Abstract
Dizocilpine maleate (MK-801) is one of several NMDA receptor antagonists that is widely used to pharmacologically model the symptoms of psychosis and schizophrenia in animals. MK-801 elicits behaviors in adult zebrafish (Danio rerio) that are phenotypically consistent with behaviors observed in humans and rodents exposed to tbhe drug. However, the molecular and cellular processes that mediate the psychotomimetic, cognitive and locomotive behaviors of MK-801 are unclear. We exposed zebrafish larvae to MK-801 to assess their merit as a model organism to elucidate the behavioral effects of NMDA receptor blockade. Zebrafish larvae were acutely immersed in MK-801 to assess the effect on spontaneous swimming. MK-801 caused a time- and dose-dependent increase in larval swim speed, and the peak response (a five-fold increase in swim speed) was evoked by a three h exposure to a 20 uM dose. Zebrafish larvae did not exhibit sensitivity to the locomotor effects of MK-801 until 5 dpf, suggesting a critical role for developmental in sensitivity to the drug. Exposure to the low potency NMDA antagonist, memantine, did not alter the swim speed of zebrafish larvae. Co-immersion in D1 or D2 dopamine receptor antagonists did not disrupt the time course or magnitude of the increase in swim speed, suggesting dopaminergic signaling is not required for the locomotor actions of MK-801. Our findings of the behavioral actions of MK-801 in zebrafish larvae are consistent with previous observations in mammals and imply that the physiological, cellular and molecular processes disrupted by MK-801 are conserved in zebrafish larvae. These data suggest that the zebrafish larvae is a valid and useful model to elucidate neurobehavioral aspects of NMDA receptor antagonism and may provide insight to the neurobiology of psychosis and schizophrenia.
N-methyl-D-aspartate (NMDA) receptors are a subclass of ionotropic glutamate receptor that mediate excitatory transmission throughout the central nervous system (Dingledine, Borges, Bowie, & Traynelis, 1999). Pharmacological blockade of NMDA receptors with dissociative anesthetics like PCP and ketamine exacerbate the symptoms of schizophrenia and evoke a myriad of schizophreniform behaviors in healthy humans that are isomorphic with the idiopathic disease (Javitt & Zukin, 1991; Luby, Cohen, Rosenbaum, Gottleib, & Kelley, 1959). Stimulants such as cocaine and amphetamine can also induce schizophrenia-like symptoms (Ellinwood, Sudilovsky, & Nelson, 1973), but the spectrum of symptoms induced by NMDA receptor antagonists more fully recapitulate the positive, negative, and cognitive symptoms of schizophrenia (Krystal et al., 1994).
NMDA receptor antagonists are used extensively to model schizophrenia in rodents (Coyle, Tsai, & Goff, 2003; Javitt, 2004; Jentsch & Roth, 1999). Animal models of schizophrenia are useful for drug screening, but they can also provide insight to the pathophysiology of the disease. MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine maleate)] is a potent PCP congener (Wong, Kemp, Priestly, Knight, Woodruff, & Iversen, 1986) that blocks NMDA receptors and reproduces most symptoms of schizophrenia (Javitt & Zukin, 1991), including pronounced psychomotor disturbances like head twitching, and head weaving (Breese, Knapp, & Moy, 2002; Gilmour et al., 2009).
There is compelling evidence that the behavioral, locomotor and cognitive actions of MK-801 are retained in adult zebrafish since the drug disrupts shoaling behavior (Echevarria, Hammack, Pratt, & Hosemann, 2008), increases circling and swimming behavior and decreases performance in conditioned place preference experiments (Swain, Sigstad, & Scalzo, 2004).
Zebrafish possess several inherent advantages to elucidate the behavioral and molecular neuropharmacology of NMDA receptor blockade, including a high fecundity, physiological similarity to mammals, adaptability to multiple experimental techniques, and an amenability to high throughput approaches and forward genetic screens (Pyati, Look, & Hammerschmidt, 2007). MK-801 was recently shown to disrupt respiratory physiology in zebrafish larvae (Turesson, Schwerte, & Sundin, 2006), but the psychomotor actions of MK-801 have not been characterized in zebrafish larvae. We therefore set out to characterize the behavioral pharmacology of MK-801-evoked locomotion in zebrafish larvae.
Method
Subjects
A breeding stock of 20 adult wild type zebrafish (AB strain) was purchased from Zebrafish International Resource Center (Eugene, OR). Male and female fish were housed together in two 20 L aquaria supplied with fish water at 28° C, pH 8.0 and a conductivity of 500 μS. Aeration and filtration was provided by a canister filter system (XP4, RENA www.rena.fr). Adult fish were kept on a 14 h light, 10 h dark photoperiod and fed a mixture of dry brine shrimp flakes (Brine Shrimp Direct, Ogden, UT) and live brine shrimp (Artemia nauplii) three times per day. Fish were acclimated to laboratory conditions for 30 days prior to breeding.
Zebrafish embryos were obtained by natural, pair-wise mating (Westerfield, 1993). Embryos were collected 4 hours post fertilization (hpf), cleaned, sorted and stored in buffered embryo media: 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2 and 0.33 mM MgSO4 at 28.5 °C.
At 1-day post fertilization (dpf), zebrafish embryos were treated with a 0.1mg/ml protease solution (P6911; Sigma Aldrich, St Louis, MO) to digest the chorion. After digestion of the chorion, embryos were washed in embryo media and stored in the dark at 28.5 °C until time of experiment. All procedures were approved by Institutional Animal Care and Use Committee (IACUC), Charles Drew University.
General procedure
Zebrafish were digitally video recorded to assess the behavioral effects of acute exposure to MK-801 on spontaneous (fictive) locomotion. Slight differences in the early development of zebrafish larvae can impact locomotor capacity (Kimmel, Patterson, & Kimmel, 1974), so larvae were arranged into groups of ten to minimize the impact of individual variance in locomotor response to drugs. Data points, therefore, represent an average response of groups of larvae rather than an average response of individual larvae.
Locomotion assay
On the day of experimentation, zebrafish larvae were arranged into groups of 10 and transferred to the wells of a 12-well multiplate (diameter = 22 mm). For visualization and video recording, multiwell plates were placed atop a light box (PortaTrace, Gagne, Inc.;Johnson City, NY, USA) for illumination. Since changes in illumination can evoke transient changes in activity (Burgess & Granato, 2007a), zebrafish were exposed to continuous illumination for at least 10 min prior to recordings. The spontaneous locomotor activity of each group of animals was recorded by a digital video camera suspended above the set up which captured video at a frame rate of 30 frames per second (Sanyo Xacti, Sanyo North America, San Diego, CA). This experimental set up only captures displacement in the X- and Y- planes, so larvae were suspended in a minimal volume (2.0 mL) throughout this study to minimize the depth of the liquid (∼5 mm) and restrict locomotion in the z-axis. Physical stimuli can also evoke locomotor responses in zebrafish larvae (Ribera & Nusslein-Volhard, 1998), so larvae were left undisturbed for at least 10 min prior to video recording. The activity of each group of larvae was video recorded for five min to capture basal locomotor activity. The media in each well was then replaced with 2.0 mL of drug-supplemented media (experimental groups) or vehicle-supplemented media (control groups). Subsequent video recordings were obtained either hourly or after 4 h of exposure to monitor drug-induced changes in locomotion. All drugs were purchased from Tocris Bioscience (Ellisville, MO).
Locomotor analysis
Native digital video files (MPEG-4) were transferred to a personal computer and reformatted to MPEG-1 format with IMTOO MPEG converter software (www.imtoo.com). The behavioral analysis software package, GroupScan 1.00 (Clever Sys, Renton, VA), was used to calculate the average locomotor speed (mm/sec) for the group of larvae. The locomotor activity was measured as the average swim speed of larvae housed in the wells of a 12 well, multi-well plate. Data were exported to SigmaPlot 10.0 (Systat Software, San Jose, CA) for plotting, analyses of variance (ANOVA) and Tukey's test. Statistical significance was set at p < 0.05. Each point represents the mean ± SEM (n = 4-8). Changes in locomotor activity (Δ locomotor activity) were calculated by subtracting the average speed obtained prior to drug exposure (t = 0 h) from the average speed obtained after a given period of drug exposure.
Results
The zebrafish nervous system is sufficiently developed by 5 dpf to permit free swimming larvae to interact with the environment (Budick & O'Malley, 2000; Kimmel, Ballard, Kimmel, Ullmann, & Schilling, 1995). Our initial characterization of the locomotor effect of MK-801 was therefore performed with 5 dpf larvae. Immersion in 50 uM MK-801 increased the spontaneous locomotion of zebrafish larvae as early as 1 h after exposure. The average swim speed of zebrafish larvae immersed in MK-801 increased nearly five-fold, from 0.44 ± 0.31 mm/sec (mean ± SEM) to 1.97 ± 0.20 mm/sec (p < 0.05) (Fig. 1). The increase in swim speed was time-dependent and the response peaked after 3 h of exposure. The gain in swim speed persisted for the course of the experiment. All subsequent video recordings were obtained after 4 h of exposure to drugs to ensure the maximal behavioral response was captured.
Figure 1.
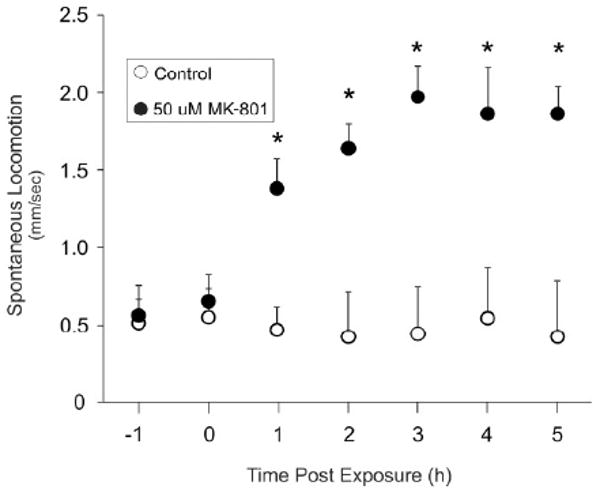
Temporal effects of MK-801 on locomotor activity of zebrafish larvae. Results are presented as means + S.E.M. N = 4 groups of larvae per condition, F(5, 12) = 5.60, p = 0.0068, *vs 0h, p < 0.05 by Tukey's HSD).
The locomotor response to MK-801 was dose-dependent. A 5 μM dose of MK-801 elicited a statistically significant increase in spontaneous locomotion of 0.29 ± 0.7 mm/sec (p < 0.05). The maximal increase in average swim speed, 1.35 ± 0.13 mm/sec, was evoked by a 20 μM dose of MK-801 (Fig. 2). Higher doses evoked smaller increases in swim speed and conferred an “inverted U” shape to the dose-response curve. The EC50 for the increase in swim speed was 10.3 μM.
Figure 2.
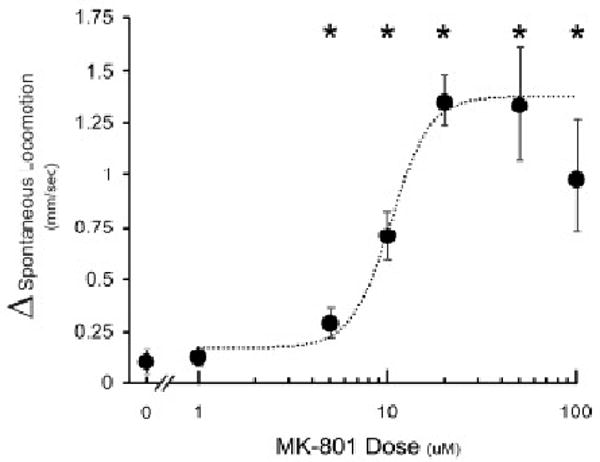
Dose-dependency of the locomotor effect of MK-801. Changes in spontaneous locomotion (Δ spontaneous locomotion) were calculated by (AVG Speed at t4h − AVG Speed at t0h) at each dose. Values are presented as means ± S.E.M. Dashed line represents a non-linear regression of the data (Sigma Plot 10); calculated EC50 = 10.3μM; R2 = 0.9917. N = 4-8 groups of larvae per concentration, F(5, 18) = 8.00. p = 0.0004, *dose vs vehicle (0 μM), p < 0.05 by Tukey's HSD).
Memantine is a low potency NMDA receptor antagonist that evokes a minimal locomotor response in mammals when administered at very high doses (Gilmour et al., 2009). Memantine did not increase the swim speed of zebrafish larvae at any dose tested (Fig. 3), including a 500 μM dose (data not shown).
Figure 3.
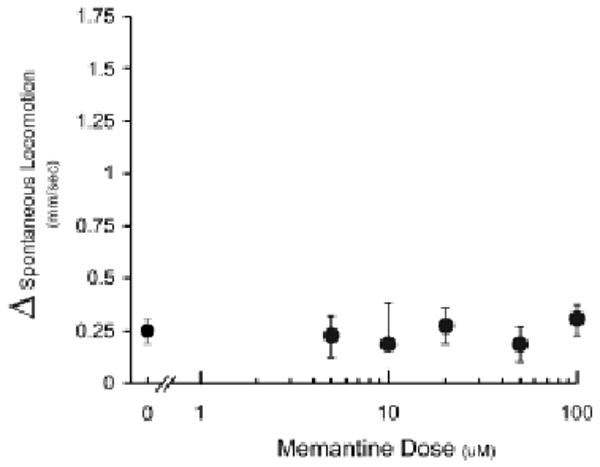
Effect of memantine on spontaneous locomotion of zebrafish larvae. Changes in spontaneous locomotion (Δ spontaneous locomotion) were calculated by (AVG Speed at t4h – AVG Speed at t0h) at each dose. Values are presented as means ± S.E.M. N = 4 groups of larvae per concentration [F(5, 18) = 0.30, p = 0.91].
Rodents display locomotor responses to MK-801 as early as postnatal day 3 or 4 (Rajachandran, Goodwin, & Spear, 1991). In our study, zebrafish larvae were exposed to MK-801 on dpf 2-7 to characterize the development of behavioral sensitivity to the drug. Developmental age was vital for the locomotor response to MK-801 as locomotor responses could only be evoked from zebrafish larvae older than 5 dpf (Fig. 4). Interestingly, the amplitude of the locomotor response to MK-801 was not developmentally-sensitive since the magnitude of the gain in swim speed was equivalent among larvae aged 5, 6, or 7 dpf (Fig. 4).
Figure 4.
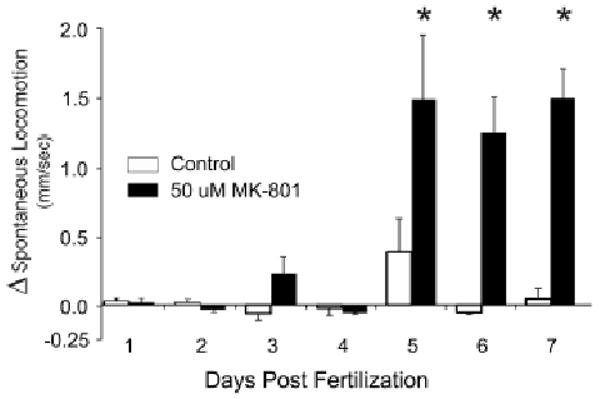
Effect of development on sensitivity to locomotor effects of MK-801. Changes in spontaneous locomotion (Δ spontaneous locomotion) were calculated by (AVG Speed at t4h – AVG Speed at t0h) at the developmental times indicated. Values are presented as means + S.E.M. N = 4-8 groups of larvae per concentration, F(5, 18) = 6.88. p = 0.0002, *Control vs 50 μm MK, p < 0.05 by Tukey's HSD).
In mammals, the behavioral actions of psychomotor stimulants are mediated mostly by disruptions in dopamine signaling. MK-801, however, is unique since an dopamine signaling is not required for the locomotor actions of the drug (Chartoff, Heusner, & Palmiter, 2005; Mele, Cabib, & Oliviero, 1995; Okuyama, Imagawa, & Tomigawa, 1996). The role of dopamine in the psychomotor actions of MK-801 has not been characterized in zebrafish. We therefore exposed zebrafish larvae to MK-801 solutions supplemented with a D1 dopamine receptor antagonist (SKF-83566) (Arnt & Hyttel, 1986) or a D2 dopamine receptor antagonist (sulpiride) (Usuda, Nishikori, Noshiro, & Maeno, 1981) to determine the role of dopamine in MK-801-evoked locomotion.
Concurrent exposure to dopamine receptor antagonists, SKF-83566 and sulpiride, either alone or together, did not attenuate the locomotor response to MK-801 (Fig. 5). This suggests that neither D1–like nor D2–like dopamine receptor activation is required.
Figure 5.
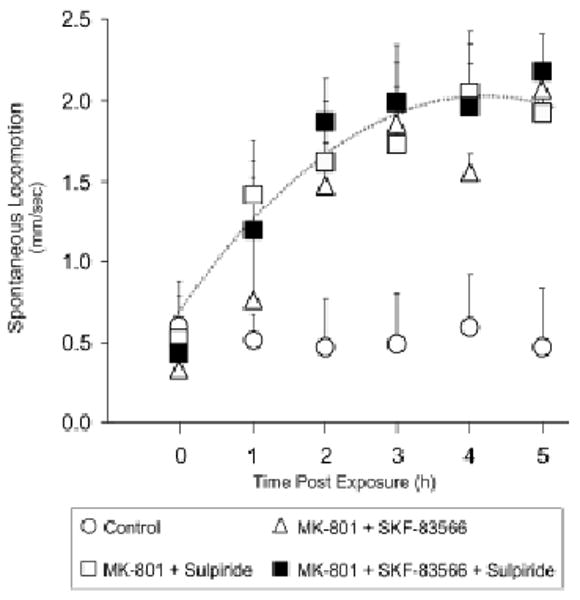
Effect of dopamine receptor antagonists on the locomotor effects of MK-801. Groups of zebrafish larvae (5dpf) were concurrently exposed to 50 μM MK-801 + 50 μM Sulpiride; 50 μM MK-801 + 50 μM SKF-83566; or 50 μM MK-801 + 50 μM Sulpiride + 50 μM SKF-83566. Dashed line represents the time course of the locomotor response to 50 μM MK-801 shown in Fig. 1 for compairson. Values are presented as means + S.E.M. N = 4 groups of aniamls per drug combination [F(5, 18) = 14.27. p = 0.0001].
Discussion
This study demonstrates that the neurobehavioral actions NMDA receptor antagonism are conserved in zebrafish larvae, and that our findings are qualitatively comparable to findings previously described in rodents (Loscher & Honack, 1992) and adult zebrafish (Swain et al., 2004). For example, acute MK-801 exposure elicits robust response in both fish and rodent models, but the low potency antagonist, memantine, does not evoke a locomotor response in either species (see Fig. 3 and Gilmour et al., 2009). The EC50 of the locomotor response to MK-801 in zebrafish larvae is 10 uM, a value that is consistent with the EC50 of MK-801-evoked behaviors previously described in adult zebrafish (Echevarria et al., 2008; Swain et al., 2004) and rodents (Gilmour et al., 2009). Interestingly, the locomotor actions of MK-801 are dose-dependent and biphasic in both rodents and fish; low concentrations are stimulatory while high concentrations of the drug are are sedating. In mammals, dopamine is not required to elicit the psychomotor effects of MK-801 (Chartoff et al., 2005). To our knowledge the role of dopamine in the zebrafish response to MK-801 has not previously been described. In this study we demonstrate that consistent with findings in mammalian models, dopamine is not required for the locomotor actions of MK-801 in zebrafish larvae Our data demonstrate several similarities between the MK-801-evoked behaviors of mammals and fish, however, some differences were observed.
For example, MK-801 evokes locomotor responses in neonatal rats as early as postnatal day 3 (Rajachandran et al., 1991), but larval zebrafish did not respond to the drug until 5 dpf (Fig. 3). This was a very curious finding since zebrafish larvae respond to tactile stimuli as early as 27 hpf (Ribera & Nusslein-Volhard, 1998) and mRNA transcripts for NMDA receptor subunits are detected as early as 24 hpf (Cox, Kucenas, & Voigt, 2005). However, our data are consistent with a previous report which demonstrated that MK-801 does not evoke physiological changes in zebrafish respiration until 6 dpf (Turesson et al., 2006). These data imply that the glutamatergic circuits responsible for the behavioral and physiological actions of MK-801 are not active until after 5 dpf.
Prepulse inhibition (PPI) is a sensorimotor gating mechanism in which a weaker prestimulus (prepulse) inhibits the reaction of an organism to a subsequent strong, startling stimulus (pulse). Sensorimtor gating deficits are a hallmark of schizophrenia and are frequently used as diagnostic tools in clinical and preclinical investigations (Bloom & Kupfer, 1995). PPI involves glutamatergic pathways and PCP and MK-801 have both been shown to disrupt it (Geyer, 1998). Since PPI has been demonstrated in zebrafish larvae as early as 3 dpf (Burgess & Granato, 2007b), it might be insightful to assess the effects of MK-801 on PPI in 3 or 4 dpf zebrafish larvae. Such an investigation may help distinguish the glutamatergic circuits that modulate PPI from those that mediate the locomotor actions of MK-801.
Overall, the behavioral and pharmacological data presented in this report demonstrate the considerable phenotypic similarities in MK-801-induced behaviors of mammals and zebrafish. We interpret these results to suggest a high degree of conservation in the underlying cellular and molecular mechanisms that mediate MK-801-evoked behaviors. Since the psychomotor actions of MK-801 actions are not completely understood (Breese et al., 2002) we propose that the zebrafish larvae is a highly suitable model to elucidate fundamental aspects of psychomotor stimulants.
Acknowledgments
This work was supported by NIH (R24DA017298) Minority Institution Drug Abuse Research Program (MIDARP) Program to Charles Drew University (PI, Theodore C. Friedman).
Contributor Information
John Chen, Pomona College, U.S.A..
Roshni Patel, Pomona College, U.S.A..
Theodore C. Friedman, Charles Drew University of Medicine and Science, U.S.A.
Kevin S. Jones, Charles Drew University of Medicine and Science, U.S.A.
References
- Arnt J, Hyttel J. Inhibition of SKF 38393- and pergolide-induced circling in rats with unilateral 6-OHDA lesion is correlated to dopamine D-1 and D-2 receptor affinities in vitro. Journal of Neural Transmission. 1986;67(3-4):225–240. doi: 10.1007/BF01243350. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Kupfer DJ. Psychopharmacology: The fourth generation of progress. New York: Raven Press; 1995. [Google Scholar]
- Breese GR, Knapp DJ, Moy SS. Integrative role for serotonergic and glutamatergic receptor mechanisms in the action of NMDA antagonists: Potential relationships to antipsychotic drug actions on NMDA antagonist responsiveness. Neuroscience & Biobehavioral Reviews. 2002;26(4):441–455. doi: 10.1016/s0149-7634(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. Journal of Experimental Biology. 2000;203(Pt 17):2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. Journal of Experimental Biology. 2007a;210(Pt 14):2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. The Journal of Neuroscience. 2007b;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30(7):1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Cox JA, Kucenas S, Voigt MM. Molecular characterization and embryonic expression of the family of N-methyl-D-aspartate receptor subunit genes in the zebrafish. Developmental Dynamics. 2005;234(3):756–766. doi: 10.1002/dvdy.20532. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Annals of the New York Academy of Sciences. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Echevarria DJ, Hammack CM, Pratt DW, Hosemann JD. A novel behavioral test battery to assess global drug effects using the zebrafish. International Journal of Comparative Psychology. 2008;21(2):19–34. [Google Scholar]
- Ellinwood EH, Jr, Sudilovsky A, Nelson AM. Evolving behavior in the clinical and experimental amphetamine (model) psychosis. American Journal of Psychiatry. 1973;130(10):1088–1093. doi: 10.1176/ajp.130.10.1088. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Behavioral studies of hallucinogenic drugs in animals: Implications for schizophrenia research. Pharmacopsychiatry. 1998;31(Suppl 2):73–79. doi: 10.1055/s-2007-979350. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT, et al. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: Implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology. 2009;205(2):203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Molecular Psychiatry. 2004;9(11):984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Developmental Psychobiology. 1974;7(1):47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JB, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Loscher W, Honack D. The behavioural effects of MK-801 in rats: Involvement of dopaminergic, serotonergic and noradrenergic systems. European Journal of Pharmacology. 1992;215(2-3):199–208. doi: 10.1016/0014-2999(92)90029-4. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottleib JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. A M A Archives of Neurology & Psychiatry. 1959;81(3):363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Mele A, Cabib S, Oliviero A. Effects of the NMDA-antagonist, MK-801, on stress-induced alterations of dopamine dependent behavior. Psychopharmacology. 1995;117(3):313–317. doi: 10.1007/BF02246106. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Tomisawa A. Behavioral evidence for modulation by sigma ligands of (+)MK-801-induced hyperlocomotion in monoamine-depleted mice. Neuropharmacology. 1996;35(4):467–474. doi: 10.1016/0028-3908(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Look AT, Hammerschmidt M. Zebrafish as a powerful vertebrate model system for in vivo studies of cell death. Seminars in Cancer Biology. 2007;17(2):154–165. doi: 10.1016/j.semcancer.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Rajachandran L, Goodwin GA, Spear LP. Psychopharmacological effects of MK-801 in infant and preweanling rat pups. Pharmacology, Biochemistry, & Behavior. 1991;40(2):291–295. doi: 10.1016/0091-3057(91)90555-g. [DOI] [PubMed] [Google Scholar]
- Ribera AB, Nusslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. The Journal of Neuroscience. 1998;18(22):9181–9191. doi: 10.1523/JNEUROSCI.18-22-09181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicology & Teratology. 2004;26(6):725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Turesson J, Schwerte T, Sundin L. Late onset of NMDA receptor-mediated ventilatory control during early development in zebrafish (Danio rerio) Comparative Biochemistry & Physiology Part A: Molecular & Integrative Physiology. 2006;143(3):332–339. doi: 10.1016/j.cbpa.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Usuda S, Nishikori K, Noshiro O, Maeno H. Neuroleptic properties of cis-N-(1-benzyl-2-methylpyrrolidin-3-yl)-5-chloro-2-methoxy-4-methylaminob enzamide (YM-09151-2) with selective antidopaminergic activity. Psychopharmacology. 1981;73(2):103–109. doi: 10.1007/BF00429198. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: A guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- Wong EH, Kemp JA, Priestly T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


