Non-coding small RNA (sRNA) molecules regulate gene expression networks in all domains of life (1, 2), but inferences regarding the roles of sRNAs in adaptive evolution have been indirect. Here we show that a spontaneous adaptive mutation in the bacterium Myxococcus xanthus restored a defective social trait – multicellular fruiting body development – by deactivating a previously undiscovered sRNA, here designated ‘Pxr’. We show that Pxr acts as a pivotal regulatory checkpoint that blocks Myxococcus development until nutrients have been depleted.
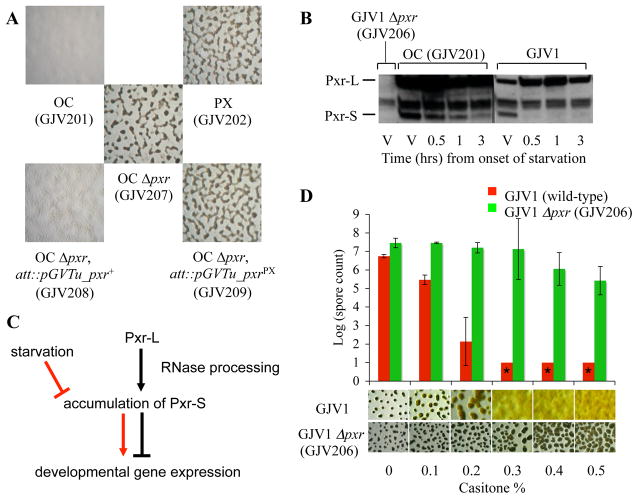
Upon starvation, M. xanthus cells aggregate, exchange multiple signals and construct spore-bearing fruiting bodies (3). In a previous study, an M. xanthus “cheater” strain (OC, ‘Obligate Cheater’) that is defective at development evolved into a strain (PX, ‘Phoenix’) with restored developmental proficiency that emerged after a cheater-induced population crash (4). Development was restored in PX by a single C→A mutation in the intergenic region upstream of an acetyltransferase gene (Mxan_1079) (Fig. S1A) (4). PX development does not require Mxan_1079, as disruption of this gene in strain PX (Fig. S2, GJV204, Table S1) does not impair development. Changing the C→A mutation in GJV204 back to the wild-type allele (A→C) abolishes development (Fig. S2, GJV205), indicating that the region between Mxan_1078 and Mxan_1079 encodes a genetic element, pxr, that controls development.
The pxr region contains a putative σ54 promoter (Fig. S1A) preceding a sequence that appears to encode a non-coding sRNA similar in structure to some Escherichia coli sRNAs (Fig. S1B) (2). The only detected homolog of this sRNA gene was found in the myxobacterium Stigmatella aurantiaca (Fig. S1B). Two pxr-specific sRNA forms, Pxr-L (long) and Pxr-S (short), are present in vegetative cells of the developmentally proficient “wild-type” strain GJV1 and the defective strain OC (GJV201) but are absent from pxr-deletion mutants of GJV1 (GJV206, Fig. 1B) and OC (GJV207, Fig. S1C).
Figure 1.
(A) pxr negatively regulates M. xanthus development. (B) Temporal patterns of Pxr production in OC and GJV1. “V” indicates vegetative cells and the asterisk indicates non-Pxr RNA detected in GJV1 Δpxr (GJV206). (C) Model of the Pxr developmental checkpoint in M. xanthus. Pxr-L and Pxr-S are both produced at high levels during vegetative growth (black) and Pxr-S blocks development. Upon starvation (red), Pxr-S accumulation is eliminated and development proceeds. (D) pxr inhibits development in the presence of nutrients. The graph and images show spore production and developmental phenotypes, respectively, at different casitone levels. Asterisks indicate zero spores at the limit of detection and error bars represent 95% confidence intervals.
Deletion of pxr from OC restores development (Fig. 1A, GJV207). Subsequent ectopic expression of the pxr+ allele in GJV207 suppressed development (Fig. 1A, GJV208), demonstrating that Pxr negatively regulates this process. Ectopic expression of the pxrPX allele in GJV207 failed to block development (Fig. 1A, GJV209), indicating that the C→A mutation in strain PX deactivates Pxr function, thereby allowing development to proceed.
We compared the temporal patterns of Pxr accumulation during starvation in GJV1 and OC (Fig. 1B). Pxr-L is present at high levels during both vegetative growth and starvation in both GJV1 and OC (Fig. 1B). In contrast, Pxr-S is present at high levels in vegetative cells of both GJV1 and OC but is greatly diminished in GJV1 soon after the onset of starvation (Fig. 1B). This suggests that Pxr-S, but not Pxr-L, is the primary negative regulator of development when nutrients are abundant and that the rapid reduction of Pxr-S upon starvation allows development to proceed (Fig. 1C). Under this model, OC does not develop because it fails to sufficiently reduce Pxr-S. Indeed, Pxr-S remains present in OC through at least 23 hrs of starvation (Fig. S3A) but remains absent from GJV1 (Fig. S3B). Pxr-S may originate from processing of Pxr-L in a manner analogous to processing of eukaryotic microRNAs (1).
The transition from vegetative growth to development is a critical juncture in the life history of fruiting myxobacteria (5). We tested whether pxr might govern this transition in M. xanthus by deleting this gene from the wild-type GJV1 and comparing the developmental phenotypes of GJV1 and the mutant (GJV206) at several nutrient (casitone) levels (Fig. 1D). GJV1 spore production drops precipitously with increasing casitone and no spores or fruiting bodies are made at 0.3–0.5% casitone. In contrast, GJV206 forms fruiting bodies even at 0.5% casitone and produces nearly as many spores at high casitone levels as GJV1 does under complete starvation. These results show that pxr is a key developmental gatekeeper that blocks M. xanthus development when food is abundant.
Supplementary Material
Acknowledgments
We thank L. Sogaard-Andersenn, L. Kroos,… and for helpful discussion and C. Fuqua for manuscript suggestions. This work was supported by NIH grant GM079690 to G.J.V.
References and Notes
- 1.Stefani G, Slack FJ. Nat Rev Mol Cell Biol. 2008;9:219. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman LS. Trends Genet. 2005;21:399. doi: 10.1016/j.tig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Shimkets LJ, Reichenbach H, Dworkin M. In: The Prokaryotes. Dworkin M, editor. Vol. 7. Springer; New York: 2005. pp. 31–115. [Google Scholar]
- 4.Fiegna F, Yu YTN, Kadam SV, Velicer GJ. Nature. 2006;441:310. doi: 10.1038/nature04677. [DOI] [PubMed] [Google Scholar]
- 5.Harris BZ, Kaiser D, Singer M. Genes Dev. 1998;12:1022. doi: 10.1101/gad.12.7.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.