1. Introduction
Transcutaneous electrical nerve stimulation (TENS) involves the application of electrical currents to the skin for pain control; it is a noninvasive modality that is commonly used by health care professionals to control both acute and chronic pain arising from several conditions [3,18,20,24,49,52–53]. TENS emerged and became widely accepted after the publication of the gate control theory of pain [44]. Both high-and low-frequency TENS cause hypoalgesia through the release of endogenous opioids in the central nervous system [9,34,58]. At the spinal level and the rostral ventral medulla there are different opioids released with different stimulation frequencies and thus different opioid receptors activated to produce analgesia with high or low frequency TENS [62]. Low frequencies, usually below 10Hz, activate μ-opioid receptors and high frequencies, above 50Hz, activate δ-opioid receptors [9,34,58].
Repeated stimulation of opioid receptors by repeated administration of morphine or opioid analgesics can lead to an analgesic tolerance defined as a decrease in analgesic effectiveness with repeated use [43]. In a similar way, repeated utilization of therapeutic electrophysical agents that reduce pain through release of endogenous opioids could have a gradual diminution of their analgesic effect. Chandran and Sluka [9] demonstrated in rats that repeated administration of low and high frequency TENS leads to a development of opioid tolerance with a corresponding cross-tolerance to intrathecally administered μ- and δ-opioid agonists, respectively. In clinical practice, TENS is usually applied daily over many weeks. Approximately 30% of patients fail to respond to TENS and, of the patients who respond initially, only a third continue to obtain pain relief after two years [4]. Solomon et al. [63] showed that people who had been taking opioids long enough to develop tolerance prior to surgery did not respond to TENS when used postoperatively. Although commonly accepted that TENS reduces its efficacy with repeated application, the development of tolerance to TENS has not been investigated and confirmed in human subjects. With these concerns in mind, the aim of the present investigation was to examine the analgesic tolerance to TENS in human subjects.
2. Methods
2.1. Subjects
One-hundred healthy, TENS-naïve, pain-free subjects (48 men, 52 women; mean age 31.75 ± 12.05 years; age range 18–60 years) were recruited from the staff and students of the University of Iowa after approval was obtained from the local Institutional Review Board. The sample size was calculated using data from previous studies on PPT and TENS [51]. Considering a significance level of 5%, power of 80%, 4 treatment groups, and an effect size of 0.4, it was calculated that 25 participants were required in each group. Subjects were screened and excluded if they had altered skin sensation or history of recent trauma in upper limbs, cardiac pacemaker, pregnancy, and if they were receiving any type of pain medication. After the participants provided written informed consent they were stratified by gender and randomly assigned to 1 of 4 groups: control (n = 25), placebo TENS (n = 25), low frequency TENS (n = 25) and high frequency TENS (n = 25). Randomization was performed using the sequentially numbered, opaque sealed envelopes (SNOSE) allocation concealment method [22,55]. The envelopes were stored in a secure cabinet that only the allocation investigator had access to and were opened immediately prior to intervention allocation.
Demographic information including age, race, gender, height, and weight were recorded. There were no significant differences between groups based on age, race, gender, or body mass index (BMI) (Table 1).
Table 1.
Characteristics of the study participants
| Control (n = 25) | Placebo TENS (n = 25) | Low Frequency TENS (n = 25) | High Frequency TENS (n = 25) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 12 (48%) | 12 (48%) | 12 (48%) | 12 (48%) |
| Female | 13 (52%) | 13 (52%) | 13 (52%) | 13 (52%) |
| Age (mean ± SD) | 30.48 ± 2.04 | 30.4 ± 2.53 | 38.2 ± 2.85 | 27.92 ± 1.67 |
| BMI (mean ± SD) | 25.21 ± 0.92 | 26.16 ± 0.62 | 25.46 ± 1.09 | 25.59 ± 1.09 |
| Race | ||||
| Caucasian | 21 (84%) | 24 (96%) | 21 (84%) | 22 (88%) |
| African American | 1 (4%) | 0 (0%) | 0 (0%) | 1 (4%) |
| Asian | 1 (4%) | 1 (4%) | 3 (12%) | 1 (4%) |
| Other | 2 (8%) | 0 (0%) | 1 (4%) | 1 (4%) |
2.2. Pressure Pain Threshold
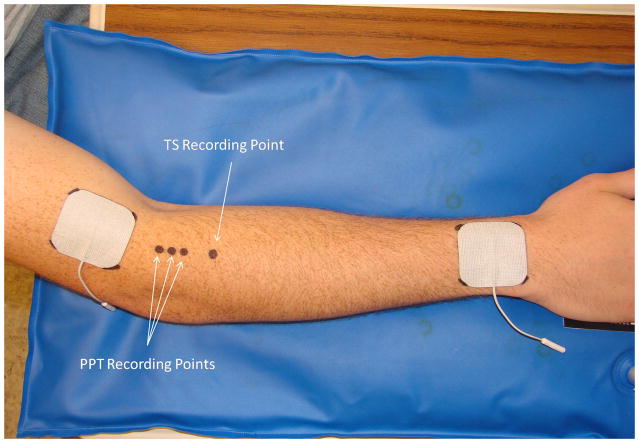
Pressure-pain threshold (PPT) has been reported to reflect mainly pressure pain sensitivity of deeper tissues [36–38]. PPT measurements were recorded by an assessor who was blind to group allocation using a Somedic Type II digital pressure algometer (Somedic Inc, Hörby, Sweden) from three marked spots along the extensor mass of the non-dominant forearm (2, 3 and 4 cm below the elbow crease) (Fig. 1). The pressure was applied perpendicularly to the skin at a rate of 50kPa/s through a flat circular probe measuring 1cm2 and covered with 1mm of rubber to avoid painful skin stimuli due to sharp metal edges [37–38]. Subjects were instructed to press the algometer button when pressure was first perceived as pain and the algometer was retracted at this point. The average of the PPT scores recorded at the 3 points was used as the final value at each measurement time. Each subject had 2 practice trials on the non-testing forearm followed by the data collection round.
Figure 1.
This figure illustrates the placement of TENS electrodes and recording sites for PPT and TS.
2.3. Tonic experimental pressure pain stimulus
Pain intensities to tonic pressure were used to determine pressure temporal summation. A temporal summation (TS) area was marked on the posterior aspect of the non-dominant forearm, over the extensor mass, 6cm below the crease of the elbow (Fig. 1). Temporal summation was measured with a custom-built device incorporating a pressure transducer and a lever with a movable weight to grade the pressure applied (kPa). The forearm was secured in place with a vacuum pillow (Versa Form, Sammons Preston, Bolingbrook, IL) and the pressure stimulus was delivered through a 1cm2 probe. Temporal summation was tested at an 8/20 pain rating for 2 minutes with subjects rating their pain every 10 seconds on a 0 to 20 numeric rating scale (0 = no pain and 20 = the worst pain imaginable) starting at time zero. The area under the curve was calculated for data analysis using the following formula:
Where: A = area under the curve; Pi = pain intensity at moment i; P0 = pain intensity at moment 0; Δt = time frame between the measurements of pain intensity (10 seconds).
The 0–20 numeric rating scale (NRS) was used because it was found to be easier to use and associated with higher compliance and lower failure rates when compared to the Visual Analogue Scale [28]. It has established validity and reliability for assessing acute pain [23,30,46].
2.4. TENS Procedure
The subject’s skin was cleansed with mild soap and water, and 2 square self-adhesive electrodes (5 × 5cm) (StimCare Premium Electrodes, Empi Inc., St. Paul, MN) were placed 1cm proximal to the elbow crease and 1cm proximal to the wrist crease on the dorsum of the non-dominant upper limb. The corners of the electrodes were marked on the skin using a permanent marker to allow exactly the same electrodes positioning during the 5 consecutive days of the study (Fig. 1). Subjects were instructed not to wash off these markings during the week of testing.
Two active units were used: one was set at low frequency (4Hz) and another was set at high frequency (100Hz). These active units applied TENS (100μs pulse duration) at maximal tolerable intensity for 20 minutes to the non-dominant forearm, daily for 5 days. This duration of TENS application was based on other studies showing the effect of TENS in experimental pain models [10–12,51,72]. Maximal tolerable intensity was the greatest intensity the subject could tolerate that was not painful. In some cases this intensity produced a motor contraction. The average amplitude employed in the low frequency TENS group was 30.64±1.59mA and in the high frequency TENS group was 25.79±1.29mA. Previous work from our laboratory in animals shows that TENS activates large diameter afferents at just below or just above motor threshold. Nociceptors were not activated until we get to 2x motor threshold, a clearly painful stimulus in humans [50]. In humans, a similar pattern of activation is observed in the ranges that we used. Levin and Hui-Chan [41] performed recordings from the median nerve in human subjects and showed that high frequency TENS (100Hz) applied at three times the sensory threshold activates only large-diameter Aβ fibers. Similarly, low frequency TENS (4Hz), at maximal tolerable intensity, activates only Aβ afferent fibers, whereas Aδ activation only occurs at intensities above maximal tolerable intensity. Thus, we believe that intensities we used in the present study only activated Aβ afferent fibers. The pulse amplitude applied on Day 1 was noted and on all subsequent days the same dose of TENS was applied, i.e. all TENS parameters were kept constant for the rest of the week. The placebo TENS was applied using a sham unit that looked similar to the active unit. This unit actively applied TENS (continuous mode, 100Hz, 100μs) at maximal tolerable intensity for 30 seconds and then the current ramped off over the next 15 seconds. The same parameters used on day 1 were used for the rest of the week. All devices were Rehabilicare Maxima TENS units and they were identical in appearance (Empi Inc., St. Paul, MN). They delivered a rectangular, balanced, asymmetrical, biphasic pulsed current.
The TENS units were calibrated using a digital oscilloscope (TDS 430A, Tektronix Inc, Beaverton, OR) prior to starting the study. For each pulse amplitude setting on the devices, peak to peak voltage was measured across a 1kΩ resistor to calculate the corresponding current in mA.
TENS applications were performed by an investigator who did not participate in outcome assessments. During the pain measurements, the intensity of TENS was decreased and kept at a sensory intensity level to ensure the pain assessor was kept blind to the subject’s group allocation.
2.5. Activation of Diffuse Noxious Inhibitory Control
The cold pressor test was used on day 5 to induce pain and to trigger the diffuse noxious inhibitory control (DNIC) response [35,65]. The conditioning stimulus consisted of the immersion of the subjects’ testing side lower extremity in a bucket of ice water (4°C) to just above the ankle. Pain intensity rating was measured 20 seconds after immersion on a 0–20 scale. PPT was recorded 30 seconds after immersion from the three spots over the non-dominant forearm as described before. Before they removed the foot from the water, subjects were asked to rate their pain again on a 0–20 scale. The percentage of PPT change was calculated considering the PPT values recorded on day 5 (baseline).
2.6. General Overview of Protocol
One day 1 after obtaining consent and demographic information, the subjects were randomized into one of four groups. They were asked to remain seated in a comfortable upright position during all procedures. The non-dominant forearm was cleansed, the PPT and TS areas were marked as described before, and the TENS electrodes were applied to subjects allocated in active or placebo TENS groups. PPTs were assessed followed by TS measurement and the pain assessor left the room. In the placebo TENS, low frequency TENS, and high frequency TENS groups, the leads were connected to the electrodes and the treatment was applied for 20 minutes. Subjects in the control group were informed they should rest for 20 minutes. After a 20 minute treatment interval, the pain assessor returned, TS and PPTs were reassessed and subject’s weight and height were measured.
On days 2 to 4, PPTs were recorded before and after a 20 minute treatment (or rest in control group) interval.
On day 5, the PPTs and TS were recorded before and after the 20 minute TENS application (or rest in control group). After TENS, the DNIC response was assessed.
2.7. Blinding Assessment
At the conclusion of testing, the TENS investigator asked the subject “Do you think you received active TENS, placebo TENS or don’t know?” The pain assessor was asked “Do you think the subject received active TENS, placebo TENS or don’t know?” Their responses to these questions were recorded and used to gauge the adequacy of subject and investigator blinding [51].
2.9. Statistical Analyses
Changes in PPT were calculated each day as percentage (%) of baseline (Pre TENS), where no change was equivalent to 0%. Positive % values represent hypoalgesia and negative values represent hyperalgesia. Changes in temporal summation for pressure stimuli were calculated as a difference in the area under the curve for the duration of testing (i.e. 120 seconds) post-TENS to pre-TENS. Descriptive statistics were calculated for all variables and tests for normal distribution (Shapiro Wilk) were carried out. The PPT percentage change data were normally distributed and were therefore analyzed using a repeated measures ANOVA with between-subjects factors. A one-way ANOVA for independent samples compared differences between groups at each time period. Post hoc testing was performed with a Tukey’s test for differences between groups. Chi squared test was used to compare the % of summators and non-summators on days 1 and 5. Repeated measures ANOVA was also used to compare differences across time during TS, between summators and non-summators, and between days 1 and 5 for groups. A paired t-test compared the difference in TS between days 1 and 5 for each group and the pain intensity during DNIC test at 20 seconds and at the end of test. The PPT percentage of change data recorded during DNIC test were not normally distributed and were therefore analyzed using Kruskal-Wallis test. Associations among the pain intensity and % of PPT change during DNIC test were assessed using Pearson’s product-moment correlation coefficients. Statistical significance was set at p < 0.05. All analyses were performed using SPSS (version 17.0; SPSS Inc, Chicago, IL). Data are presented as mean ± S.E.M.
3. Results
3.1 PPT Data
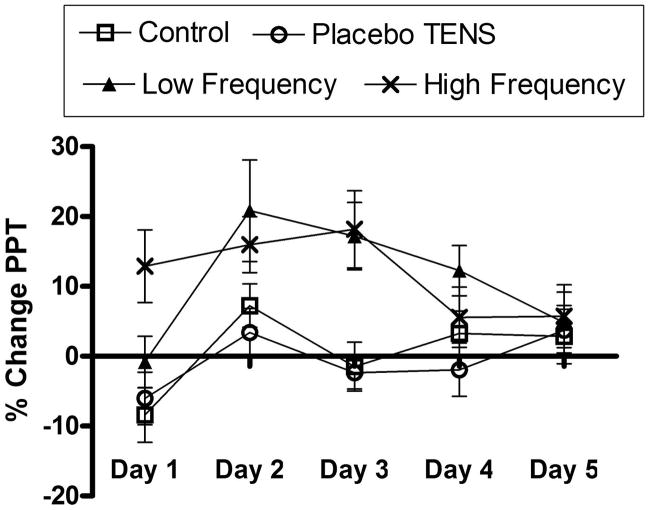
Mean PPT percentage of change (± S.E.M.) for all experimental groups on each day are shown in Figure 2. The repeated measures ANOVA with between-subjects factors revealed differences over days (p = 0.006) and between groups (p > 0.001). There was no significant interactive effect between days and groups (p = 0.091). Post hoc Tukey tests indicated a significant hypoalgesic effect in the low frequency TENS group when compared to the control group (p = 0.024) and the placebo TENS group (p = 0.004). Similarly the high frequency TENS group showed a significant difference when compared with control and placebo TENS groups (p = 0.012, p = 0.002). No significant differences were found between control and placebo TENS groups (p = 0.945) or between low and high frequency TENS groups (p = 0.995).
Figure 2.
Percentage of change in pressure pain threshold (PPT) for each experimental group during the 5 consecutive days.
For comparisons between groups on each day, control and placebo TENS groups were combined since there were no significant differences between them. One-way ANOVA for independent samples identified that significant differences occurred between the combined control and placebo TENS groups and the other experimental groups from day 1 to day 4 with values ranging from p < 0.0001 to p = 0.0443. On day 5, no differences were observed between groups (p = 0.8766), representing a decrease in hypoalgesic effect presented by active TENS groups. Post hoc Tukey tests are summarized in Table 2.
Table 2.
Summary of Tukey post hoc tests for statistical comparison between low and high frequency TENS groups with the combined control + placebo TENS groups at each day.
| Day | Group | Comparison group | Mean difference between groups | P Value |
|---|---|---|---|---|
| 1 | Control + Placebo | Low Frequency | −6.39 | > 0.05 |
| High Frequency | −20.11 | < 0.001 | ||
| Low Frequency | High Frequency | −13.72 | > 0.05 | |
| 2 | Control + Placebo | Low Frequency | −15.60 | < 0.05 |
| High Frequency | −10.73 | > 0.05 | ||
| Low Frequency | High Frequency | 4.86 | > 0.05 | |
| 3 | Control + Placebo | Low Frequency | −19.13 | < 0.01 |
| High Frequency | −20.10 | < 0.001 | ||
| Low Frequency | High Frequency | −0.96 | > 0.05 | |
| 4 | Control + Placebo | Low Frequency | −11.65 | < 0.05 |
| High Frequency | −4.97 | > 0.05 | ||
| Low Frequency | High Frequency | 6.68 | > 0.05 | |
| 5 | Control + Placebo | No significant differences |
3.2. Mechanical Temporal Summation Data
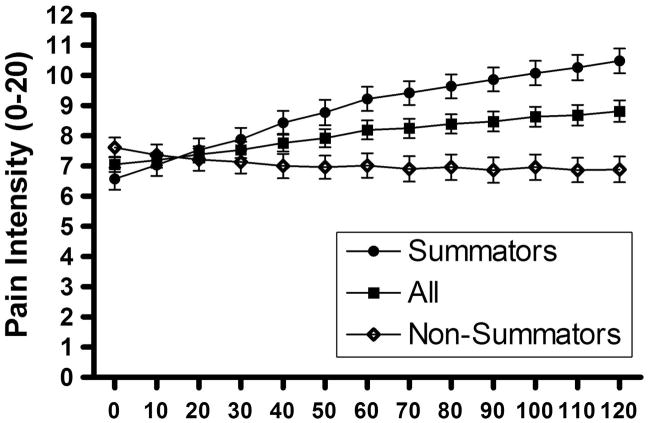
One subject was excluded from analysis due to a large variation in pressure readings for mechanical temporal summation testing. Figure 3 shows the pain intensity during temporal summation to tonic pressure in all subjects. There was a significant increase in pain over time (p < 0.0001). Further, the subjects were divided in summators (n = 53) and non-summators (n = 46) (summators were defined as having an area under the curve above 100 pain intensity. sec). There was a significant higher increase in pain intensity over time in summators when compared with non-summators (p = 0.001). Chi squared test revealed no significant difference in percentage of summators on day 1 compared to day 5 (p = 0.1070).
Figure 3.
Pain intensity during temporal summation to tonic pressure in all subjects, summators and non-summators.
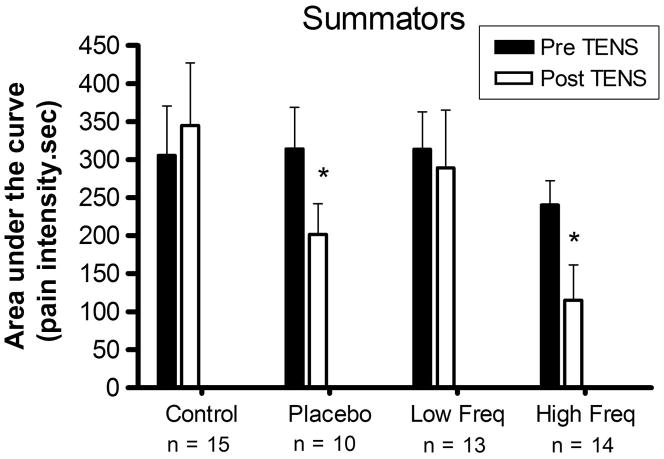
When considering all subjects (summators and non-summators), there were no significant changes in area under the curve pre and post TENS on days 1 and 5 (p > 0.05). However, considering only the summators, both placebo TENS and high frequency TENS groups presented a decrease in mechanical temporal summation on day 1 after treatment (p = 0.0325, p = 0.0120) (Fig. 4).
Figure 4.
Area under the curve after temporal summation to tonic pressure in summators subjects on day 1 (* indicates significant difference with the pre TENS area).
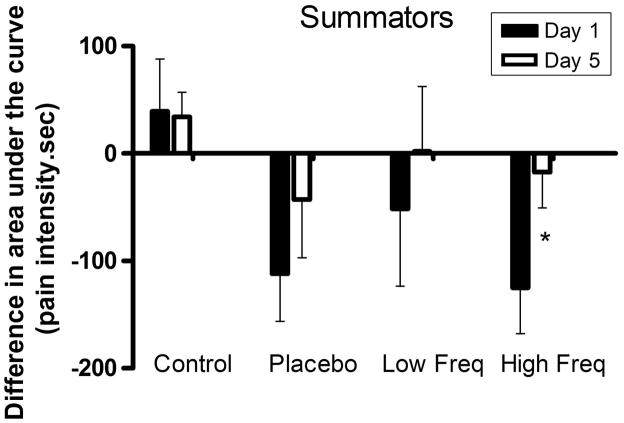
Repeated measures ANOVA did not show significant differences in the difference scores in the area under the curve (area under the curve post TENS – area under the curve pre TENS) between groups (p = 0.072) and across time (p = 0.132) in summators. Nevertheless, when comparing the difference in the area under the curve on day 1 to day 5, the high frequency TENS group showed a significant decrease in difference scores (p = 0.036) on day 5 (Fig. 5) suggesting reduced effectiveness of high frequency TENS on temporal summation.
Figure 5.
Difference scores (post TENS area – pre TENS area) in area under the curve after temporal summation to tonic pressure in summators subjects on days 1 and 5 (* indicates significant difference with the day 1).
3.3. DNIC Data
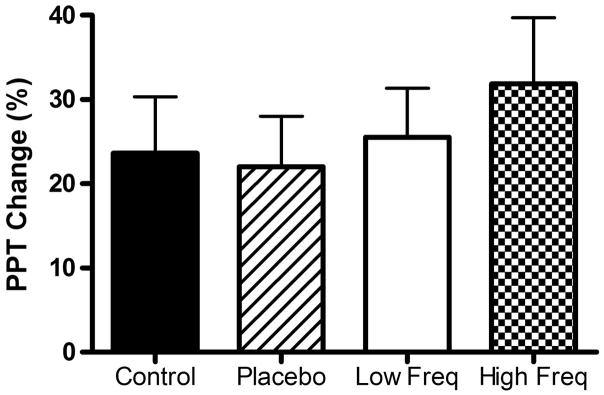
As described before, pain intensity was measured during the cold pressor test, 20 seconds after immersion and at the end of test, on a 0–20 scale. The mean pain rating for all subjects at 20 sec was 8.95 ± 0.48, and at the end was 12.19 ± 0.50. There was a significant difference in pain intensity between the 2 times (p < 0.0001). There was also a positive correlation between the pain intensity during the cold pressor test and the percentage of change in PPT (r = 0.289, p = 0.004) (Fig. 6). There was no significant difference in the percentage of change in PPT between the study groups (p = 0.6858) (Fig. 7).
Figure 6.
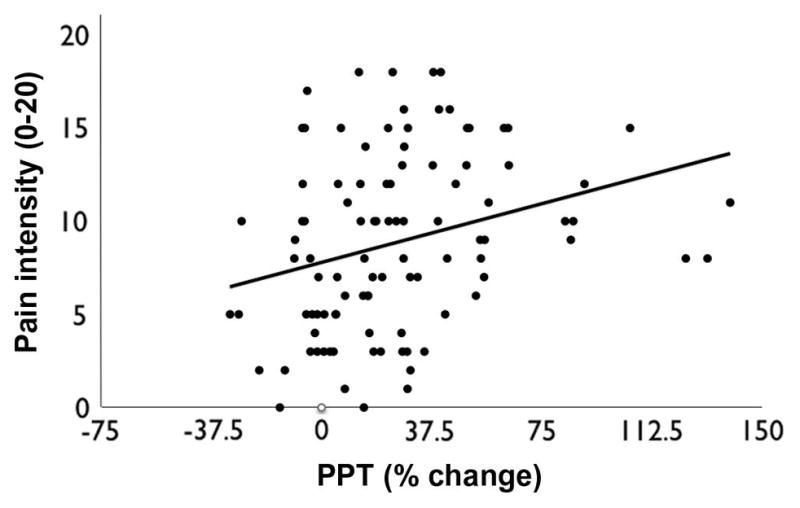
Scatter plot and correlation between pain intensity and percent change in PPT during DNIC test.
Figure 7.
Percentage of change in PPT during cold pressor test in all groups.
3.4. Assessment of Blinding
The pain assessor correctly identified that subjects received an active TENS unit in 4% (1/25) of cases in both the high and low frequency TENS groups. The same rate was observed in the placebo TENS group where the assessor correctly identified the placebo unit in 4% (1/25) of time. In other words, the assessor was blinded 96% of the time when recording pain measures in placebo, low frequency and high frequency TENS groups, indicating successful blinding (p < 0.0001).
Subjects were blinded to the treatment 20% (5/25) of the time in the high frequency TENS group, 36% (9/25) in the low frequency TENS group, and 48% (12/25) in the placebo TENS group. The rate of blinding in the placebo TENS group was no different than chance (random 50:50 probability) (p = 0.8415).
4. Discussion
Patients with chronic pain who initially respond to TENS may become long-term users [8,26,32–33]. Since animal studies show that TENS activates opioids receptors to produce analgesia, repeated TENS applications could cause analgesic tolerance similar to long-term use of opioids [43]. Although analgesic tolerance to TENS has been demonstrated in rats [9,17,19], this is the first study designed to address specifically the analgesic tolerance to TENS in humans. The results show that repeated daily application of either high- or low-frequency TENS with the same daily dose (intensity, frequency, pulse duration, and treatment duration) and electrode position results in a decrease in its hypoalgesic effect by the 4th and 5th consecutive days, respectively. These data parallel findings in animal studies which show the tolerance-like effect to repeated TENS results from tolerance at centrally-located opioid receptors [9]. Clinical studies in human subjects confirm that opioid receptors play a role in TENS analgesia. These studies show release of endogenous opioids in human subjects during TENS, low and high frequency TENS analgesia is blocked by systemic naloxone and that TENS is less effective in patients who are opioid tolerant [27,39–40,54,56,63]. Clinically, 32% of long-term users of TENS with a variety of chronic pain conditions report a decline in TENS efficacy from the time the unit was issued [32]. However, the length of TENS utilization necessary to cause analgesic tolerance in these users was not reported. It is possible that, in a clinical setting, the development of analgesic tolerance can be delayed if TENS parameters or treatment schedule are changed. We previously [19] showed in rats that mixed- (low and high frequency simultaneously) or daily alternating-frequency (4/100Hz) TENS delays the occurrence of analgesic tolerance. Alternatively, increasing the pulse amplitude, and thereby increasing the dose, could also alleviate analgesic tolerance to TENS. Future experiments need to confirm if changing stimulation parameters in human subjects can prevent analgesic tolerance.
Prior studies compared efficacy of different frequencies of TENS, and mixed TENS frequencies on a variety of outcomes. Alternating TENS frequency (2Hz, 100μs, 3.5s × 100Hz, 700μs, 2.5s) results in a greater increase in heat pain thresholds in healthy subjects when compared with 2Hz, 100Hz or control (no TENS) [64]. However, 100Hz TENS was more effective for increasing mechanical pain threshold [64]. When comparing different pulse patterns of TENS on ice-pain thresholds in healthy subjects, continuous high frequency TENS (80Hz) produced the greatest mean elevation when compared to burst, modulation (burst with amplitude modulation), and random frequencies (14–188Hz); although all pulse patterns increased ice-pain thresholds when compared to controls [31]. In direct contrast, Chen et al. [10] found no differences in hypoalgesic effect between constant-frequency TENS (80Hz) and frequency-modulated TENS, where the frequency varied from 20Hz to 100Hz. It is possible that both applications (constant-frequency and frequency-modulated TENS) activated only high frequency mechanisms while prior studies activated low frequency mechanisms [17,19,57–59,61–62]. Unfortunately, few studies have utilized placebo controls for comparison. While Johnson et al. [31] compared different frequencies against no-treatment controls, Chen and Johnson [10] did not utilize a control group. The current study showed analgesia with both low (4Hz) and high (100Hz) frequency TENS when compared to placebo or a no-treatment control group, but no difference between the two active TENS groups. However, on Day 1, 4Hz had no effect on PPTs while 100 Hz increased the PPTs. This finding suggests that low frequency TENS has a delayed hypoalgesic effect when compared with high frequency TENS. Low frequency TENS increased the PPT on days 2, 3 and 4, whereas high frequency TENS increased PPT on days 1, 2 and 3. These results are in accordance with other authors who compared different TENS frequencies on PPT, performing only one treatment session. In these studies, high frequency TENS was more effective than low frequency TENS for PPT increase [12,14,64,69–70]. Animal studies show that high and low frequency TENS activate different neuropharmacological mechanisms in the central nervous system [59–61]. Therefore, it is possible that mechanisms activated by high frequency TENS promote a faster hypoalgesic response than low frequency TENS in an experimental model.
Increasing intensity of stimulation is another potential mechanism by which we could alleviate tolerance. Prior studies show that low intensity TENS does not produce analgesia [1,6,13–14,45,52,71] and the intensity of stimulation is correlated with the degree of analgesia [51]. We propose that continuously increasing intensity of stimulation within a single session or daily could increase dose and prevent the development of analgesic tolerance.
The current study used a novel type of sham TENS unit to deliver placebo TENS that has been recently validated [51]. This sham unit applied TENS (100Hz, 100μs) at maximal tolerable intensity for 30 seconds and then the current ramped off over the next 15 seconds. The fact that the sham unit delivers electrical current for a short period of time increases the rate of subject blinding [15,51]. This time is considered too brief to have any definite physiological effect [15]. Our success in blinding the pain assessor (96%) was similar to previous studies that used this novel type of TENS placebo [15,51]. The pain assessor correctly identified that subjects were receiving active TENS treatment only once in both high and low frequency TENS groups; this was because it was possible to notice mild muscle contractions during PPT recordings in these subjects. The success of subject blinding in the placebo TENS group (48%) is in accordance with a previous study (40%) [51]. However, this level of blinding was lower than observed by Cowan et al. [15] (71%) when using the current intensity at sensory threshold. It is possible that the use of a maximal tolerable intensity in the present study was responsible for this difference, making it easier for the subjects to notice when the current ramped off.
The mechanism of analgesic tolerance is not completely understood and a number of neurotransmitters and receptors have been described. The neuropeptide cholecystokinin (CCK) has been implicated in the development of tolerance to TENS [17]. CCK is an endogenous opioid antagonist that activates CCK-A and CCK-B receptors [17,74]. Prior work shows that both high and low frequency TENS tolerance can be prevented by blockade of CCK-A and CCK-B receptors, respectively [17]. NMDA receptors in the central nervous system have also been implicated in opioid analgesia and blockade of NMDA receptors during TENS prevents the development of analgesia tolerance [29]. It has also been suggested that tolerance is a consequence of an adaptive change by the nervous system to regular repetitive stimuli produced by TENS [32]. This hypothesis led to an incorporation of parameter modulation (such as frequency and amplitude) in most TENS devices; as described before, the usefulness of these modulations still lacks strong scientific evidence. Analgesic tolerance to TENS, however, appears to result from tolerance at opioid receptors in the central nervous system, and uses known opioid-tolerance mechanisms.
The temporal summation protocol used in the present study reflects the central excitability from deep tissue pressure pain. It is believed that temporal summation is a consequence of wind-up of dorsal horn neurons [73]. High frequency TENS decreased temporal summation on day 1 when compared to pre-TENS temporal summation; however, this decrease was not different from placebo TENS. Interestingly the reduction in temporal summation in both groups was not present on day 5, likely a result of opioid tolerance. Since TENS and placebo effects are opioid-mediated [2,5,16,42,68,75], we suggest that repetitive activation of opioid receptors leads to analgesic tolerance by reducing the effectiveness on temporal summation. It is not clear, however, why subjects receiving low frequency TENS did not show a difference in temporal summation since μ-opioid agonists, the receptor involved in low frequency TENS analgesia, reduce temporal summation in human subjects [25] and wind-up in animals [21]. It is possible that strong muscle contractions observed in this group could have sensitized the extensor mass of forearm impairing the TENS-induced hypoalgesic effect.
An important but not surprising finding is that tolerance to TENS did not affect the DNIC response leading us to conclude that descending systems were not involved in analgesic tolerance. However, it should be pointed out that while both DNIC and TENS utilize endogenous opioid mechanisms to produce analgesia, they do this through activation of different pathways. Specifically, DNIC activates neurons in the subnucleus reticularis dorsalis (SRD) in the caudal-dorsal medulla [7,66–67] which are rich in μ-opioid receptors [47–48]. On the other hand, TENS utilizes the periaqueductal grey (PAG) and rostral ventral medulla (RVM) to produce an opioid-mediated analgesia [16,34,62].
In summary, the findings presented in this novel study, support that both high and low frequency TENS produce analgesic tolerance by the 4th and 5th day of treatment respectively. These data extend and validate prior studies in animals showing tolerance to TENS [9,17,29]. Future studies should be performed to find ways to delay and prevent the occurrence of tolerance to repeated TENS in humans.
Acknowledgments
Supported by the National Institute of Health R03 NR010405, the Marsha and Ralph Congdon Faculty Development Fellowship in Acute Care for the Chronically Ill, Coordination for the Improvement of Higher Level Personnel (CAPES), the Institute for Clinical and Translational Science, and the University of Iowa - National Center for Research Resources (NCRR) 5 UL1 RR024979-3. Special thanks to Shannon Lehmann for assistance with recruitment and scheduling.
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aarskog R, Johnson MI, Demmink JH, Lofthus A, Iversen V, Lopes-Martins R, Joensen J, Bjordal JM. Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiother Res Int. 2007;12(4):251–263. doi: 10.1002/pri.384. [DOI] [PubMed] [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubin M, Marks R. The efficacy of short-term treatment with transcutaneous electrical nerve stimulation for osteo-arthritic knee pain. Physiotherapy. 1995;81(11):669–675. [Google Scholar]
- 4.Bates JA, Nathan PW. Transcutaneous electrical nerve stimulation for chronic pain. Anaesthesia. 1980;35(8):817–822. doi: 10.1111/j.1365-2044.1980.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7(2):181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595(2):353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 8.Chabal C, Fishbain DA, Weaver M, Heine LW. Long-term transcutaneous electrical nerve stimulation (TENS) use: impact on medication utilization and physical therapy costs. Clin J Pain. 1998;14(1):66–73. doi: 10.1097/00002508-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102(1–2):195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Johnson MI. An investigation into the effects of frequency-modulated transcutaneous electrical nerve stimulation (TENS) on experimentally-induced pressure pain in healthy human participants. J Pain. 2009;10(10):1029–1037. doi: 10.1016/j.jpain.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Chen CC, Johnson MI. A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants. Clin Physiol Funct Imaging. 2010;30(4):260–268. doi: 10.1111/j.1475-097X.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Johnson MI. An investigation into the hypoalgesic effects of high- and low-frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. J Pain. 2010;11(1):53–61. doi: 10.1016/j.jpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002;99(1–2):253–262. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- 14.Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106(1–2):73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 15.Cowan S, McKenna J, McCrum-Gardner E, Johnson MI, Sluka KA, Walsh DM. An investigation of the hypoalgesic effects of TENS delivered by a glove electrode. J Pain. 2009;10(7):694–701. doi: 10.1016/j.jpain.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desantana JM, da Silva LF, de Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantana JM, da Silva LF, Sluka KA. Cholecystokinin receptors mediate tolerance to the analgesic effect of TENS in arthritic rats. Pain. 2010;148(1):84–93. doi: 10.1016/j.pain.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSantana JM, Santana-Filho VJ, Guerra DR, Sluka KA, Gurgel RQ, da Silva WM., Jr Hypoalgesic effect of the transcutaneous electrical nerve stimulation following inguinal herniorrhaphy: a randomized, controlled trial. J Pain. 2008;9(7):623–629. doi: 10.1016/j.jpain.2008.01.337. [DOI] [PubMed] [Google Scholar]
- 19.Desantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89(4):754–760. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desantana JM, Sluka KA, Lauretti GR. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clin J Pain. 2009;25(1):12–19. doi: 10.1097/AJP.0b013e31817d1070. [DOI] [PubMed] [Google Scholar]
- 21.Dickenson AH, Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurones in the rat dorsal horn. Pain. 1986;24(2):211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- 22.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20(2):187–191. doi: 10.1016/j.jcrc.2005.04.005. discussion 191–183. [DOI] [PubMed] [Google Scholar]
- 23.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emmiler M, Solak O, Kocogullari C, Dundar U, Ayva E, Ela Y, Cekirdekci A, Kavuncu V. Control of acute postoperative pain by transcutaneous electrical nerve stimulation after open cardiac operations: a randomized placebo-controlled prospective study. Heart Surg Forum. 2008;11(5):E300–303. doi: 10.1532/HSF98.20081083. [DOI] [PubMed] [Google Scholar]
- 25.Enggaard TP, Poulsen L, Arendt-Nielsen L, Hansen SH, Bjornsdottir I, Gram LF, Sindrup SH. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92(1–2):277–282. doi: 10.1016/s0304-3959(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 26.Fishbain DA, Chabal C, Abbott A, Heine LW, Cutler R. Transcutaneous electrical nerve stimulation (TENS) treatment outcome in long-term users. Clin J Pain. 1996;12(3):201–214. doi: 10.1097/00002508-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47(3):295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 28.Herr KA, Spratt K, Mobily PR, Richardson G. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain. 2004;20(4):207–219. doi: 10.1097/00002508-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9(3):217–225. doi: 10.1016/j.jpain.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 31.Johnson MI, Ashton CH, Bousfield DR, Thompson JW. Analgesic effects of different pulse patterns of transcutaneous electrical nerve stimulation on cold-induced pain in normal subjects. J Psychosom Res. 1991;35(2–3):313–321. doi: 10.1016/0022-3999(91)90086-4. [DOI] [PubMed] [Google Scholar]
- 32.Johnson MI, Ashton CH, Thompson JW. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain. 1991;44(3):221–229. doi: 10.1016/0304-3959(91)90089-G. [DOI] [PubMed] [Google Scholar]
- 33.Johnson MI, Ashton CH, Thompson JW. Long term use of transcutaneous electrical nerve stimulation at Newcastle Pain Relief Clinic. J R Soc Med. 1992;85(5):267–268. doi: 10.1177/014107689208500508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298(1):257–263. [PubMed] [Google Scholar]
- 35.Knudsen L, Drummond PD. Cold-induced limb pain decreases sensitivity to pressure-pain sensations in the ipsilateral forehead. Eur J Pain. 2009;13(10):1023–1029. doi: 10.1016/j.ejpain.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Kosek E, Ekholm J, Hansson P. Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scand J Rehabil Med. 1999;31(2):89–93. doi: 10.1080/003655099444597. [DOI] [PubMed] [Google Scholar]
- 37.Leffler AS, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain. 2002;6(2):149–159. doi: 10.1053/eujp.2001.0312. [DOI] [PubMed] [Google Scholar]
- 38.Leffler AS, Hansson P, Kosek E. Somatosensory perception in patients suffering from long-term trapezius myalgia at the site overlying the most painful part of the muscle and in an area of pain referral. Eur J Pain. 2003;7(3):267–276. doi: 10.1016/S1090-3801(02)00138-6. [DOI] [PubMed] [Google Scholar]
- 39.Leonard G, Coutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. 2010:1–9. doi: 10.1016/j.jpain.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain. 2010 doi: 10.1016/j.pain.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Levin MF, Hui-Chan CW. Conventional and acupuncture-like transcutaneous electrical nerve stimulation excite similar afferent fibers. Arch Phys Med Rehabil. 1993;74(1):54–60. [PubMed] [Google Scholar]
- 42.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Maguma HT, Thayne K, Davis B, Taylor DA. Correlation of the time course of development and decay of tolerance to morphine with alterations in sodium pump protein isoform abundance. Biochem Pharmacol. 2010;79(7):1015–1024. doi: 10.1016/j.bcp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 45.Olsen MF, Elden H, Janson ED, Lilja H, Stener-Victorin E. A comparison of high- versus low-intensity, high-frequency transcutaneous electric nerve stimulation for painful postpartum uterine contractions. Acta Obstet Gynecol Scand. 2007;86(3):310–314. doi: 10.1080/00016340601040928. [DOI] [PubMed] [Google Scholar]
- 46.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20(2):88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Pinto M, Castro AR, Tshudy F, Wilson SP, Lima D, Tavares I. Opioids modulate pain facilitation from the dorsal reticular nucleus. Mol Cell Neurosci. 2008;39(4):508–518. doi: 10.1016/j.mcn.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Pinto M, Sousa M, Lima D, Tavares I. Participation of mu-opioid, GABA(B), and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: implications for descending modulation of nociceptive transmission. J Comp Neurol. 2008;510(2):175–187. doi: 10.1002/cne.21793. [DOI] [PubMed] [Google Scholar]
- 49.Platon B, Andrell P, Raner C, Rudolph M, Dvoretsky A, Mannheimer C. High-frequency, high-intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. Pain. 2010;148(1):114–119. doi: 10.1016/j.pain.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain. 2005;6(10):673–680. doi: 10.1016/j.jpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, Miller CA, Polehna AC, Ruggle RC, Vance CG, Walsh DM, Sluka KA. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11(3):230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4(8):455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 53.Rutjes AW, Nuesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, Brosseau L, Reichenbach S, Juni P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst Rev. 2009;(4):CD002823. doi: 10.1002/14651858.CD002823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF beta-endorphin content in patients without pain problems. Pain. 1981;10(2):169–172. doi: 10.1016/0304-3959(81)90192-5. [DOI] [PubMed] [Google Scholar]
- 55.Scales DC, Adhikari NK. Maintaining allocation concealment: following your SNOSE. J Crit Care. 2005;20(2):191–193. doi: 10.1016/j.jcrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Sjolund BH, Eriksson MB. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res. 1979;173(2):295–301. doi: 10.1016/0006-8993(79)90629-2. [DOI] [PubMed] [Google Scholar]
- 57.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77(1):97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 58.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840–846. [PubMed] [Google Scholar]
- 59.Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4(2):185–193. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- 60.Sluka KA, Lisi TL, Westlund KN. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch Phys Med Rehabil. 2006;87(8):1137–1140. doi: 10.1016/j.apmr.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sluka KA, Vance CG, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem. 2005;95(6):1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- 62.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003;4(3):109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 63.Solomon RA, Viernstein MC, Long DM. Reduction of postoperative pain and narcotic use by transcutaneous electrical nerve stimulation. Surgery. 1980;87(2):142–146. [PubMed] [Google Scholar]
- 64.Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil. 2007;88(10):1344–1349. doi: 10.1016/j.apmr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008;1230:73–79. doi: 10.1016/j.brainres.2008.06.120. [DOI] [PubMed] [Google Scholar]
- 66.Villanueva L. Diffuse Noxious Inhibitory Control (DNIC) as a tool for exploring dysfunction of endogenous pain modulatory systems. Pain. 2009;143(3):161–162. doi: 10.1016/j.pain.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain. 1996;67(2–3):231–240. doi: 10.1016/0304-3959(96)03121-1. [DOI] [PubMed] [Google Scholar]
- 68.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh DM, Foster NE, Baxter GD, Allen JM. Transcutaneous electrical nerve stimulation. Relevance of stimulation parameters to neurophysiological and hypoalgesic effects. Am J Phys Med Rehabil. 1995;74(3):199–206. [PubMed] [Google Scholar]
- 70.Walsh DM, Lowe AS, McCormack K, Willer JC, Baxter GD, Allen JM. Transcutaneous electrical nerve stimulation: effect on peripheral nerve conduction, mechanical pain threshold, and tactile threshold in humans. Arch Phys Med Rehabil. 1998;79(9):1051–1058. doi: 10.1016/s0003-9993(98)90170-8. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, Gold J, Wender RH. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth Analg. 1997;85(2):406–413. doi: 10.1097/00000539-199708000-00029. [DOI] [PubMed] [Google Scholar]
- 72.Ward AR, Lucas-Toumbourou S, McCarthy B. A comparison of the analgesic efficacy of medium-frequency alternating current and TENS. Physiotherapy. 2009;95(4):280–288. doi: 10.1016/j.physio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 73.You HJ, Lei J, Arendt-Nielsen L. Selective inhibitory effects of pregabalin on peripheral C but not A-delta fibers mediated nociception in intact and spinalized rats. Neuroscience. 2009;164(4):1845–1853. doi: 10.1016/j.neuroscience.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 74.Zhang LX, Li XL, Wang L, Han JS. Rats with decreased brain cholecystokinin levels show increased responsiveness to peripheral electrical stimulation-induced analgesia. Brain Res. 1997;745(1–2):158–164. doi: 10.1016/s0006-8993(96)01095-5. [DOI] [PubMed] [Google Scholar]
- 75.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]