SUMMARY
Background
Several aspects of the inflammatory response to a single insult, i.e., exposure to 2 ppm of ozone (O3) for 3 h or 6 h, are less pronounced in surfactant protein A deficient (SP-A −/−) mice (KO) than in wild type mice (WT). It was hypothesized that a mild insult, specifically low doses of lipopolysaccharide (LPS), would adversely affect host defense and differentially potentiate O3-induced injury in WT and KO mice.
METHODS
WT and KO mice were treated with different doses of LPS or LPS (2 ng) + O3 (2 ppm) or filtered air (FA) for 3 h, then sacrificed 4 h following exposure (O3, FA) or 20 h after LPS treatment alone. Several endpoints of inflammation were measured in bronchoalveolar lavage (BAL).
RESULTS
1) At 20 h after LPS treatment alone, both WT and KO mice exhibited signs of inflammation, but with differences in the macrophage inflammatory protein 2 (MIP-2) response pattern, total cells (at 0.5 ng LPS) and basal levels of oxidized protein and phospholipids; 2) After LPS + O3, KO compared to WT showed decrease in polymorphonuclear leukocytes (PMNs) and MIP-2 and increase in phospholipids, and after LPS + FA an increase in total cells; 3) WT after LPS + FA showed an increase in SP-A with no further increase after LPS + O3, and an increase in oxidized SP-A dimer following O3 or LPS + O3.
CONCLUSIONS
LPS treatment has negative effects on inflammation endpoints in mouse BAL long after exposure and renders KO mice less capable of responding to a second insult. LPS and O3 affect SP-A, quantitatively and qualitatively, respectively.
Keywords: polymorphonuclear leukocytes, cytokines, lipopolysaccharide, oxidation, macrophage, ozone
“Duty is liberating. It forces you to transcend your own limitations and makes you do things that may not come naturally, but must be done, because they are right.”
David Rockefeller
INTRODUCTION
Lipopolysaccharide (LPS) or endotoxin is a major component of the outer membrane of gram-negative bacteria, which consists of Lipid A, a core polysaccharide, and O antigen, and is a strong inducer of innate immunity. Inhalation of endotoxin results in the recruitment of inflammatory cells and enhances the production of a variety of proinflammatory cytokines. A significant number of studies have shown that an endotoxin response is mediated through the interaction of the CD14/TLR4/MD2 receptor complex, which is linked intracellularly to NFκB pathways involved in the production of cytokines in many cell types, especially macrophages1–4. Recent studies demonstrated that TLR-4 is also necessary for full development of ozone (O3) induced lung injury5,6. Inhalation of high concentrations (100 µg) of LPS before O3 exposure has been shown to potentiate the effect of O3 and induce lung inflammation7,8.
O3 is one of the major components of air pollution. It is a highly toxic constituent of photochemical smog and it reacts primarily with molecules at the air-fluid boundary because its low solubility in water limits its ability to diffuse into the tissues. The biological effects of O3 are associated with oxidative stress in the lower respiratory tract and it may damage the epithelium, both directly and by triggering an inflammatory cascade that causes indirect damage 9–11. O3-induced changes in the lung include an influx and activation of immune cells, increase in proinflammatory cytokines and chemokines, including the chemokines, monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-27,8,12–14. O3 exposure also causes surfactant dysfunction15,16, although the mechanism for this remains unclear.
Pulmonary surfactant is a complex mixture of lipids and proteins. In addition to preventing alveolar collapse, its components play an important role in the first line of defense against various insults. Surfactant protein A (SP-A) is a major protein component of lung surfactant involved broadly in surfactant-related functions and in innate immunity17–20. Concerning innate immunity, SP-A has been shown to enhance phagocytosis of a number of microorganisms21–27, regulate complement activation28, and augment the production of reactive oxygen and nitrogen species (RONS), a mechanism for killing pathogens22,29,30. Deficits in these functions in SP-A deficient (SPA −/−) (KO) mice make them susceptible to pneumonia27,30–32. Evidence also indicates SP-A involvement in the regulation of inflammation, particularly with regard to lung injury and repair, by regulation proinflammatory cytokines2,14,33, clearance of apoptotic cells34,35, and regulation of collagen and matrix metalloproteinases36,37. Several studies have shown that various SP-A functions are adversely affected by oxidation38–41 or nitration42–45. The authors recently reported that oxidation of SP-A increased immediately after O3, before the oxidation of other proteins14, and also that during aging in the rat, SP-A undergoes disproportionately increased carbonylation compared to other bronchoalveolar lavage (BAL) proteins, suggesting increased susceptibility to oxidation46. It was postulated that, on the one hand, decrements in SP-A function may occur due to oxidation and, on the other hand, SP-A, by way of this susceptibility to oxidation, may play a role in limiting tissue damage by scavenging excess RONS14.
Acute lung injury has been shown to alter the levels of pulmonary surfactant in BAL, although questions remain about the mechanisms responsible for this, because both increases and decreases in the levels of phospholipids have been reported after LPS insults16,47–49. The lipids in surfactant, while primarily responsible for the reduction of surface tension can also influence the behaviour of immune cells. Surfactant lipids suppress the activation of immune cells and inhibit the production and release of proinflammatory cytokines50,51. However, both SP-A and the surfactant lipids can be oxidized by O3 or endogenous RONS39,40,44,52–55, altering their function and potentially contributing to the inflammation resulting from O3 exposure.
Exposure to O3 is associated with changes in innate immunity and inflammatory processes in both KO and wild type (WT) mice, but differences between the two types were observed, indicating a role of SP-A in the first line of defense. Because exposure to multiple toxicants may result in injuries or responses that are not predicted by evaluation of exposure to individual toxicants, the present study was undertaken in order to investigate the effect of a low dose of LPS prior to O3 exposure in WT mice with intact defenses and SP-A KO mice in which the innate host defense molecule, SP-A, has been ablated. The focus was on the early events occurring 4 hours after O3 treatment, a time point that in previous studies14 showed significant changes in several relevant endpoints.
OBJECTIVES
The aim of the study was to determine whether mice treated with a low concentration of LPS are sensitized, altering their response to an oxidative stress such as that produced by the environmental pollutant O3.
MATERIALS AND METHODS
Animals
The study was conducted on pathogen-free male WT and SP-A KO male mice on the C57BL/6 genetic background. WT mice were obtained from Jackson Laboratories (Bar Harbor, ME) and kept under standard environmental conditions in the animal facility at the Penn State College of Medicine. KO mice were maintained as specific pathogen-free mice under barrier/sterile conditions in the animal facility at the Penn State College of Medicine. Both WT and KO mice were fed rodent chow and tap water ad libitum. The body weight of the mice was in the range of 20–25 g. The Institutional Animal Care and Use Committee at the Penn State College of Medicine approved this study.
LPS dose response
Five to six week old WT male mice (n=4 mice/time point) received a single dose (0.2, 0.5, 1, 2, 50, or 200 ng) of smooth LPS (Escherichia coli 055:B5; Sigma, St. Louis, MO) in 50 µl saline intrapharyngeally. Control mice received equal volumes of saline intrapharyngeally, in parallel. Treatments were administered by placing the 50 µl dose in the pharynx of anaesthetized mice. The mice aspirated the bolus and recovered from the instillation with no obvious ill effects. Based on the WT data, two low doses of LPS shown to have stimulatory effects on inflammation were selected for study in KO mice. Five to six week old KO mice (n=4 mice/time point) received a single dose of LPS (0.5 or 2 ng/mouse/50 µl in saline). Control mice received equal volumes of saline intrapharyngeally. Both WT and KO mice were sacrificed at 20 h after LPS treatment by anaesthetization with halothane and exsanguination. The 20 h time point was selected on the basis of a number of reports that showed LPS-induced injury to occur within 24 h of LPS treatment56–58. The lungs were subjected to BAL with normal saline.
LPS treatment followed by O3 exposure in WT and KO mice
The 2 ng dose of LPS was selected for subsequent experimentation because it provided an effect on several outcomes from a low concentration of LPS. Five to six week old WT and KO mice (20–25 g) (n=4 mice/time point) received a single dose of LPS (2 ng) in 50 µl saline intrapharyngeally. Control mice received equal volumes of saline intrapharyngeally, in parallel. Thirteen hours after LPS treatment, the animals were exposed to either filtered air (FA) or O3 at a concentration of 2 ppm for 3 hours. These exposures were conducted in parallel at room temperature and 50% humidity as described elsewhere14. In brief, four mice were put in glass containers with wire mesh lids and then placed in a closed glass exposure chamber. The O3 was generated by an electric discharge ozonizer (Model OZ2SS-SS, Ozotech, Yreka, CA) and its concentration was monitored with an ultraviolet ozone analyzer (Model 400A, Advanced Pollution Instrumentation, San Diego, CA). This system is highly efficient at delivering O3 in specific concentrations between 0.1 ppm and 10 ppm59. Four hours after the termination of exposure, the mice were sacrified (total time of 20 hours after LPS treatment) by anesthetization and exsanguination. The lungs were subjected to BAL with normal saline.
Cell count and biochemical studies of BAL fluid
BAL fluid was obtained by instilling the lung 3 times with a volume of saline equal to 80% of lung vital capacity (for a total of 1.5 ml) through a tracheal cannula. The total fluid recovery was approximately 90% of the instilled volume and did not differ significantly between the experimental and control groups. The BAL fluid was centrifuged (150 × g, 5 min, 4°C) and the cell pellet was resuspended in 0.9% sodium chloride (NaCl). On each sample a total cell count was performed using a haemocytometer and a cytocentrifuge preparation was used to obtain differential cell counts. The cell-free supernatant was frozen and stored at a temperature of −80°C for subsequent biochemical analysis. Total protein concentration was determined using the Micro BCA Protein Assay (Pierce Biotechnology, Rockford, IL). For determination of total phospholipids, a 100 µl sample of BAL supernatant was lyophilized and assayed using the Phospholipids B Assay (WAKO Chemicals Inc, Richmond, VA).
Cytokine ELISAs
The concentration of MCP-1 and MIP-2 was measured on cell-free BAL samples with commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, McKinley Place, NE). In brief, 500 µl of cell-free BAL were lyophilized and then reconstituted to 100 µl with the assay diluent provided in the kit. Assays were performed according to the manufacturer’s recommendations, and the plates were read at 450 nm in a SPECTRA Fluor Plus ELISA plate reader (Tecan US, Research Triangle Park, NC) after the appropriate colour development period. The detection limits of these ELISA kits are 1.5 pg/ml for MIP-2 and 2 pg/ml for MCP-1.
SP-A Dot Blot
SP-A levels in the BAL were determined by protein dot blot. Protein dot blotting was done by diluting 25 µl of the cell-free BAL fluid in 975 µl of 0.02 M tris-buffered saline (pH 7.5; TBS) solution and applying 200 µl aliquots to Bio-Rad Trans Blot transfer medium (nitrocellulose, 0.45 µm) under vacuum in a 96 well dot blot apparatus (Bio-Rad, Hercules, CA). Blots were blocked overnight in 0.01 M Na/K phosphate buffered saline (pH 7.2; PBS) containing 1% bovine serum albumin (BSA). Following blocking, blots were incubated in a solution containing polyclonal rabbit anti-SP-A IgG60 (1:10,000) in PBS with 1% BSA, 0.05% Tween-20 for 1 h at RT and washed 3 times for 10 min with PBS, 0.5% Tween-20. The blots were then incubated for 1 h at room temperature with secondary antibody (goat anti-rabbit IgG HRP conjugate; 1:25,000) (Bio-Rad) and then washed as in the previous step. Antibody binding was detected by enhanced chemiluminescence (ECL). Blots were incubated for 1 min with 10 ml of each ECL solution (PerkinElmer Life Sciences, Boston, MA), and then wrapped in cellophane wrap, the excess solution removed, and exposed to Kodak X-Omat XAR film (Eastman Kodak Co., Rochester, NY). The film was developed and SP-A levels were quantified by laser densitometry.
Detection of oxidized protein in BAL
Oxidized proteins were detected using the OxyBlot Oxidized Protein Detection Kit (Intergen, Purchase, NY) as described previously14 with certain modifications. This kit detects carbonyl groups that have been introduced into proteins through oxidation. In brief, 25 µl of BAL samples were denatured by adding an equal volume of 12% SDS. Samples were then derivatized with 2.5 µl of 10X 2, 4-dinitriphenylhydrazine (DNPH) solution and incubated for 10 min at room temperature. Derivatization was stopped with the addition of 25 µl of neutralization solution. Samples were then analyzed by dot blot. Aliquots containing the DNPH-derivatized proteins were brought up to a volume of 500 µl with 0.01 M phosphate buffered saline (pH 7.2) and 200 µl of each sample was blotted onto nitrocellulose by vacuum using a 96-well dot-blot apparatus (Bio-Rad). Immunodetection of oxidized proteins was performed according to the manufacturer’s instructions, although the rabbit anti-DNP and goat anti-rabbit IgG (HRP-conjugated) antibodies supplied were used at one half of the recommended concentrations. ECL was used to detect antibody binding and the blots were exposed to XAR film (Eastman Kodak Co., Rochester, NY). The film was developed and carbonylated protein levels were quantified by laser densitometry.
Oxidized and non-oxidized SP-A protein in BAL by Western Blot
BAL samples in 200 µl aliquots were concentrated by lyophilization. Protein samples were then subjected to electrophoresis on 12.5% SDS polyacrylamide gels. Two separate gels were run and then the separated proteins were transferred to nitrocellulose membrane (nitrocellulose, 0.45 µm) for SP-A detection and on polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) for oxidized SP-A in a buffer of 25 mM Tris base, 192 mM glycine, 20% methanol. Immunodetection of SP-A protein was made as described in the previous section of SP-A BAL protein dot blots. Oxidized SP-A protein was detected as described previously61, with some modifications. The membrane was dehydrated for 1 min with 100% methanol and then washed for 5 min in 0.02 M Tris (pH 7.5) with 20% methanol, 5 min with 2 N hydrochloric acid (HCl), and then treated with 100 µg DNPH/ml in 2 N HCl for 5 min. The membrane was again washed 3 times with 2 N HCl, 7 times with 100% methanol, and once with 0.02 M TBS (5 min each wash). The membrane was blocked overnight in 5% powdered milk (Carnation) in 0.02 M TBS, 0.05% Tween-20. All the post-transfer steps were made in 100 ml of solution at room temperature. Immunodetection of oxidized proteins was made, as described in the previous section on BAL protein dot blots.
Statistical analysis
All analyses for the linear trend model between dose and main outcome and non-parametric comparisons were performed using SAS V9.1 (SAS Institute, Cary, NC) and a p-value of less than 0.05 was considered significant. LPS dose was converted to log scale prior to analyses in order to satisfy the distributional assumption associated with the linear model. All the other data were analyzed by t-test (Sigma Stat; SPSS; Chicago, IL).
RESULTS
Behavioural Observations
Both WT and SP-A mice exposed to different doses of LPS appeared to behave similarly to those treated with saline. The mice that received a low dose of LPS followed by O3 exposure behaved differently from those that received LPS followed by FA exposure. Shortly after the start of the O3 exposure period the fur of these mice became ruffled, and after 30 min to 1 hour, the O3-exposed mice became less active, curled up, and appeared to sleep for the duration of the exposure period. Following the exposure, their activity returned to normal within the first hour. Mice exposed to LPS and FA remained active throughout the exposure period.
LPS dose response study for WT mice
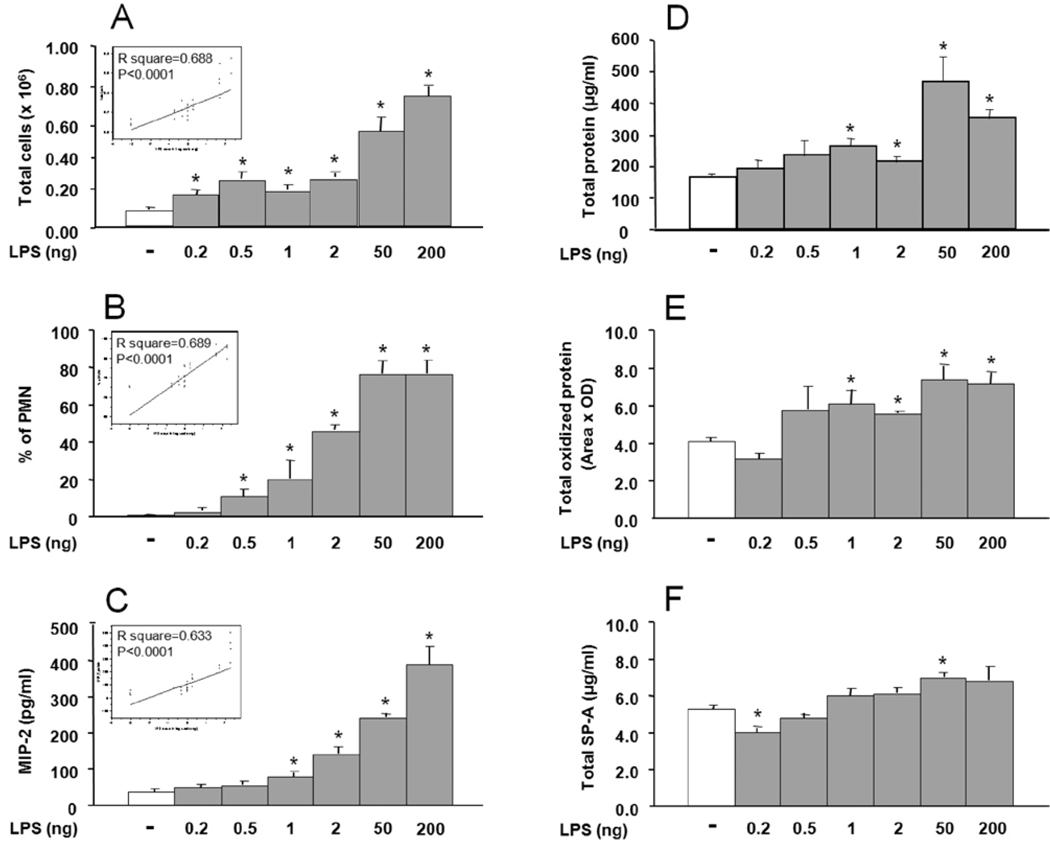
The analysis of BAL revealed several changes in the mice treated with LPS compared to the saline treated controls (Figure 1). These changes included a significant increase (p<0.05) in the mean total BAL cell count (Fig. 1A), in the percentage of polymorphonuclear (PMN) leukocytes (Fig. 1B), and in the mean level of MIP-2 (Fig. 1C) starting for each end point at LPS dosage of ≥ 0.2 ng, ≥0.5 ng, and at ≥1 ng, respectively. Total protein (Fig. 1D) and its levels of oxidation (Fig. 1E), measured by carbonyl content for total oxidized protein, were also higher in LPS treated mice and reached significance (p<0.05) at doses of LPS of 1 ng and higher. SP-A protein levels in BAL measured by protein dot blot for the most part remained at normal levels with a decrease and increase observed at 0.2 and 50 ng LPS, respectively, (Fig. 1F). No consistent change was observed in total phospholipids at any dose of LPS (not shown). The increases correlated with LPS dose (Figs. 1A, 1B, 1C) for total cells (r2=0.688, p<0.0001), percentage of PMNs (r2=0.689, p<0.0001), and the level of MIP-2 protein (r2=0.633, p<0.0001). For the other parameters, no correlation was observed.
FIGURE 1.
Lipopolysaccharide (LPS) effect on total cell and polymorphonuclear leukocyte (PMN) count, macrophage inflammatory protein 2 (MIP-2) content, total protein and total oxidized protein, and total surfactant protein A (SP-A) in bronchoalveolar lavage (BAL) of wild type (WT) mice. The mice received LPS at different concentrations (0.2, 0.5, 1, 2, 50, and 200 ng) intrapharyngeally in 50 µl saline. Control animals received 50 µl saline in parallel. BAL was collected 20 h after LPS treatment. Values are expressed as mean ± S.D., for total cells (A), PMNs (B), MIP-2 (C), total protein (D), total oxidized protein (E), and total SP-A protein (F). Statistically significant (p ≤0.05) differences between LPS treated (gray bar) and control (open bar) mice (n=4 for each group) are indicated by an asterisk (*). The figure in the box represents the correlation between LPS dose and the indicated read-outs.
Comparison of LPS effect in WT and KO mice
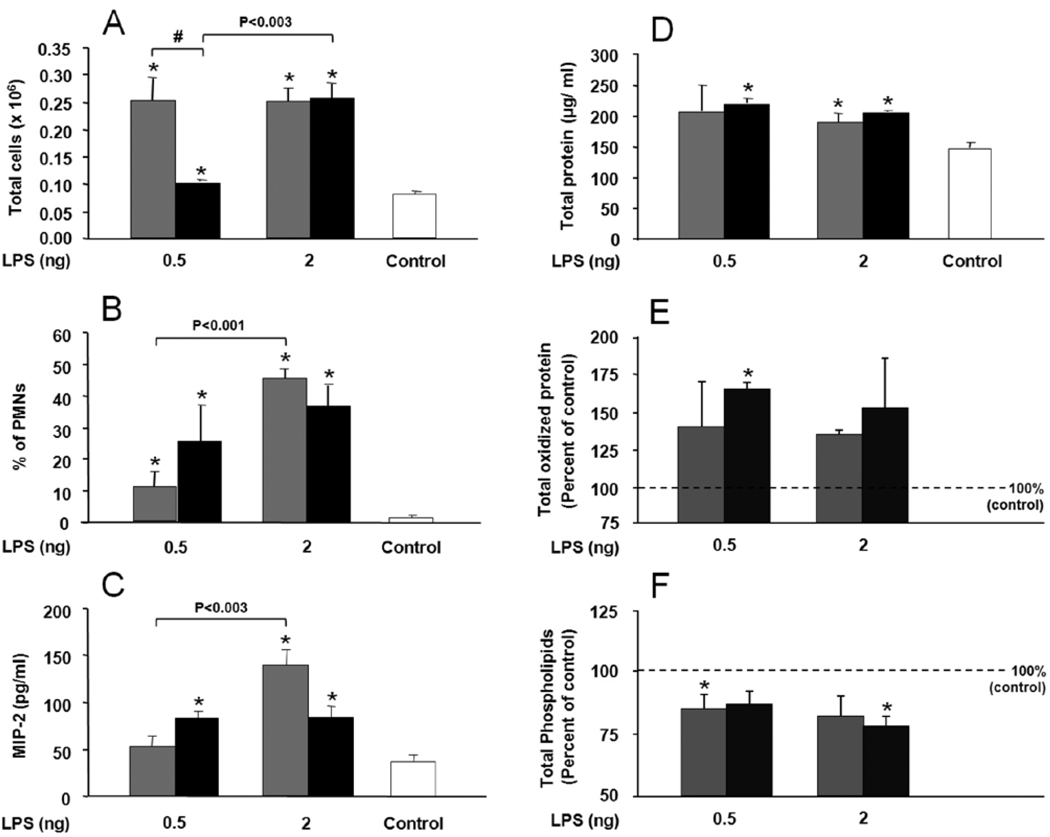
KO mice LPS treated (0.5 ng or 2.0 ng/mouse) also showed signs of inflammation in the BAL (Figure 2). Significant differences were observed for both LPS doses in several endpoints (Figs. 2A–2D) compared to measurements in the BAL of control saline-treated mice. Control values for total oxidized protein (Fig. 2E) and total phospholipids (Fig. 2F) differed from one another so control values at were set at 100% and the LPS samples were calculated as a percentage of the control. Oxidized protein values increased after LPS and there was a trend toward greater increases in KO mice, although statistical significance was observed only at 0.5 ng LPS. With respect to phospholipids, LPS administration was associated with reduced levels and significant differences occurred at 0.5 ng LPS for WT, and at 2 ng LPS for KO mice.
FIGURE 2.
Lipopolysaccharide (LPS) effect on total cell and polymorphonuclear leukocyte (PMN) count, macrophage inflammatory protein 2 (MIP-2) content, total protein and oxidized protein, and total phospholipids in bronchoalveolar lavage (BAL) of surfactant protein A deficient (SP-A −/−) mice (KO) in comparison with wild type (WT) mice. The mice received intrapharyngeal instillation of selected LPS doses (0.5 ng and 2 ng) in 50 µl saline. Control mice received 50 µl saline in parallel. BAL was collected 20 h after LPS treatment. Values are expressed as mean ± S.D. (n=4) for WT (gray bars), KO (black bars). Filtered air (FA) control values are represented by a single bar (open bar) when no differences were seen between KO and WT mice (panels A, B, C, D). For panels E and F, where differences in basal levels between WT and KO were observed, the control values were set to 100% (dotted line). Data are shown for total cells (A), percentage of PMNs (B), MIP-2 (C), total protein (D), total oxidized protein (E), and total phospholipids (F). Statistically significant (p ≤0.05) differences between LPS treated and control mice are indicated by an asterisk (*), and between WT and KO mice by the sign (#) above a connecting bar. Differences between LPS doses in WT or KO are shown by a connecting bar with the p-value above the bar.
On comparison of the KO data and WT data certain differences emerged: 1) Significant differences in the total number of cells (Fig. 2A) were observed between WT and KO at exposure to 0.5 ng of LPS but not 2 ng of LPS, indicating an inability of KO to respond to low levels of LPS, but this inability was overcome in the face of a more severe insult (i.e. 2 ng LPS); 2) PMN recruitment as assessed by the percent of PMNs (Fig. 2B) in BAL is gradual in WT; significant differences were observed between the two doses of LPS and between the LPS treated animals and the controls. For the KO no significant differences between the two doses of LPS were observed although PMN recruitment was increased in LPS treated animals compared to the controls. A decrease in macrophage/monocyte cell count in WT and KO (not shown) followed a pattern similar to that described for PMNs; 3) MIP-2 levels (Fig. 2C) for each group, for the most part, tended to parallel the observations made for PMNs, except for a lack of significant difference in WT between mice treated with 0.5 ng of LPS and controls. Taken together, these observations indicate that the response of KO mice to low levels of LPS is limited; 4) With regard to BAL protein (Fig. 2D), although no differences were observed between WT and KO mice in total protein levels, there was a trend toward greater changes in total oxidized protein in KO mice than in WT mice; 5) A difference (decrease) in total phospholipids (Fig. 2F) was observed between control and LPS treated mice, but not between WT and KO mice.
Effect of O3 or FA on BAL cells and cytokines in LPS treated WT and KO mice
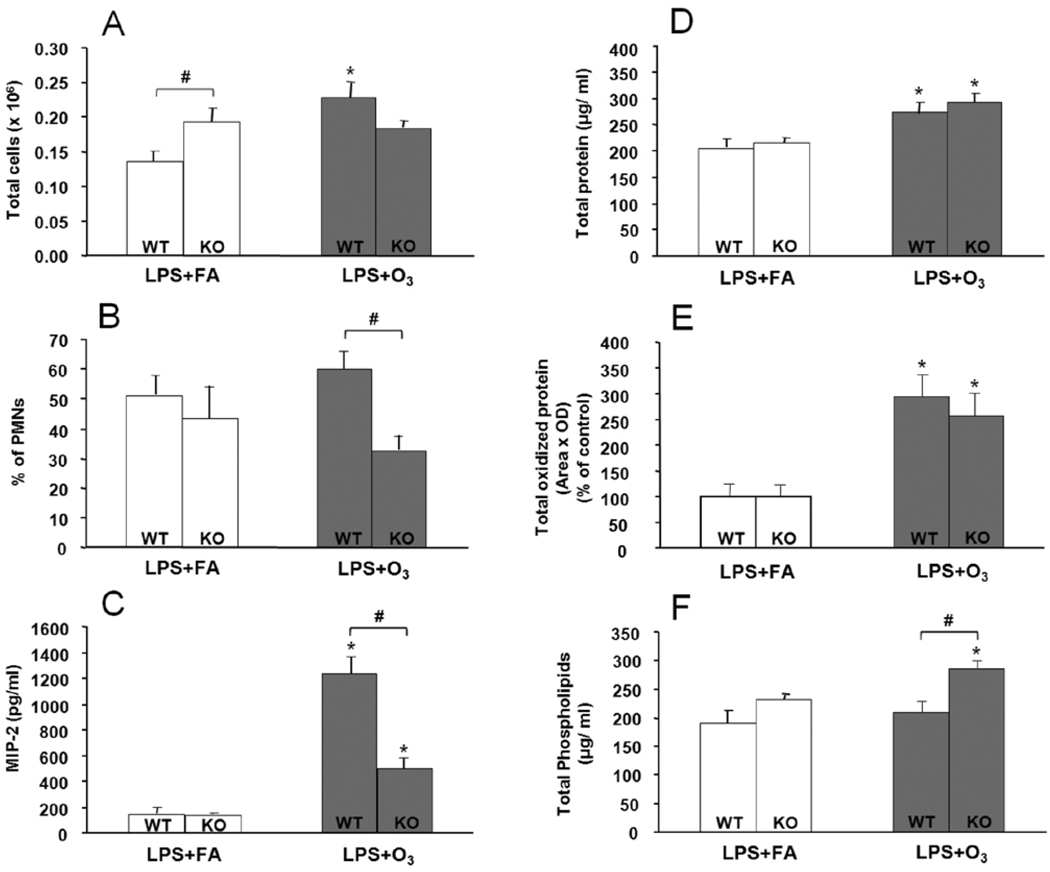
Exposure of WT mice to LPS (2 ng) + O3 resulted in significant increases (p<0.05) in several parameters in the BAL (Figure 3) compared to LPS + FA exposure. These differences included significant changes in the total cell count (Fig. 3A), levels of MIP-2 (Fig. 3C), and MCP-1 (not shown), total protein (Fig. 3D), and total oxidized protein (Fig. 3E) in BAL. No significant differences were observed between the groups of WT mice in the percentage of PMNs (Fig. 3B) or phospholipids (Fig. 3F).
FIGURE 3.
Effect of lipopolysaccharide (LPS) + filtered air (FA) or LPS + ozone (O3) on total cell count, polymorphonuclear leukocyte (PMN) count, MIP-2, phospholipids, total protein, and total oxidized protein content in the bronchoalveolar lavage (BAL) of wild type (WT) and surfactant protein A deficient (SP-A −/−) mice (KO). The mice received 2 ng of LPS intrapharyngeally in 50 µl saline, and 13 h later they were exposed to either FA or O3 (2 ppm for 3 h). BAL was collected 4 h following the conclusion of FA or O3 exposures, or a total time of 20 h after LPS treatment. Values are expressed as mean ± S.D. (n=4) for LPS+ FA (open bars), and LPS + O3 (black bars). Data are shown for total cells (A), percentage of PMNs (B), MIP-2 (C), total protein (D), total oxidized protein (E), and phospholipids (F). Significant (p ≤0.05) differences between LPS + FA and, LPS + O3 treated mice are indicated by an asterisk (*), and between WT and KO with the pound sign (#) above a connecting bar (p≤0.05).
Exposure of KO mice to LPS (2 ng) + O3 resulted in significant (p<0.05) increases compared to the LPS + FA treated group, in the levels of MIP-2 (Fig. 3C) and MCP-1 (not shown), phospholipids (Fig. 3F), total protein (Fig. 3D) and oxidized protein (Fig. 3E). This LPS response pattern was similar to that observed in the WT mice, except that in the KO mice a significant increase in phospholipid levels in response to LPS + O3 was observed compared to the LPS + FA mice, but no change in total BAL cell count.
Significant differences were observed between WT and KO mice after LPS + O3 treatment in the percent of PMNs (Fig. 3B), MIP-2 (Fig. 3C), and phospholipid (Fig. 3F). No changes were observed between WT and KO mice with regards to the levels of MCP-1 (not shown), total protein (Fig. 3D), and total oxidized protein (Fig. 3E). Differences between WT and KO mice after LPS + FA were observed only for total cells (Fig. 3A).
The impact of LPS and O3 on SP-A content and levels of oxidized SP-A
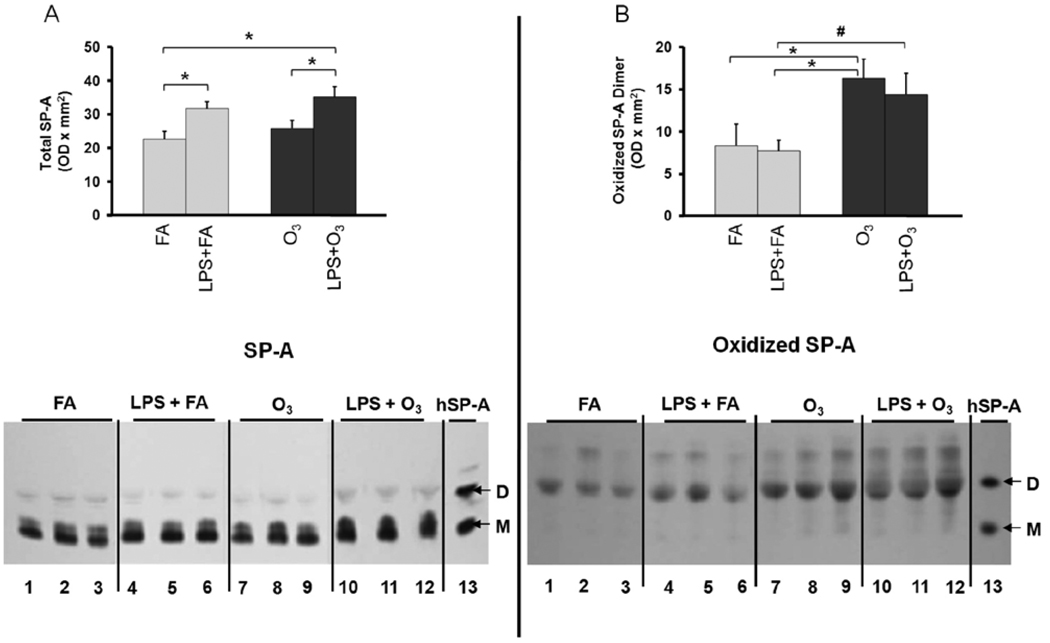
Small but significant increases in total SP-A content were observed in response to LPS + FA compared to FA alone (Fig. 4A). Similar increases were observed in response to LPS + O3 compared to O3 alone, indicating that LPS is the dominant factor for this increase. When the levels of oxidized SP-A were examined, significant differences were observed in the dimeric form of SP-A in response to O3 and in response to LPS + O3 (Fig. 4B). No significant differences were observed between LPS + FA and FA alone, indicating that changes in the oxidation state of SP-A are primarily due to O3 exposure.
FIGURE 4.
The effects of lipopolysaccharide (LPS) with or without ozone (O3), O3 alone or filtered air (FA) alone on the level of surfactant protein A (SP-A) (Panel A) and oxidized SP-A (Panel B) in the bronchoalveolar lavage (BAL) of wild type (WT) mice. The mice received 2 ng of LPS intrapharyngeally in 50 µl saline, then 13 h later they were exposed to either FA (gray bars) or O3 (2 ppm for 3 h) (black bars) and subjected to BAL 4 h after ozone exposure or 20 h after LPS treatment. SDS gel electrophoresis was performed on concentrated samples from 200 µl of BAL fluid and the proteins transferred to membranes as described in Methods. Levels of SP-A (Panel A; top) and oxidized SP-A (Panel B; top) were measured by densitometry of Western blots. For each condition n=6 except for FA, where n=5. At the bottom of panels A and B a representative immunoblot (Panel A) and an oxyblot (Panel B) are shown. A reference lane is shown that contains immunostained human alveolar proteinosis SP-A (hSP-A) with bands of monomeric (M) and dimeric (D) SP-A marked in each panel. The position of the oxidized SP-A dimer bands was determined by comparison with an identical immunoblot stained with an antiserum to SP-A. The values depicted are the mean ± S.D. Significant (p≤0.05) differences between LPS + FA and LPS + O3 treated mice are indicated by the pound sign (#) and other comparisons by an asterisk (*).
DISCUSSION
The authors have previously reported14 that WT mice and KO mice of the same genetic background showed signs of inflammation in BAL fluids after exposure to O3 (2 ppm) for three or six hours. Several aspects of the inflammatory response were less pronounced in KO mice, and it was speculated that this occurs because in the absence of SP-A the alveolar macrophage is not primed by SP-A, rendering it less capable of mounting an effective inflammatory response. Other researchers reported that high dose LPS (100 µg) inhalation, prior to O3 exposure of WT mice, appeared to sensitize the mice to O3 differentially at different postnatal ages, with regard to inflammatory markers8. The present study was designed to investigate the hypothesis that low doses of instilled LPS adversely affect the inflammatory processes in the lung and potentiate differentially the impact of O3-induced injury in mice with intact innate immunity (i.e., WT mice) and those with compromised innate immunity (i.e., KO mice).
The results showed that: 1) a low dose of LPS (2 ng) alters the cellular, cytokine, total protein and oxidized protein content of the BAL of WT mice at 20 h after LPS treatment; 2) differences are observed between WT and KO mice after LPS exposure (0.5 or 2 ng) in the total cell count, the MIP-2 response pattern, and the base levels of oxidized protein and phospholipid content; 3) when 3 h ozone exposure follows 13 h after LPS (2 ng) treatment, differences between WT and KO mice are observed in PMNs, MIP-2 protein and phospholipid content of BAL; 4) in WT an increase in total SP-A is observed in response to LPS + FA or LPS + O3 but not in response to O3 alone. An increase in oxidized SP-A dimer is observed in response to O3 or LPS + O3 but not in response to LPS + FA; 5) LPS treatment has no apparent impact on behaviour, whereas during the course of O3 exposure, both WT and KO mice become less active. These findings together allow the conclusion that: 1) low level LPS insult can have long lasting effects on BAL composition; 2) a second stimulus may result in more pronounced effects that those produced by each stimulus alone; and 3) SP-A plays a role in the regulation of inflammation.
The differences between the WT and KO mice in response to LPS are long-lasting, as they were detectable 20 h after LPS treatment, and are limited to the total BAL cell count and the pattern of the MIP-2 response. Although the reasons for this are not fully understood, these findings support the theory that in WT mice the alveolar cells, and most likely the macrophages, in the face of a weak threat (i.e. 0.5 ng LPS) can, via small non-significant changes in MIP-2 expression, mediate a significant recruitment of cells into the alveolar space. When the insult is stronger (i.e., 2 ng LPS), increases in molecules such as MIP-2, a macrophage-produced neutrophil chemoattractant molecule, are necessary for adequate cell recruitment. When the activity of the alveolar cells is compromised with regard to the first line of defense, as could be the case with the KO mice, the low level threat (0.5 ng LPS) appears to be perceived as a higher level threat (i.e. 2 ng LPS) and this results in an increase in molecules such as MIP-2. The increased MIP-2 production in the KO mice in response to a low level insult, failed to translate to the increase of total cells in the alveolar space seen in the WT mice that could presumably help to eliminate the perceived threat. Together these observations are consistent with the idea that KO mice exhibit a compromised and/or limited response compared to WT mice. It is possible that the alveolar macrophages of the KO mice, in contrast to those of the WT mice, are not adequately prepared to effectively mount a response to LPS. It has been postulated that the alveolar macrophages in the absence of SP-A are hypoactive due to lack of priming by SP-A18. Inability of KO mice alveolar macrophages to function at a level similar to that of the WT mice has been observed recently with regards to phagocytosis of K. pneumoniae27 and in several other studies where SP-A KO mice have been used. These include the inability of SP-A in KO mice to clear P. aeruginosa infection32,62 and group B streptococcus63, and the ability of SP-A to modulate inflammatory processes, and minimize lung injury and mortality after experimental bone marrow transplantation64–66.
When a second insult (i.e. O3 exposure) was imposed upon the LPS-treated mice, a significant decrease in PMNs and MIP-2 protein content was observed in the BAL of KO mice compared to WT mice, although both types of mice showed a significant increase in MIP-2 compared to LPS + FA treated mice. In contrast, when a single insult was present (either LPS (2 ng) or O3 exposure14) no differences in MIP-2 protein or PMNs (in LPS only) were observed between WT and KO mice, even though both types showed increase in MIP-2 protein and PMNs compared to non-treated controls. In the earlier O3 study14, although no difference between WT and KO mice was observed in response to O3 in BAL MIP-2 protein, the MIP-2 mRNA content was significantly lower in the KO than in the WT mice. These observations indicate that prior LPS treatment may up-regulate MIP-2 expression via a translational control. On the other hand O3 exposure in the KO mouse may negatively affect posttranscriptional processes involved in MIP-2 expression. These possibilities could explain the lower levels of MIP-2 protein observed in response to LPS + O3 in the KO mice compared to the WT mice, and implicate SP-A in the regulation of MIP-2.
The data from the earlier O3-exposure study14 indicate also that translation of MIP-2 may be halted entirely in the KO mouse, as assessed by the lack of difference in mRNA content between O3-exposed and control KO mice. Moreover, although LPS and O3 may each have an individual impact on MIP-2 production, the mechanisms via which this occurs are likely to differ or overlap. In the WT mice the change in MIP-2 levels with the combined exposure (LPS + O3) was greater than the sum of changes resulting from individual exposures (LPS or O3 alone). The MIP-2 values for the controls were 36 pg/ml ± 16, for LPS treated 148 pg/ml ± 38, and for those following O3 exposure (without prior LPS treatment) 627 pg/ml ± 20514. However, after exposure to both LPS and O3, MIP-2 levels reached 1,234 pg/ml ± 277, raising the possibility of synergism between the two agents. This synergism appears to be significantly compromised in the KO mouse, indicating a role for SP-A in the regulation of inflammation, and specifically the production of MIP-2.
Increased SP-A content in WT mice was observed in response to LPS and in agreement with the earlier O3 study14 no differences in SP-A content were observed in response to O3 exposure, indicating that the SP-A increase observed in response to LPS + O3 is primarily mediated by LPS. On the other hand, an increased level of oxidized SP-A was observed in response to O3, in both the present study and the earlier report14, but not in response to LPS, indicating that the oxidative stress mediated by O3 exposure is responsible for the increased content of oxidized SP-A in response to LPS + O3. The differences in SP-A content and oxidized SP-A observed in response to LPS and O3, both separately and in combination, indicate further that each agent operates via a different mechanism, and that LPS affects SP-A quantitatively, perhaps by interfering with its regulation, whereas O3 affects SP-A qualitatively by increasing oxidation of its dimeric form. At first glance it appears that LPS and O3 work at cross-purposes in terms of SP-A, as LPS increases the production and O3 increases the oxidation of SP-A. Recent studies have shown that oxidized SP-A exhibits reduced function27,39–41 and therefore even if the SP-A content is increased, the functional SP-A content may not change under the experimental conditions of this study. SP-A, however, has been shown to be oxidized earlier than the total BAL protein content14,46, indicating that SP-A may play a protective role in response to an oxidative stress by scavenging RONS and serving as a sacrificial antioxidant67,68. According to this scenario, SP-A may serve as the sacrificial molecule, as its high susceptibility to oxidation may scavenge reactive oxidants and protect other molecules from becoming inactivated by oxidation. Alternatively, as postulated previously14 the oxidation of dimeric SP-A may be important in signalling, and thus some of the defects observed in the KO mice may be due to lack of SP-A-mediated signalling in time of stress. Moreover, deficits of immune cell function, in both macrophages and neutrophils, and in the regulation of RONS production have been observed in KO mice, thereby implicating SP-A in these processes14,29–31.
In summary, the findings of this study lead to several conclusions: 1) A low dose of LPS can have a long-lasting effect on several aspects of BAL properties including cell count, cytokines, protein content, and other indices. 2) SP-A plays a role in the processes of inflammation in response to LPS and/or O3 exposure. LPS appears to have an impact on SP-A regulation, affecting the quantitative content of SP-A and the O3 effect on SP-A dimer oxidation, thus altering SP-A qualitatively, which may have an impact on its function. 3) SP-A appears to play a role in the regulation of MIP-2 perhaps via its ability to “prime” alveolar macrophages for an appropriate response in the face of an insult. LPS and O3 exposure probably modulate MIP-2 expression via different mechanisms. 4) Exposure to a low dose of LPS and O3 revealed some unexpected outcomes that were not observed with either insult alone, indicating that these agents operate via different and/or overlapping mechanisms. Finally, the present findings support the theory that in the absence of SP-A, alveolar macrophages may not be able to elicit an appropriate response to insulting agents, implicating SP-A in a role of adequately preparing or priming macrophages to respond.
Acknowledgements
The authors thank Julie Graham for typing. This work was supported by ES0098882 from the National Institute of Environmental Health Sciences.
REFERENCES
- 1.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996 Jul;60(1):8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Song M, Phelps DS. Interaction of surfactant protein A with lipopolysaccharide and regulation of inflammatory cytokines in the THP-1 monocytic cell line. Infect Immun. 2000 Dec;68(12):6611–6617. doi: 10.1128/iai.68.12.6611-6617.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broad A, Jones DE, Kirby JA. Toll-like receptor (TLR) response tolerance: a key physiological “damage limitation” effect and an important potential opportunity for therapy. Curr Med Chem. 2006;13(21):2487–2502. doi: 10.2174/092986706778201675. [DOI] [PubMed] [Google Scholar]
- 4.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005 Jan;3(1):36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 5.Kleeberger SR, Reddy SP, Zhang LY, Cho HY, Jedlicka AE. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2001 Feb;280(2):L326–L333. doi: 10.1152/ajplung.2001.280.2.L326. [DOI] [PubMed] [Google Scholar]
- 6.Kleeberger SR, Reddy S, Zhang LY, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: role of toll-like receptor 4. Am J Respir Cell Mol Biol. 2000 May;22(5):620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 7.Johnston CJ, Oberdorster G, Gelein R, Finkelstein JN. Endotoxin potentiates ozone-induced pulmonary chemokine and inflammatory responses. Exp Lung Res. 2002 Sep;28(6):419–433. doi: 10.1080/01902140290092029. [DOI] [PubMed] [Google Scholar]
- 8.Johnston CJ, Holm BA, Finkelstein JN. Sequential exposures to ozone and lipopolysaccharide in postnatal lung enhance or inhibit cytokine responses. Exp Lung Res. 2005 May;31(4):431–447. doi: 10.1080/01902140590918605. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa MG. Biochemical basis of ozone toxicity. Free Radic Biol Med. 1990;9(3):245–265. doi: 10.1016/0891-5849(90)90035-h. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy CH, Hatch GE, Slade R, Mason RP. Application of the EPR spin-trapping technique to the detection of radicals produced in vivo during inhalation exposure of rats to ozone. Toxicol Appl Pharmacol. 1992 May;114(1):41–46. doi: 10.1016/0041-008x(92)90094-9. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007 Jul;4(3):240–246. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol. 1998 Jan;274(1 Pt 1):L39–L46. doi: 10.1152/ajplung.1998.274.1.L39. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll KE, Simpson L, Carter J, Hassenbein D, Leikauf GD. Ozone inhalation stimulates expression of a neutrophil chemotactic protein, macrophage inflammatory protein 2. Toxicol Appl Pharmacol. 1993 Apr;119(2):306–309. doi: 10.1006/taap.1993.1074. [DOI] [PubMed] [Google Scholar]
- 14.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007 Apr 1;220(1):72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie WD, van Schaik SM, Vargas I, Enhorning G. Ozone affects breathing and pulmonary surfactant function in mice. Toxicology. 1998 Jan 16;125(1):21–30. doi: 10.1016/s0300-483x(97)00158-3. [DOI] [PubMed] [Google Scholar]
- 16.Muller B, Seifart C, Barth PJ. Effect of air pollutants on the pulmonary surfactant system. Eur J Clin Invest. 1998 Sep;28(9):762–777. doi: 10.1046/j.1365-2362.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 17.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 18.Floros J, Phelps DS. Pulmonary surfactant protein A; structure, expression, and its role in innate host defense. In: Nakos G, editor. Surfactant-Update of Intensive Care Medicine. Greece: Ioannina; 2002. pp. 87–102. [Google Scholar]
- 19.Phelps DS. Surfactant regulation of host defense function in the lung: a question of balance. Pediatr Pathol Mol Med. 2001 Jul-Aug;20(4):269–292. [PubMed] [Google Scholar]
- 20.Wright JR. Pulmonary surfactant: a front line of lung host defense. J Clin Invest. 2003 May;111(10):1453–1455. doi: 10.1172/JCI18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Iwaarden JF, van Strijp JA, Ebskamp MJ, Welmers AC, Verhoef J, van Golde LM. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am J Physiol. 1991 Aug;261(2 Pt 1):L204–L209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]
- 22.Madan T, Eggleton P, Kishore U, Strong P, Sarma PU, et al. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997 Aug;65(8):3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen MJ, Harbeck R, Smith B, Voelker DR, Mason RJ. Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect Immun. 1999 Sep;67(9):4563–4569. doi: 10.1128/iai.67.9.4563-4569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding J, Umstead TM, Floros J, Phelps DS. Factors affecting SP-A-mediated phagocytosis in human monocytic cell lines. Respir Med. 2004 Jul;98(7):637–650. doi: 10.1016/j.rmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005 Jan;288(1):L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- 26.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, et al. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect Immun. 2007 Mar;75(3):1403–1412. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008 Dec 4;9(1):77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watford WT, Wright JR, Hester CG, Jiang H, Frank MM. Surfactant protein A regulates complement activation. J Immunol. 2001 Dec 1;167(11):6593–6600. doi: 10.4049/jimmunol.167.11.6593. [DOI] [PubMed] [Google Scholar]
- 29.Hickman-Davis JM, Gibbs-Erwin J, Lindsey JR, Matalon S. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am J Respir Cell Mol Biol. 2004 Mar;30(3):319–325. doi: 10.1165/rcmb.2003-0246OC. [DOI] [PubMed] [Google Scholar]
- 30.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002 Mar;282(3):L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 31.Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, et al. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect Immun. 2004 Oct;72(10):6002–6011. doi: 10.1128/IAI.72.10.6002-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998 Oct;19(4):700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 33.Alcorn JF, Wright JR. Surfactant protein A inhibits alveolar macrophage cytokine production by CD14-independent pathway. Am J Physiol Lung Cell Mol Physiol. 2004 Jan;286(1):L129–L136. doi: 10.1152/ajplung.00427.2002. [DOI] [PubMed] [Google Scholar]
- 34.Reidy MF, Wright JR. Surfactant protein A enhances apoptotic cell uptake and TGF-beta1 release by inflammatory alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003 Oct;285(4):L854–L861. doi: 10.1152/ajplung.00439.2002. [DOI] [PubMed] [Google Scholar]
- 35.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002 Oct 1;169(7):3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez de Lara L, Becerril C, Montano M, Ramos C, Maldonado V, Melendez J, et al. Surfactant components modulate fibroblast apoptosis and type I collagen and collagenase-1 expression. Am J Physiol Lung Cell Mol Physiol. 2000 Nov;279(5):L950–L957. doi: 10.1152/ajplung.2000.279.5.L950. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez de Lara LG, Umstead TM, Davis SE, Phelps DS. Surfactant protein A increases matrix metalloproteinase-9 production by THP-1 cells. Am J Physiol Lung Cell Mol Physiol. 2003 Oct;285(4):L899–L906. doi: 10.1152/ajplung.00082.2003. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004 Mar;286(3):L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ Health Perspect. 2002 Jan;110(1):79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004 Apr 13;43(14):4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- 41.Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008 Jan;294(1):L121–L130. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med. 2002 Dec 15;33(12):1703–1713. doi: 10.1016/s0891-5849(02)01170-x. [DOI] [PubMed] [Google Scholar]
- 43.Haddad IY, Zhu S, Ischiropoulos H, Matalon S. Nitration of surfactant protein A results in decreased ability to aggregate lipids. Am J Physiol. 1996 Feb;270(2 Pt 1):L281–L288. doi: 10.1152/ajplung.1996.270.2.L281. [DOI] [PubMed] [Google Scholar]
- 44.Zhu S, Haddad IY, Matalon S. Nitration of surfactant protein A (SP-A) tyrosine residues results in decreased mannose binding ability. Arch Biochem Biophys. 1996 Sep 1;333(1):282–290. doi: 10.1006/abbi.1996.0392. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S, Kachel DL, Martin WJ, 2nd, Matalon S. Nitrated SP-A does not enhance adherence of Pneumocystis carinii to alveolar macrophages. Am J Physiol. 1998 Dec;275(6 Pt 1):L1031–L1039. doi: 10.1152/ajplung.1998.275.6.L1031. [DOI] [PubMed] [Google Scholar]
- 46.Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am J Physiol Lung Cell Mol Physiol. 2009 Jan;296(1):L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- 47.Fehrenbach H, Brasch F, Uhlig S, Weisser M, Stamme C, Wendel A, et al. Early alterations in intracellular and alveolar surfactant of the rat lung in response to endotoxin. Am J Respir Crit Care Med. 1998 May;157(5 Pt 1):1630–1639. doi: 10.1164/ajrccm.157.5.9611070. [DOI] [PubMed] [Google Scholar]
- 48.Viviano CJ, Bakewell WE, Dixon D, Dethloff LA, Hook GE. Altered regulation of surfactant phospholipid and protein A during acute pulmonary inflammation. Biochim Biophys Acta. 1995 Dec 7;1259(3):235–244. doi: 10.1016/0005-2760(95)00167-0. [DOI] [PubMed] [Google Scholar]
- 49.Quintero OA, Korfhagen TR, Wright JR. Surfactant protein A regulates surfactant phospholipid clearance after LPS-induced injury in vivo. Am J Physiol Lung Cell Mol Physiol. 2002 Jul;283(1):L76–L85. doi: 10.1152/ajplung.00418.2001. [DOI] [PubMed] [Google Scholar]
- 50.Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am J Physiol. 1997 Jun;272(6 Pt 1):L1070–L1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 51.Walti H, Polla BS, Bachelet M. Modified natural porcine surfactant inhibits superoxide anions and proinflammatory mediators released by resting and stimulated human monocytes. Pediatr Res. 1997 Jan;41(1):114–119. doi: 10.1203/00006450-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Haagsman HP, van Golde LM. Synthesis and assembly of lung surfactant. Annu Rev Physiol. 1991;53:441–464. doi: 10.1146/annurev.ph.53.030191.002301. [DOI] [PubMed] [Google Scholar]
- 53.Oosting RS, van Greevenbroek MM, Verhoef J, van Golde LM, Haagsman HP. Structural and functional changes of surfactant protein A induced by ozone. Am J Physiol. 1991 Aug;261(2 Pt 1):L77–L83. doi: 10.1152/ajplung.1991.261.2.L77. [DOI] [PubMed] [Google Scholar]
- 54.Putman E, van Golde LM, Haagsman HP. Toxic oxidant species and their impact on the pulmonary surfactant system. Lung. 1997;175(2):75–103. doi: 10.1007/pl00007561. [DOI] [PubMed] [Google Scholar]
- 55.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem Res Toxicol. 2002 Jul;15(7):896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 56.Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997 Mar 10;202(1):49–57. doi: 10.1016/s0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]
- 57.Wagner JG, Van Dyken SJ, Wierenga JR, Hotchkiss JA, Harkema JR. Ozone exposure enhances endotoxin-induced mucous cell metaplasia in rat pulmonary airways. Toxicol Sci. 2003 Aug;74(2):437–446. doi: 10.1093/toxsci/kfg120. [DOI] [PubMed] [Google Scholar]
- 58.Delayre-Orthez C, Becker J, de Blay F, Frossard N, Pons F. Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2005 Dec;138(4):298–304. doi: 10.1159/000088867. [DOI] [PubMed] [Google Scholar]
- 59.Umstead TM, Phelps DS, Wang GR, Floros J, Tarkington BK. In vitro exposure of proteins to ozone. Toxicology Mechanisms and Methods. 2002;12(1):1–16. doi: 10.1080/15376510209167932. [DOI] [PubMed] [Google Scholar]
- 60.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000 May;278(5):L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 61.Robinson CE, Keshavarzian A, Pasco DS, Frommel TO, Winship DH, Holmes EW. Determination of protein carbonyl groups by immunoblotting. Anal Biochem. 1999 Jan 1;266(1):48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- 62.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006 Jun;34(6):704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, et al. Surfactant protein-A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999 Feb;20(2):279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 64.Yang S, Milla C, Panoskaltsis-Mortari A, Hawgood S, Blazar BR, Haddad IY. Surfactant protein A decreases lung injury and mortality after murine marrow transplantation. Am J Respir Cell Mol Biol. 2002 Sep;27(3):297–305. doi: 10.1165/rcmb.2002-0035OC. [DOI] [PubMed] [Google Scholar]
- 65.Haddad IY, Milla C, Yang S, Panoskaltsis-Mortari A, Hawgood S, Lacey DL, et al. Surfactant protein A is a required mediator of keratinocyte growth factor after experimental marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2003 Sep;285(3):L602–L610. doi: 10.1152/ajplung.00088.2003. [DOI] [PubMed] [Google Scholar]
- 66.Hickman-Davis JM, Wang Z, Fierro-Perez GA, Chess PR, Page GP, Matalon S, et al. Surfactant dysfunction in SP-A−/− and iNOS−/− mice with mycoplasma infection. Am J Respir Cell Mol Biol. 2007 Jan;36(1):103–113. doi: 10.1165/rcmb.2006-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim IG, Park SY, Oh TJ. Dithiothreitol induces the sacrificial antioxidant property of human serum albumin in a metal-catalyzed oxidation and gamma-irradiation system. Arch Biochem Biophys. 2001 Apr 1;388(1):1–6. doi: 10.1006/abbi.2000.2255. [DOI] [PubMed] [Google Scholar]
- 68.Sayed AA, Cook SK, Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J Biol Chem. 2006 Jun 23;281(25):17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]