure (COMM), a self-report assessment of past-month aberrant medication-related behaviors, has been validated in specialty pain management patients. The performance characteristics of the COMM were evaluated in primary care (PC) patients with chronic pain. It was hypothesized that the COMM can identify patients with prescription drug use disorder (PDD). English-speaking adults awaiting PC visits at an urban, safety-net hospital, who had chronic pain and had received any opioid analgesic prescription in the past year were administered the COMM. The Composite International Diagnostic Interview served as the “gold-standard”, using DSM-IV criteria for PDD and other substance use disorders (SUDs). A receiver operating characteristics (ROC) curve demonstrated the COMM’s diagnostic test characteristics. Of the 238 participants, 27 (11%) met DSM-IV PDD criteria, while 17 (7%) had other SUDs, and 194 (82%) had no disorder. The mean COMM score was higher in those with PDD than among all others (i.e., those with other SUDs or no disorder, mean 20.4 [SD 10.8] vs. 8.4 [SD 7.5], p<0.0001). A COMM score of ≥13 had a sensitivity of 77% and a specificity of 77% for identifying patients with PDD. The area under the ROC curve was 0.84. For chronic pain patients prescribed opioids, the development of PDD is an undesirable complication. Among PC patients with chronic pain prescribed prescription opioids, the COMM is a promising tool for identifying those with PDD.
Introduction
Multiple studies have shown dramatic increases in prescriptions for opioid analgesics for chronic non-cancer chronic pain in the United States.[12,25,31]Although more Americans use marijuana, the number of first time abusers of prescription pain medications has recently exceeded the number of new marijuana users.[1] With the initial diagnosis and management of chronic, non-cancer pain falling largely under the domain of the primary care physician (PCP), many of these doctors report they are not adequately trained to recognize and manage patients at high-risk for, or suffering from, prescription drug use disorder (PDD).[6] Experts in addiction and pain debate what constitutes PDD in a chronic pain population.[15,30] Although there is some consensus regarding clinical features that patients with PDD typically exhibit, no single “gold-standard” exists for diagnosing PDD in primary care (PC) patients with chronic pain. [2,4,5,9,29]
Current practice guidelines recommend utilizing the Current Opioid Misuse Measure (COMM) to assess patients that are prescribed opioid therapy.[7] Developed by experts in pain and addiction, the COMM is a patient self-report assessment of past-month aberrant medication-related behaviors, defined as behaviors that are concerning for addiction or taking a medication in a way other than how it is prescribed. [5,27] Aberrant medication-related behaviors may include PDD as well as unintentional misuse, purposeful diversion, or addiction to substances other than pain medication. The COMM validation study was conducted with patients treated in specialty pain management clinics, and a score of nine or greater was determined to be suggestive of prior thirty-day prescription opioid misuse.
The diagnostic capabilities of the COMM have not been evaluated among PC patient populations. Diagnostic tests may perform differently when utilized in clinical settings other than those in which they are validated. The COMM may serve as a practical means of monitoring PC patients treated with opioid therapy for the development of PDD, however, it remains to be determined whether this group of patients may be accurately assessed for PDD with this tool. Using a DSM-IV diagnosis of PDD as a “gold-standard”, the diagnostic performance characteristics of the COMM were evaluated among a sample of PC patients with chronic pain who had received prescription opioids in the past year. The research team chose a broad sample of those at risk for PDD because the clinical challenges are not limited to those using daily current opioids. It was hypothesized that the COMM can identify participants with PDD and can distinguish them from all others. Secondly, as an exploratory aim, it was hypothesized that the COMM can differentiate those with PDD, some of whom may have a comorbid illicit drug disorder and/or comorbid past year alcohol dependence, from participants with a lone other substance use disorder (SUD) (i.e. lone illicit drug disorder and/or past year alcohol dependence), a prior disorder (PDD or SUD) or no disorder. A receiver operating characteristics (ROC) curve was constructed in order to evaluate whether the established COMM threshold score of nine is suggestive of PDD in this patient sample.
Methods
Study Design
This was a cross-sectional study of PC patients with chronic pain, defined as lasting for three months or longer, completed at the PC clinics of an urban, safety-net, academic medical center.[3,18] The study consisted of two parts, an interview with a trained member of the research team and a subsequent electronic medical record review for abstraction of prescription opioid data to meet entry criteria.
Setting
Patients waiting for scheduled PC visits were recruited by trained research interviewers. Interviewers were physicians, master-level professionals, college graduates, and college students who underwent 60+ hours of interview training. All participants were approached in the waiting rooms of an academic, urban, safety-net hospital primary care practice.[18] Safety-net hospitals in the United States care for poor and vulnerable populations who may be uninsured or underinsured, and includes disproportionate numbers of underrepresented minorities. [5]
Recruitment and Enrollment
Between February, 2005 and August, 2006, 2,194 patients were approached, of whom 822 (37.4%) were eligible for the study based on explicit criteria (i.e. were 18 – 60 years of age, spoke English, endorsed pain of at least three months duration, reported use of any analgesic medication [ including over-the-counter or prescription] in the prior month, and had a scheduled PC appointment). Over 75% of those eligible (620/822) agreed to participate in the study. Electronic medical record entries from 12 months prior to study entry were reviewed looking for documentation of an opioid prescription. Standardized chart abstraction forms were used and the electronic medical records were comprehensive. They included notes from all clinic visits, all emergency department records, all inpatient discharge summaries, phone notes, and an institutional prescription database. Patients were eligible for inclusion in this study if they had at least one prescription for any of the following opioids in the prior year: Butorphanol/Stadol; Codeine/Tylenol# 2,3,4; Fentanyl oral/Actiq; Fentanyl transdermal (Duragesic); Hydrocodone (Vicodin, Norco, Zydone, Maxidone, Lortab, Lorcet, Hydrocet, Co-Gesic, Anexsia); Hydromorphone (Dilaudid); Meperidine (Demerol); Methadone (for pain, not maintenance treatment); Morphine-immediate release (MSIR); Morphine-extended release (MSContin); Nalbuphine (Nubain); Oxycodone-immediate release (Percocet, Roxicet, Endocet, Tylox, OxyIR, Roxicodone); Oxycodone-Long acting (Oxycontin); Pentazocine (Talwin); Propoxyphene (Darvon, Darvocet); Levorphanol (Levo-Dromoran); Oxymorphone (Numorphan, Opana, Opana ER). Thus, the 238 patients that were prescribed an opioid pain reliever in the prior 12 months were the study sample for this analysis. Informed consent was obtained from eligible patients, and participants were compensated $10. The Boston University Medical Center Institutional Review Board approved the study.
Measures and Key Variables
Unless otherwise noted, all variables are obtained from subject interview.
Study Terminology
For the purpose of this study, PDD will be used to describe participants who meet DSM- IV criteria for current (past year) prescription opioid abuse or dependence.[2] During the interview portion, participants were assessed using the Composite International Diagnostic Interview (CIDI) v.2.1 module on Drug Disorders.[21] Using the CIDI, PDD was defined as meeting DSM-IV criteria for current (past year) prescription opioid abuse or dependence.[21] Criteria for abuse included social, physical or legal consequences from use. The criteria for dependence included compulsive use, health consequences, and physical dependence (i.e., tolerance or withdrawal). Physical dependence alone did not suffice to meet the diagnosis. Participants with PDD could also have comorbid other SUDs.
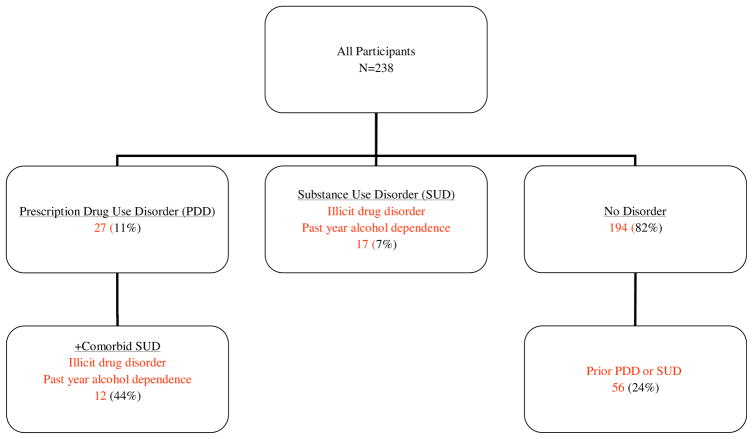
Other SUD will describe participants who meet DSM-IV criteria for any current (past year) illicit drug abuse or dependence and/or past year alcohol dependence.[21] These were assessed using the CIDI v.2.1 module on Drug Disorders (illicit drugs) and the CIDI-short form (CIDI-SF) for alcohol dependence.[21] Participants with PDD may also have another SUD (i.e. comorbid illicit drug disorder and/ or past year alcohol dependence), but will only be analyzed in the group labeled PDD. Prior drug disorder was defined as meeting DSM-IV criteria for prior (more than 12 months ago) prescription drug disorder and/ or illicit drug disorder.[21] Current alcohol abuse and past alcohol use disorders were not measured using the full CIDI; instead the CIDI-SF was used to reduce respondent time burden. Nicotine dependence was not included in the variable SUD. (Figure 1). For the main analysis, the participants with Current PDD were compared to all others, which included some with other SUDs. For the exploratory analyses, participants were assigned to one of the following groups: Current PDD, Current Other SUD (have SUD other than PDD), Prior SUD (with or without PDD) and No Lifetime Disorder.
Figure 1.
Participants and DSM-IV Diagnoses
Chronic pain has many different definitions, but experts agree that it is pain that persists for months or years.[26] For the purpose of this study, chronic pain is defined as pain of at least three months duration.
COMM Measure
During the interview portion of the study, each participant was administered a forty-question, beta version of the Current Opioid Misuse Measure.[5] Subsequently, the COMM was narrowed down to seventeen questions during its validation study.[5] The seventeen questions include one newly constructed question. Specifically, question K23 from the beta version, “how often has something happened that’s worried you about how you’re handling your medications?” was changed to COMM question 10, “How often have you been worried about how you’re handling your medications?” and COMM question 11, “How often have others been worried about how you’re handling your medications?” For the study presented, participants’ scores were calculated based on the sixteen questions present in the beta-version that were retained in the final COMM questionnaire. Some of the participants' scores would have been higher had not that question been omitted.
Other Variables
The following key variables were examined: 1) socio-demographic factors including age (in years), gender, race/ethnicity (Black, Hispanic, White, other), income (≥ or < $20,000), employment (unemployed or receiving disability payments vs. other), education (< high school, high school or more), marital status (partnered, divorced, single), health insurance (Medicaid/Medicare vs. others- including private and uninsured); 2) lifetime post-traumatic stress disorder (PTSD) diagnosis from the CIDI v. 2.1 PTSD module;[21] 3) current major depression from the Patient Health Questionnaire (PHQ) for Depression, a 9-item validated measure correlated with past two week major depression;[17] 4) family history of SUD (single question about 1st degree relatives having alcohol or drug problems); 5) current cigarette smoking (taken from the visit closest to the interview date during the electronic medical record review).[18]
Statistical Analysis
Descriptive statistics were calculated using frequencies, means, medians, and standard deviations. To describe the level of opioid medication prescription, we grouped the participants by number of equivalent pills of 5 mg oxycodone (the most common opioid medication prescription) given the plethora of different types of prescriptions, including medication, strength, dosing instructions and number of fills (original plus any refills). Participants with PDD were compared to all others using t-tests for continuous data and the Fisher’s Exact test for categorical data.
As the COMM scores were not distributed normally, all statistical analyses were conducting using both parametric and non-parametric tests of difference. In order to address the first hypothesis, that the COMM can identify subjects with PDD and distinguish them from all others, the t-test and Wilcoxon rank sum test were performed to examine COMM scores by drug disorder groups (PDD versus no disorder).[10] Both parametric and non-parametric analysis of variance (F-test and Kruskal-Wallis test) were utilized to explore the second hypothesis, that the COMM can differentiate participants with PDD from subjects with a lone other SUD, a prior disorder (PDD or SUD), or no disorder. As both the parametric and non-parametric tests yielded the same statistically significant results, the mean scores are reported in this paper. Finally, a receiver operating characteristics (ROC) curve was constructed. Using this curve it was determined whether a threshold score of nine is suggestive of PDD in this patient sample. The data analysis for this paper was generated using SAS/STAT® 9.1 statistical software. Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. The Type I error level for all tests was set at 0.05.
Results
The demographic characteristics of all 238 participants, stratified by DSM-IV diagnosis of PDD or no PDD are presented. (Table 1). Among the entire subject panel, 15% of the subjects received the equivalent of 20 tablets of 5 mg oxycodone in ≤2 fills, 12.6% received 21-60 tablets in ≤3 fills, 22.7% received 61-150 tablets in ≤3 fills, and 49.6% received >150 tablets or >3 fills of any amount (e.g. 4 prescriptions of 20 tablets each). The majority of those in the last category received >6 fills. Eleven percent (27/238) met DSM-IV criteria for current PDD. There were few differences among the two groups of subjects with respect to mean age, distribution of gender and race, and education level attained. The sample had a mean age in the 40s, was largely African American, and the majority had 12 or more years of education. At least 50% of those with PDD and those with no PDD were receiving disability payments, and nearly a third of each group had lifetime post-traumatic stress disorder. Consistent with other studies examining clinical risk factors for PDD [14,18,19], participants with PDD were more likely to suffer from current depression, to smoke, or to have past year other drug disorder.
Table 1.
Demographic characteristics of participants stratified by PDD diagnosis
| Variable | Current PDD | No PDD | p - value | |
|---|---|---|---|---|
| N=27 (11%) | N=211 (89%) | |||
| N (%) | N (%) | |||
| Mean Age (SD) | 47.0 (7.9) | 46.6 (8.6) | .85 | |
| Female Gender | 15 (56%) | 118 (56%) | 1.0 | |
| Race | African American | 19 (70%) | 125 (60%) | .36 |
| Hispanic | 3 (11%) | 21 (10%) | ||
| Whit | 5 (19%) | 42 (20%) | ||
| Other | 0 | 21 (10%) | ||
| Education | < 12 yrs | 9 (33%) | 62 (29%) | .66 |
| >12 yrs | 18 (67%) | 149 (71%) | ||
| Receiving Disability Payments | 16 (59%) | 105 (50%) | .42 | |
| Lifetime PTSD | 13 (48%) | 73 (35%) | .20 | |
| Current Depression (past 2 weeks) | 17 (63%) | 74 (35%) | .006 | |
| Current Smoking | 21 (81%) | 97 (47%) | .001 | |
| Current Alcohol Dependence or Drug Disorder* | 12 (44%) | 17 (8%) | <0.001 | |
| Mean COMM Score (SD) | 20.4 (10.8) | 8.4 (7.5) | <0.001 | |
Other than prescription opioid
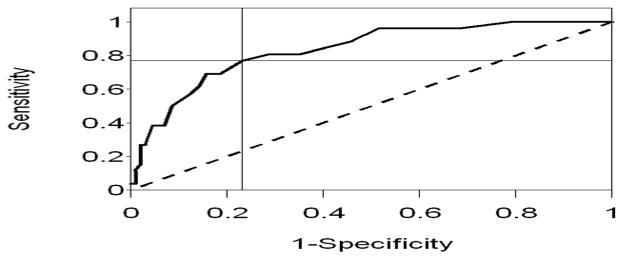
The mean COMM score for those subjects with current PDD, 20.4 (SD 10.8), was significantly higher than those with no current PDD, 8.4 (SD 7.5), p = <0.0001, as was the median COMM score 18.5 vs. 7. 5, p<0.0001. (Table 1). Among all participants, COMM scores ranged from 0 to 45. A receiver operating characteristics curve of the COMM data compared to a DSM-IV diagnosis of PDD was constructed. (Figure 2). The area under the curve was 0.84 (95% CI 0.76, 0.91). Diagnostic performance characteristics across a range of possible COMM scores are presented in Table 2. In this sample, a COMM score of thirteen has the maximum sum of sensitivity and specificity, with a sensitivity of 0.77 and a specificity of 0.77, for identifying participants with DSM-IV PDD. At this value, the positive predictive value (PPV) is 0.30 and the negative predictive value (NPV) is 0.96, while the positive and negative likelihood ratios are 3.31 and 0.30, respectively. This indicates that the probability of having PDD with a positive COMM score is 30%, while the probability of not having PDD when the COMM is normal (i.e. below the threshold value of 13) is 96%.[11]
Figure 2. Receiver operating characteristic curve.
COMM prediction score sensitivity and specificity estimates measured against a DSM-IV diagnosis of PDD. AOC = 0.84. (95% CI 0.76, 0.91) Diagonal line represents chance prediction.
Table 2.
COMM Prediction Score vs. DSM-IV Diagnosis
| COMM Score | Sensitivity | Specificity | PPV | NPV | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|---|
| 7 | 0.961 | 0.484 | 0.196 | 0.989 | 1.866 | 0.079 |
| 8 | 0.884 | 0.540 | 0.201 | 0.972 | 1.924 | 0.213 |
| 9 | 0.846 | 0.595 | 0.215 | 0.967 | 2.094 | 0.258 |
| 10 | 0.807 | 0.646 | 0.230 | 0.962 | 2.284 | 0.297 |
| 11 | 0.802 | 0.681 | 0.25 | 0.964 | 2.538 | 0.282 |
| 12 | 0.807 | 0.712 | 0.269 | 0.965 | 2.805 | 0.270 |
| 13 | 0.769 | 0.767 | 0.303 | 0.962 | 3.311 | 0.300 |
| 14 | 0.692 | 0.813 | 0.327 | 0.952 | 3.704 | 0.3784 |
| 15 | 0.692 | 0.843 | 0.367 | 0.954 | 4.421 | 0.364 |
| 16 | 0.615 | 0.858 | 0.363 | 0.944 | 4.351 | 0.447 |
| 17 | 0.576 | 0.873 | 0.375 | 0.940 | 4.569 | 0.484 |
All 238 subjects were categorized according to whether they met criteria for a DSM-IV diagnosis of current PDD (11%, 27/238), current other SUD (7%, 17/238), or no disorder (82%, 194/238). Participants with no disorder were further categorized based on whether they met criteria for a prior disorder (PDD or other SUD) (24% 56/238) or had no lifetime disorder (58% 138/238). (Figure 1). Mean COMM scores were calculated for participants with PDD, current other SUD, prior disorder (PDD or SUD), and no lifetime disorder. The mean score for those with PDD remained significantly different from all other groups, including participants with a current other SUD, while the other groups did not differ significantly from each other. (Table 3).
Table 3.
Mean COMM scores for Current PDD vs. all others
| Disorder Group | Mean COMM Score (SD) | Median Score | P-value |
|---|---|---|---|
| Current PDD | 20.4 (10.8) | 18.5 | <0.0001 |
| Current Other SUD | 13.0 (7.4) | 12.0 | |
| Prior Disorder | 9.1 (8.3) | 6.0 | |
| No Lifetime Disorder | 7.6 (6.9) | 6.0 |
Discussion
Using a DSM-IV diagnosis of PDD as a “gold-standard”, the diagnostic performance characteristics of the COMM were evaluated among a sample of PC patients with chronic pain who had received prescription opioids in the prior year. The data confirm the hypothesis that the Current Opioid Misuse Measure can distinguish those with a DSM-IV diagnosis of PDD. When compared to the findings presented in the original COMM validation study, conducted among a cohort of patients from specialty pain clinics, the diagnostic characteristics of the COMM seem different in our urban, academic PC patient sample.[5] In that original study, the threshold (cutoff) value used was nine to detect opioid misuse as defined by a composite measure (not a diagnosis of PDD). In our data, with 2 questions different from the original, a threshold value of thirteen, rather than nine, maximized the sum of sensitivity and specificity for identifying patients with DSM-IV diagnosis of PDD.
A ROC curve analysis suggests a cutoff point of thirteen to maximize the sensitivity and specificity of the COMM within this PC population. The area under the curve of 0.84 implies that the test is good, or moderately accurate, for identifying participants with DSM-IV PDD.[13] As in the original COMM validation study, the selected cutoff score results in greater sensitivity so that few cases of actual PDD are missed.[5] Changing the cutoff to obtain greater specificity limits the number of false-positives.[11] Individual clinicians, based on the overall prevalence of PDD in their own patient population, may decide to choose a COMM score that maximizes either sensitivity or specificity, rather than the sum of the two values.[11]
Results also support the exploratory hypothesis that the COMM appears to distinguish those with PDD, some of whom may have comorbid illicit drug disorders and/or past year alcohol dependence, from those with a lone other SUD, a prior drug disorder (PDD or SUD), or no disorder. The discriminatory capacity of the COMM supports the content validity of the tool.[11] This is particularly valuable as patients with other current SUDs or prior disorders (PDD or SUD), while at higher risk for opioid misuse, may not be currently abusing prescription opioids. Clinicians must appropriately monitor these patients, and the COMM appears to specifically measure PDD.[8,20]
The predictive value calculations demonstrate that primary care clinicians can feel fairly confident that patients (in a population with a comparable prevalence of PDD) with a COMM score of less than 13 do not have PDD. However, only 30% of those with a COMM score of 13 or greater will have PDD. These data reflect the fact that predictive value calculations are affected by the prevalence of disease in a population.[11] Furthermore, as concluded in the original COMM validation study, this tool appears to identify some patients who are not likely having problems with their prescription opioids.[5] Rather, some of those patients identified as positive will be false positives – patients identified as misusing their medication when they are not. Clinicians are encouraged to practice caution when interpreting the COMM scores and to take into consideration other extenuating circumstances.[5,23]
The cutoff COMM score obtained in this study was higher than that obtained in the first published validation study.[5] One possible explanation for this finding can be derived from the fact that a different “gold-standard” was chosen for the current study, using the CIDI to assess for DSM-IV criteria for PDD or other SUDs, while the original validation study compared the forty-question beta version of the COMM to the Aberrant Drug Behavior Index.[4,5,9] The Aberrant Drug Behavior Index may measure aberrant medication related behaviors that do not meet criteria for DSM-IV PDD, but are thought by experts to be indicative of prescription opioid “misuse” or “non-medical use”.[15,24,27,30] In addition, unlike the DSM-IV, the Aberrant Drug Behavior Index will label a patient as having PDD if they use an illicit substance, such as cocaine, while prescribed an opiate. Utilizing the CIDI as the “gold-standard” permitted a comprehensive assessment of participants for a variety of substance use disorders including PDD, other SUD, and prior disorder (PDD or SUD). Performing this complete analysis focused on use of this instrument for patients at high risk for PDD who may receive opioid analgesic therapy. [6,7,19,20,27] However, primary care clinicians may consider using COMM scores with lower cut-offs as a trigger to discuss potential misuse of the medication in addition to potential PDD.
The COMM was developed as a self-administered questionnaire, and could be incorporated into standard practice for patients prescribed opioids chronically.[5] It takes less than 10 minutes, and is easily scored by adding the responses. Ideal timing of the measure (e.g. every month, twice yearly) and its utility in combination with treatment contracts, urine toxicology screens, pills counts and prescription monitoring will need to be studied
Limitations and Strengths
This study has certain limitations that require consideration. For example, the cross-sectional design does not allow for patients to be followed over time, limiting the types of inferences possible. Specifically, the COMM was only administered once to study participants, so we lack test re-test reliability. In addition, there were a small number of study participants with a DSM-IV diagnosis of current PDD. However, the overall sample size was large enough to produce unambiguous and statistically significant results in each test. These findings do support the need for larger studies in which primary care patients are followed prospectively and the COMM is administered repeatedly. Since geography and culture heavily influence use of prescription opioids in clinical and addiction contexts, it is not clear whether these findings are generalizable to areas outside the US or even different primary care populations within the US.
Another aspect of the study design, relying on the electronic medical record for data regarding opioid prescriptions, might mean participants who received opioid prescriptions from providers outside of the medical center were excluded from the study. However, by obtaining primary prescription data recall bias was minimized.[22] Furthermore, implementation of the COMM is oriented toward clinical practices that prescribe opioid therapy, thus providing some assurance that the appropriate patients will ultimately benefit from its use.
The study is also limited by the fact that some participants were not prescribed chronic opioids, and the data analysis did not control for the dose or duration of opioid therapy. Consequently, these results may be less relevant to patients who are prescribed chronic or high-dose opioid therapy. Current guidelines define chronic opioid therapy as “daily or near-daily use of opioids for at least ninety days.”[7,16] It is plausible that some subjects in this study demonstrated behaviors consistent with addiction due to inadequately controlled pain. The extent to which this happened would have biased the results towards the null hypothesis. Referred to as pseudoaddiction, this preoccupation with opioids often resolves once the pain is adequately controlled.[27,28] For experts in pain and addiction, there is valid concern about patients with pseudoaddiction being inaccurately labeled as suffering from PDD.[27,28]
Finally, as with any screener, there are always false positives and false negatives. As noted by Butler et al., the COMM is only one source of patient information and should not be used as the sole means of determining whether opioid therapy is appropriate.[5]
Conclusions
Among a sample of PC patients with chronic pain had received prescription opioids in the past year, the Current Opioid Misuse Measure (COMM) can identify patients with PDD. Overall, the COMM is a unique clinical tool that demonstrates utility for PC clinicians. Not only does it serve as a validated measure for assessing PDD, but it also provides a means of tracking these behaviors to identify patients at-risk for prescription opioid misuse.
For patients with chronic pain prescribed opioids, the development of PDD is a serious complication. For primary care physicians treating patients with chronic pain with prescription opioids, the COMM is a promising tool for identifying those who may have PDD and for helping to confirm that the probability for PDD is low. Future research, in which prospective studies of the COMM are conducted in a variety of PC settings, is needed.
Acknowledgments
Andrew J. Meltzer, M.D. provided valuable comments on the manuscript. A pdf of the official COMM scale can be obtained free of charge for clinical use at www.PainEdu.org. Stephen F. Butler, PhD is Senior Vice President and Chief Science Office at Inflexxion and is one of the creators of the Current Opioid Misuse Measures (COMM)®. The other authors report no conflicts of interest.
Financial Support and Disclosure: Funding/Support: The study was funded by K23 DA016665 (Liebschutz) and K24 DA022288 from the National Institute of Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. [Google Scholar]
- 2.American Psychiatric Association, Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 3.Baxter RJ, Mechanic RE. The status of local health care safety nets. Health Aff (Millwood) 1997;16:7–23. doi: 10.1377/hlthaff.16.4.7. [DOI] [PubMed] [Google Scholar]
- 4.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Califano JA. The National Center on Addiction and Substance Abuse. Columbia University; Jul, 2005. Under the counter: The diversion and abuse of controlled prescription drugs in the US. [Google Scholar]
- 7.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton P. Should opioid abusers be discharged from opioid-analgesic therapy? Pain Med. 2008;9:383–90. doi: 10.1111/j.1526-4637.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 9.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and "problematic" substance use: evaluation of a pilot assessment tool. Journal of Pain & Symptom Management. 1998;16:355–63. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 10.Dawson B, Trapp RG. Basic and Clinical Biostatistics. New York: Lange Medical Books/ McGraw-Hill; 2004. [Google Scholar]
- 11.Fletcher RH, Fletcher SW. Clinical Epidemiology the Essentials. Baltimore: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 12.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. J Pain Symptom Manage. 2004;28:176–88. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 14.Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz NP, Adams EH, Benneyan JC, Birnbaum HG, Budman SH, Buzzeo RW, Carr DB, Cicero TJ, Gourlay D, Inciardi JA, Joranson DE, Kesslick J, Lande SD. Foundations of opioid risk management. Clin J Pain. 2007;23:103–18. doi: 10.1097/01.ajp.0000210953.86255.8f. [DOI] [PubMed] [Google Scholar]
- 16.Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke K. The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Annals. 2002;32:509–521. [Google Scholar]
- 18.Liebschutz JM, Saitz R, Weiss RD, Averbuch T, Schwarz S, Meltzer EC, Clagget-Borne E, Cabral HJ, Samet JH. Clinical Factors Associated with Prescription Drug Use Disorder in Urban Primary Care Patients with Chronic Pain. The Journal of Pain. 2010 doi: 10.1016/j.jpain.2009.10.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain & Symptom Management. 2004;28:250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Prater CD, Zylstra RG, Miller KE. Successful Pain Management for the Recovering Addicted Patient. Prim Care Companion J Clin Psychiatry. 2002;4:125–131. doi: 10.4088/pcc.v04n0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–77. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ. Epidemiology an Introduction. New York: Oxford University Press, Inc; 2002. [Google Scholar]
- 23.Savage SR. Assessment for addiction in pain-treatment settings. Clin J Pain. 2002;18:S28–38. doi: 10.1097/00002508-200207001-00004. [DOI] [PubMed] [Google Scholar]
- 24.Substance Abuse and Mental Health Services Administration (SAMHSA), Office of Applied Studies. Results from the 2006 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293; 2007. [Google Scholar]
- 25.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, Jasper JF, Singh V, Jordan AE, Johnson BW, Cicala RS, Dunbar EE, Helm S, 2nd, Varley KG, Suchdev PK, Swicegood JR, Calodney AK, Ogoke BA, Minore WS, Manchikanti L. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician. 2006;9:1–39. [PubMed] [Google Scholar]
- 27.Weaver M, Schnoll S. Addiction Issues in Prescribing Opioids for Chronic Nonmalignant Pain. Journal of Addiction Medicine. 2007;1:10. doi: 10.1097/ADM.0b013e3180473bec. [DOI] [PubMed] [Google Scholar]
- 28.Weissman DE, Haddox JD. Opioid pseudoaddiction--an iatrogenic syndrome. Pain. 1989;36:363–6. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- 29.Wu SM, Compton P, Bolus R, Schieffer B, Pham Q, Baria A, Van Vort W, Davis F, Shekelle P, Naliboff BD. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. Journal of Pain & Symptom Management. 2006;32:342–51. doi: 10.1016/j.jpainsymman.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–32. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 31.Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zang F, Simoni-Wastila L, Sullivan SD. Trends and Geographic Variation of Opiate Medication Use in State Medicaid Fee-for-Service Programs. Medical Care. 2006;44:1005–1010. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]