Abstract
Nine alkaloids (acridine, aristolochic acid, atropine, berberine, caffeine, nicotine, scopolamine, sparteine, and strychnine) were evaluated as feeding deterrents for gypsy moth larvae (Lymantria dispar (L.); Lepidoptera: Lymantriidae). Our aim was to determine and compare the taste threshold concentrations, as well as the ED50 values, of the nine alkaloids to determine their potency as feeding deterrents. The alkaloids were applied to disks cut from red oak leaves (Quercus rubra) (L.), a plant species highly favored by larvae of this polyphagous insect species. We used two-choice feeding bioassays to test a broad range of biologically relevant alkaloid concentrations spanning five logarithmetic steps. We observed increasing feeding deterrent responses for all the alkaloids tested and found that the alkaloids tested exhibited different deterrency threshold concentrations ranging from 0.1 mM to 10 mM. In conclusion, it appears that this generalist insect species bears a relatively high sensitivity to these alkaloids, which confirms behavioral observations that it avoids foliage containing alkaloids. Berberine and aristolochic acid were found to have the lowest ED50 values and were the most potent antifeedants.
Keywords: alkaloids, feeding behavior, gypsy moth, Lymantria dispar, deterrence, gustation, antifeedant, Lepidoptera
Introduction
Deterrent plant secondary compounds, such as alkaloids, are important mediators of insect-plant interactions. Alkaloids are typically unfavored by many herbivorous insects. They primarily serve as growth inhibitors, feeding deterrents, and often possess toxins (Saunders et al., 1992). Alkaloids are naturally occurring organic compounds of low molecular weight and contain nitrogen incorporated into a heterocyclic ring. Approximately 20-30% of all alkaloids occur in higher plants, most commonly in dicotyledonous angiosperms at concentrations of about 0.01% of the dry weight or greater (Willaman and Schubert, 1961; Seigler, 1998). While such compounds can be accumulated in any part of the plant at varying concentrations, they are most often concentrated in the most nutritious tissues (i.e., seed tissues) (Hartmann, 1991; Bernays and Chapman, 1994).
Larvae of Lymantria dispar, similar to other lepidopteran polyphagous larvae, find foliage containing alkaloids unfavorable (Barbosa and Krischik 1987; Barbosa et al., 1990). In the present study, using L. dispar larvae, we applied various alkaloids at a broad range of concentrations, spanning five logarithmetic steps to: (a) determine the concentration-response curves for each alkaloid tested and (b) determine and compare the deterrency threshold (DT) concentration (i.e., lowest concentration at which feeding on an alkaloid-treated disk was significantly different from that of a control disk) for each of the nine alkaloids tested. By estimating the ED50 value (approximate concentrations that reduced feeding by 50%) and statistically modeling our dose-response curves for each of the alkaloids tested, we were able to establish a potency rank order. The experiments carried out here build on previous work (Shields et al., 2006), where we screened a number of alkaloids, representing several subclasses, known to inhibit growth and/or deter feeding in other Lepidoptera to determine their effect on feeding in L. dispar larvae.
Knowledge about the effects of these alkaloids may prove useful in the development of natural products, such as pesticides and antifeedants for insects, as well as contribute to our understanding of the gustatory system of this insect.
Materials and Methods
Animals
L. dispar eggs (New Jersey strain) were obtained from USDA-APHIS, Otis Air National Guard Base (Falmouth, Massachusetts). The colony was maintained on a high wheat germ artificial diet (Bio-Serv, Frenchtown, NJ) at 24-26 °C, ca. 60% humidity, and a 12h light: 12h dark photoperiod regimen (Shields et al., 2003; 2006). We used fifth instar larvae that were 12-18 h postmolt, randomly selected, and deprived of food 10-14 h prior to the experiments. Late instar larvae were chosen, since they typically carry out most of the feeding in the wild (Leonard 1974).
Experimental Protocol
We used a two-choice feeding bioassay (after Jermy et al., 1968; Shields et al., 2003; 2006) to evaluate the feeding responses of L. dispar larvae. Disks (9-mm diameter cork borer) were punched out from red oak (Quercus rubra) (L.) leaves, a plant species highly favored by L. dispar larvae (Shields et al., 2003). Branches were collected consistently from the same trees between 9-11 a.m. daily (June-July 2006) and immediately placed in water. The disks were arranged circularly in a Petri dish (100-mm diameter, 15-mm depth) (Fisher Scientific, Pittsburgh, PA) in an ABABABAB fashion (A, control; B, treatment). Metal pins were pushed through the center of each disk into two pieces of dental wax (Electron Microscopy Sciences, Hatfield, PA) and disks stood ca. 5 mm above the wax. The bottom of the dishes was lined with moistened (2 ml aliquot distilled water) filter paper (90-mm2 circle, Grade 1, Whatman, Inc.) to reduce desiccation of the leaf disks.
For feeding behavioral experiments, nine alkaloids were chosen that are considered to be potent feeding deterrents in other lepidopteran larvae (e.g., Miller and Feeny, 1983; Wrubel and Bernays, 1990): acridine (acridone alkaloid; family Rutaceae), aristolochic acid sodium salt (benzylisoquinoline; family Aristolochiaceae), atropine (tropane alkaloid; family Solanaceae), berberine hemisulfate salt (benzylisoquinoline; families Menispermaceae, Berberidaceae, Lauraceae, Annonaceae, Magnoliaceae, Papaveraceae, Ranunculaceae, Rutaceae), caffeine (purine alkaloid; families Rubiaceae, Sterculiaceae, and Theaceae), (-)- nicotine (pyridine alkaloid; family Solanaceae), scopolamine hydrobromide (tropane alkaloid; family Solanaceae), (-) sparteine, (quinolizidine alkaloid; family Leguminosae), and strychnine hemisulfate, (indole alkaloid; families Apocynaceae and Loganiaceae) (MP Biomedicals, Aurora, OH, and Fisher Scientific, Pittsburgh, PA). We use the term acridine to describe a specific alkaloid. However, this term has also been used to describe compounds containing the C13N tricycle (Gröger, 1980).
Experiments were carried out at 24 °C (± 1 °C) for a maximum of 18 h (typically 5-15h) or until ca. 50% of the total area (estimated visually) of either control or test disks had been consumed. Deionized ultra filtered water, ethanol, methanol, or chloroform (Fisher Scientific, Pittsburgh, PA) were used to dissolve each test compound, which was applied at seven different concentations (in mM: 0.001, 0.01, 0.1, 1, 10, 100, 300) spanning five logarithmetic steps. Caffeine and aristolochic were not tested at the highest concentration (i.e., 300 mM), since they were incompletely soluble. Our preliminary experiments guided us in the choice of the concentration range since they indicated that behavioral responses ranged from indifference (not deterrent) to strong feeding inhibition (deterrent). A 20-μl aliquot containing solvent or solvent plus test compound was applied to all disks (controls and test disks, respectively). At the beginning of the experiment, each larva was located in the center of a Petri dish. A set of control disks, held in the absence of larvae, was retained for comparison purposes for each experiment, called control disks II. Following each experiment, all disks (control, test, and control II disks) were oven-dried at 80°C for 48 h. We determined mean consumption (mg) by subtracting the remaining mass of a test or control disk from the mass of a control disk II. The disks were weighed (Sartorius BP 211 D) (± 0.01 mg) and the values were reported as percent relative mean consumption of control consumption.
A Kruskal-Wallis one-way ANOVA was run for each of the test compounds (alkaloids) to test the null hypothesis that there was no difference in percent relative consumption among all the concentrations of each alkaloid (significance level = 0.05). The difference between control consumption (100%) and relative mean consumption of disks was tested for each concentration using a paired Wilcoxon signed rank test. A Bonferroni correction for individual comparisons (significance levels of 0.05/7 = 0.007 or 0.05/6 = 0.008, depending on the number of comparisons) was used to maintain the experiment-wide error rate of 0.05. A Wilcoxon signed rank test was also used to determine if there was a difference in relative mean consumption between the DT concentration and consumption at 50% (i.e., ED50) (significance level = 0.05). We used the GENMOD procedure of SAS to model larval feeding responses to alkaloid treatments and to compare responses between the alkaloids tested. The error term was modeled as a Poisson distribution with a log link function. A scale parameter calculated based on deviance was used to correct the statistic for over dispersion in the data. We analyzed the data using Statmost (Dataxiom Software Inc., Los Angeles, CA), SAS (SAS Institute Inc., Cary, NC), NCSS (Kaysville, Utah), Minitab (Minitab Inc., State College, PA), and Excel (Microsoft Corp., Redmond, WA). Using the data from our concentration-response curves, we determined the DT concentration and estimated the ED50 value for each alkaloid tested.
Results
We tested the effects of nine alkaloids, belonging to several alkaloid subgroups, on the feeding behavior of gypsy moth larvae, L. dispar, in a two-choice feeding biosassy. The alkaloids were applied exogenously to red oak leaf disks. We tested each alkaloid at a variety of concentrations to construct dose-response curves. All nine alkaloids had feeding deterrent effects on gypsy moth larvae. However, differences were apparent in the dose-response curves with respect to slope of the curve, DT concentration, and ED50 values.
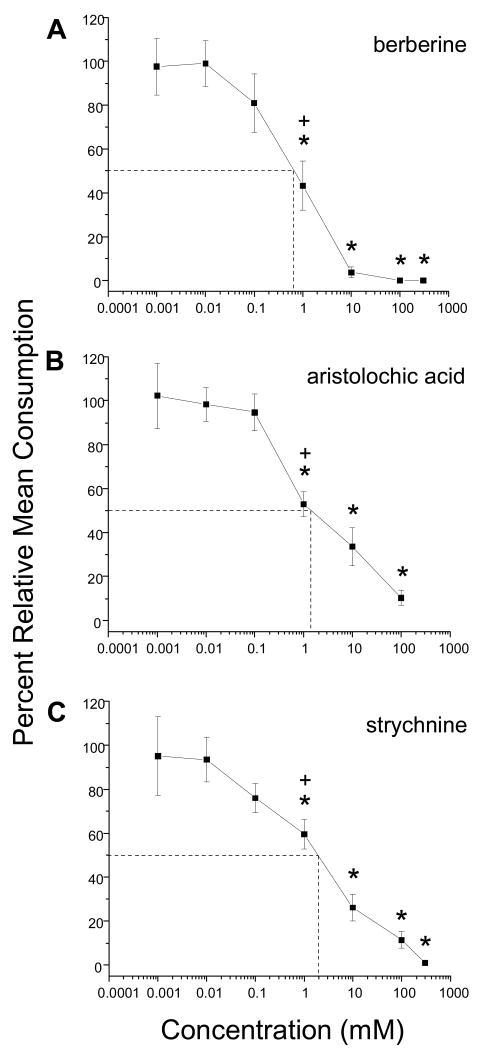
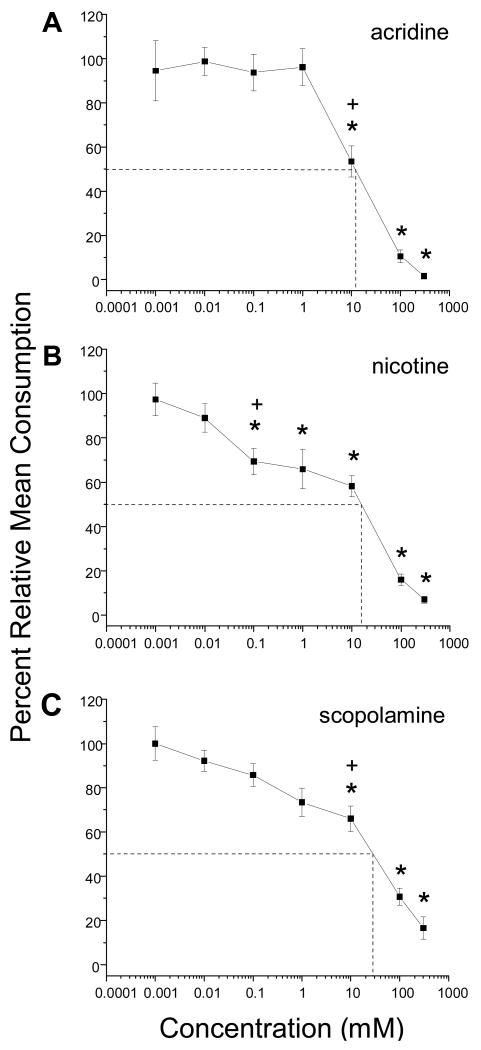
In general, we observed an increase in feeding deterrency (decrease in consumption) with increasing concentration of alkaloid tested. This resulted in differently shaped sigmoid dose response curves (Figs. 1-3). The DT concentration for each alkaloid is listed in Table 1 and indicated in Figs. 1-3. We also determined if a difference existed in relative consumption between the DT value and the ED50 value. We found no significant difference in relative mean consumption for DT and ED50 values for berberine, aristolochic acid, strychnine, sparteine, and acridine (Figs. 1-3). A significant difference between these two parameters was noted, however, for caffeine, atropine, nicotine, and scopolamine (Figs. 2, 3).
Figure 1A-C.
Concentration response curves for A) berberine, B) aristolochic acid, and C) strychnine. The alkaloids were applied to red oak leaf disks in dual choice feeding bioassays between alkaloid-treated and control leaf disks. The plus sign indicates the deterrent threshold concentration at which feeding on an alkaloid-treated disk was significantly below that on a control disk. The concentrations that are statistically significant are indicated by the asterisks. Dashed lines and their corresponding point of intersection on each concentration-response curve represent the ED50 values (approximate concentrations that reduced feeding by 50%) (0.66 mM berberine; 1.40 mM aristolochic acid; 1.89 mM strychnine). Results are derived from A, n = 9-12; B, n = 11-15; C, n = 13-36 larvae (i.e., number of replicates). Error bars represent S.E.
Figure 3A-C.
Concentration response curves for A) acridine, B) nicotine, and C) scopolamine. Details as in Fig. 1. Dashed lines and their corresponding point of intersection on each concentration-response curve represent the ED50 values (approximate concentrations that reduced feeding by 50%) (11.9 mM acridine; 15.6 mM nicotine; 28.3 mM scopolamine. Results are derived from A, n = 10-35; B, n = 13-29; C, n = 10-40 larvae or number of replicates. Error bars represent S.E.
Table 1. Varying effects of alkaloids on feeding.
| Alkaloid | Estimated concentration that reduces feeding by 50% (mM) (ED50) | Deterrency Threshold (DT) concentration |
|---|---|---|
| Berberine | 0.66a | 1.0 |
| Aristolochic acid | 1.40a, b | 1.0 |
| Strychnine | 1.89b,c | 1.0 |
| Caffeine | 2.61c | 0.1 |
| Atropine | 4.39c,d | 10 |
| Sparteine | 7.17b,d | 10 |
| Acridine | 11.9d | 10 |
| Nicotine | 15.6b,c,d | 0.1 |
| Scopolamine | 28.3e | 10 |
Figure 2A-C.
Concentration response curves for A) caffeine, B) atropine, and C) sparteine. Details as in Fig. 1. Dashed lines and their corresponding point of intersection on each concentration-response curve represent the ED50 values (approximate concentrations that reduced feeding by 50%) (2.61 mM caffeine; 4.39 mM atropine; 7.17 mM sparteine). Results are derived from A, n = 11-14; B, n = 10-25; C, n = 10-33 larvae or number of replicates. Error bars represent S.E.
After statistical modeling (see Materials and Methods) of the dose-response curves and taking into consideration the ED50 values for each alkaloid, our results suggested three main findings (Table 1): (a) berberine and aristolochic acid were the two most potent feeding deterrents; (b) scopolamine was the least potent feeding deterrent, and (c) the remaining alkaloids could be ranked (most to least potent), strychnine, caffeine, atropine, and acridine. The potency of sparteine and nicotine was difficult to assess due to the large variation observed in the feeding responses of larvae to these alkaloids; they are likely to be less potent than berberine and aristolochic acid, but more potent than scopolamine.
We also found that the percent relative mean consumption was at, or near 0% (i.e., complete feeding deterrency) at the following concentrations using the nine alkaloids tested: berberine, 100 mM, 100% feeding deterrency; aristolochic acid, 100 mM, 89.7%; strychnine, 300 mM, 99%; caffeine, 100 mM, 89.7%, atropine, 300mM, 100% sparteine, 300 mM, 92.4%; acridine, 300 mM, 98.5%; nicotine, 300 mM, 93%; and scopolamine, 300 mM, 83.4%) (Figs. 1-3).
Discussion
In this study, we determined the sensitivity (i.e., deterrency threshold concentrations, DT) of L. dispar larvae to selected alkaloids using feeding choice test bioassays. When applied exogenously to leaf disks, the alkaloids tested elicited clear concentration-dependent deterrent effects (Figs. 1-3). From these curves, we estimated the ED50 values for each of the alkaloids tested and predicted a level of potency (Table 1). These results are in partial agreement with our previous study (Shields et al., 2006), where we determined that the majority of alkaloids that we tested were, in fact, deterrent to L. dispar larvae when presented on both paper and leaf disks. One of the limitations of that study was, however, that all the alkaloids tested were presented at a 1% concentration (i.e., 1% of the dry weight of the disk) and not at similar molecular densities (i.e. molarity). We initially chose a 1% alkaloid concentration since this level falls within the range that has been reported to occur in nature for the alkaloids tested in that study (e.g., Willaman and Schubert 1961; Bernays and Chapman, 1977; Wrubel and Bernays 1990; Seigler, 1998). When we calculated the molarity of each alkaloid to determine if differences in molarity correlated with our ranking regarding potency, we found no obvious correlation between deterrency and molarity of the applied alkaloids. Subsequently, in this study, by testing a wide range of concentrations spanning five log units, we are able to obtain a better understanding of deterrency, with respect to each of the alkaloids tested.
The results of this study are in agreement with other studies that have shown the importance of alkaloids on the feeding behavior of this species (e.g., Miller and Feeny, 1983; Barbosa and Krischik, 1987; Shields et al., 2006). Barbosa & Krischik (1987) and Miller and Hanson (1989) determined that plants containing alkaloids were unfavored by gypsy moth larvae (alkaloid presence and feeding preference were correlated negatively). More specifically, tree species lacking alkaloids (e.g., Quercus rubra, Liquidambar styraciflua, Acer saccharum, Tilia americana) were more highly favored than those containing them (e.g., Fagus grandifolia and Liriodendron tulipifera) (Barbosa & Krischik 1987, Barbosa et al., 1990). Shields et al. (2003) examined the feeding preference hierarchy of gypsy moth larvae to seven tree species and found that sweet gum (L. styraciflua) and red oak (Q. rubra) were highly preferred, sugar maple (Acer saccharum) and American basswood (T. americana) were secondarily favored, American beech (F. grandifolia) and black walnut (Juglans nigra) were least favored, and tulip poplar (L. tulipifera) was strongly rejected. These findings were supported by the fact that alkaloids are absent in Q. rubra, L. styraciflua, A. saccharum, and T. americana, whereas one or more are present in F. grandifolia and L. tulipifera (Gibbs, 1974). Despite the established aversion of gypsy moth to alkaloids, field behavioral data suggests that gyspy moth can adapt to an unsuitable, alkaloid-rich host tree as an exclusive food resource for long periods (Lazarević et al., 2002), which underscores the importance of understanding this insect-plant relationship.
In agreement with the results of our study, that berberine and arisotolochic acid were potent feeding deterrents, Miller and Feeny (1983) also showed that these alkaloids caused dramatic repellent and toxic effects on L. dispar larvae. Both of these alkaloids possess a metheylenedioxyphenyl (benzodioxole) (MDP) group. Compounds possessing such groups have been shown to enhance insecticide chemical toxicity by inhibiting mixed-function oxidase enzymes (MFOs) (e.g., Hodgson et al., 1995; Feyereisen, 1999; Wheeler et al., 2001), also known as P450s, cytochrome P450 monooxygenases, polysubstrate monooxygenases, microsomal oxidases, and heme thiolate proteins (Feyereisen, 1999). MFOs can synthesize and degrade ecdysteroids and juvenile hormones, which are important for insect growth, development, and reproduction, as well as pheromone metabolism (Feyereisen, 1999). They can also catabolize and anabolize xenobiotics (i.e., drugs, pesticides, and plant toxins) and endogenous compounds (i.e., hormones, fatty acids, and steroids) (Scott, 1999). These effects of MFOs are possible because of their mode of action which involves the conversion of lipophilic compounds into more polar hydrophilic metabolites that are excreted at higher rates by the insect (Brattsten and Wilkinson, 1977). While Miller and Feeny (1983) speculated that methylenedioxyphenyl-containing alkaloids, such as berberine and aristolochic, were likely to be toxic to insects, they could not establish if these alkaloids could be detoxified by MFO enzymes and if these compounds could inhibit detoxification. To this end, Neal (1989) partially answered these questions when he tested four methylenedioxyphenyl-containing alkaloids on the polyphage, Heliothis zea. He established that while berberine was toxic to these larvae, it was only a weak monooxygenase inhibitor of O-demethylase activity, as revealed by the synergistic effect of myristicin, another MDP-containing compound with little or no toxicity by itself.
ED50 values are well established indicators of feeding deterrent activity in insects and other animals (e.g., Eichenseer and Mullin, 1997; Bernays et al., 2000). We found a significant difference in relative mean consumption for DT and ED50 values for caffeine, atropine, nicotine, and scopolamine; however no significant difference was observed for berberine, aristolochic acid, strychnine, sparteine, and acridine (Figures 1-3). Berberine, aristolochic acid, strychnine, atropine, sparteine, and acridine all had low DT concentrations and correspondingly low ED50 values (Table 1). In the case of caffeine, nicotine and scopolamine, the ED50 values were higher than the DT concentrations by one half to two orders of magnitude (Table 1).
In our study, gypsy moth larvae were able to detect the alkaloids tested with relatively high sensitivity (low DT concentration). The DT concentrations ranged from 0.1-10 mM for the nine alkaloids tested. Even though L. dispar is a polyphagous insect and feeds on a wide range of plant species (at least 12 families with many more species; Mosher, 1915; Miller and Hanson, 1989), it does not, however, indiscriminately find all plants acceptable for feeding. While L. dispar could potentially encounter alkaloids during feeding, it typically avoids foliage containing these secondary plant compounds (Mosher, 1915; Barbosa and Krischik, 1987). L. dispar larvae are capable of detecting at least some alkaloids with relatively high sensitivity and bear detoxification capacities for some of these compounds (Ahmad and Forgash, 1975; Appel and Maines, 1995). The DTs found in our study support this idea, since they are comparatively low with respect to alkaloid sensitivity reported in the literature for other polyphagous larvae (e.g., Bernays et al., 2000). Therefore, our data supports the hypothesis that L. dispar larvae are highly sensitive to some alkaloids and, subsequently, do not typically feed on these plants as part of their normal diet.
It has been shown that the chemical constituents of wheat germ-based diets affect insect behavior (i.e., induce stimulatory or deterrent effects) and can result in reduced sensitivity to feeding deterrents (Huang and Renwick, 1997). This would suggest that gypsy moth larvae reared on wheat germ diet (as in this study) may have even lower DT and ED50 values when reared on natural diet (i.e., leaves) than reported here. Again, this would support the idea of relatively high alkaloid sensitivity in this polyphagous feeder.
This study provides new information on the sensitivity of L. dispar larvae to a variety of alkaloids. We have evaluated the deterrency of these alkaloids without addressing their neural modes of action. It was not possible to address this question with our experimental design, since the feeding behavioral bioassays extended over several hours and we could not rule out that several mechanisms, including gustation, olfaction, somatosensory, and viscerosensory (e.g., habituation, pre- and post-ingestive feedback mechanisms, and/or toxicity) could have been responsible for the feeding deterrency, as suggested in other studies (e.g., Glendinning et al., 2001; Wheeler et al., 2001; Glendinning, 2002). Determining the underlying neural mechanisms of alkaloid feeding deterrency will be a challenging, but rewarding aim of future studies. We are currently carrying out elelctrophysiological studies with L. dispar larvae to address some of these issues.
Acknowledgments
We gratefully acknowledge Dr. Thomas Heinbockel, Howard University College of Medicine, Washington, D.C. for critically reviewing this manuscript. Many thanks to Mr. Howard Kaplon and Dr. Joel Snodgrass for guidance with the statistical analyses. We also thank USDA-APHIS, Otis Air National Guard Base (Falmouth, Massachusetts) and Robert Bennett (USDA, Beltsville, MD) for kindly supplying us with egg masses and Janelle Akomah for helping with this project. This work was supported by research grants (1 R15 DC007609-01) to V.D.S. and NIH (GM-58384).
Contributor Information
Kristen P. Smith, Email: kpsmith@towson.edu.
Nicole S. Arnold, Email: narnol2@towson.edu.
References
- Ahmad S, Forgash AJ. NADPH-cytochrome-c-reductase: changes in specific activity in gypsy moth larvae. Journal of Insect Physiology. 1975;21:85–88. doi: 10.1016/0022-1910(75)90071-2. [DOI] [PubMed] [Google Scholar]
- Appel HM, Maines LW. The influence of host plant on gut conditions of gypsy moth (Lymantria dispar) caterpillars. Journal of Insect Physiology. 1995;41:241–246. [Google Scholar]
- Barbosa P, Krischik VA. Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth Lymantria dispar. American Naturalist. 1987;130:53–69. [Google Scholar]
- Barbosa P, Gross P, Provan GJ, Pacheco DY, Stermitz FR. Allelochemicals in foliage of unfavored tree hosts of the gypsy moth, Lymantria dispar L. 1. Alkaloids and other components of Lirodendron tulipifera L. (Magnoliaceae), Acer rubrum L. (Aceraceae), and Cornus florida L. (Cornaceae) Journal of Chemical Ecology. 1990;16:1719–1730. doi: 10.1007/BF01014103. [DOI] [PubMed] [Google Scholar]
- Bernays EA, Chapman RF. Deterrent chemicals as a basis of oliphagy in Locusta migratoria (L.) Ecological Entomology. 1977;2:1–18. [Google Scholar]
- Bernays EA, Chapman RF. Host-Plant Selection by Phytophagous Insects. Chapman & Hall; New York: 1994. [Google Scholar]
- Bernays EA, Oppenheim S, Chapman RF, Kwon H, Gould F. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: a behavioral test of the hypothesis with two closely related caterpillars. Journal of Chemical Ecology. 2000;26:547–563. [Google Scholar]
- Brattsten LB, Wilkinson CF. Herbivore-plant interactions: mixed function oxidases and secondary plant substances. Science. 1977;196:1349–1352. doi: 10.1126/science.196.4296.1349. [DOI] [PubMed] [Google Scholar]
- Eichenseer H, Mullin C. Antifeedant comparisons of GABA/glycinergic antagonists for diabroticite leaf beetles (Coleoptera: Chrysomelidae) Journal of Chemical Ecology. 1997;23:71–82. [Google Scholar]
- Feyereisen R. Insect P450 enzymes. Annual Review of Entomology. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- Gibbs RD. Chemotaxonomy of flowering plants. Vol. 4. McGill-Queen's Univ. Press; Montreal, Canada: 1974. [Google Scholar]
- Glendinning JI, Domdom S, Long E. Selective adaptation to noxious foods by a herbivorous insect. The Journal of Experimental Biology. 2001;204:3355–3367. doi: 10.1242/jeb.204.19.3355. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomologia Experimentalis et Applicata. 2002;104:15–25. [Google Scholar]
- Gröger D. Alkaloids Derived from Tryptophan and Anthranilic Acid. In: Bell EA, Charlwood BV, editors. Secondary Plant Products. Springer-Verlag; New York: 1980. pp. 128–159. [Google Scholar]
- Hartmann T. Alkaloids. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. 2nd. Vol. 1. Academic Press; San Diego: 1991. pp. 79–121. [Google Scholar]
- Hodgson E, Rose RL, Ryu DY, Falls G, Blake BL, Levi PE. Pesticide-metabolizing enzymes. Toxicology Letters. 1995;82/83:73–81. doi: 10.1016/0378-4274(95)03469-2. [DOI] [PubMed] [Google Scholar]
- Huang XP, Renwick JAA. Feeding deterrents and sensitivity suppressors for Pieris rapae larvae in wheat germ diet. Journal of Chemical Ecology. 1997;23:51–70. [Google Scholar]
- Jermy T, Hanson FE, Dethier VG. Induction of specific food preference in lepidopterous larvae. Entomologia Experimentalis et Applicata. 1968;11:211–230. [Google Scholar]
- Lazarević J, Perić-Maturuga V, Stojković B, Tucić N. Adaptation of the gypsy moth to an unsuitable host plant. Entomologia Experimentalis et Applicata. 2002;102:75–86. [Google Scholar]
- Leonard DE. Recent developments in ecology and control of the gypsy moth. Annual Review of Entomology. 1974;19:197–229. [Google Scholar]
- Miller JS, Feeny P. Effects of benzylisoquinoline alkaloids on the larvae of polyphagous Lepidoptera. Oecologia (Berlin) 1983;58:332–339. doi: 10.1007/BF00385232. [DOI] [PubMed] [Google Scholar]
- Miller JC, Hanson PE. Laboratory feeding tests on the development of gypsy moth larvae with references to plant taxa and allelochemicals. Agricultural Experiment Station, Station Bulletin 674 1989 [Google Scholar]
- Mosher FH. Food plants of the gypsy moth in America. U S D A Bulletin No 250. 1915:1–39. [Google Scholar]
- Neal JJ. Methylenedioxyphenyl-containing alkaloids and autosynergism. Phytochemistry. 1989;28:451–453. [Google Scholar]
- Saunders JA, O'Neill NR, Romeo JT. Alkaloid Chemistry and Feeding Specificity of Insect Herbivores. In: Pelletier SW, editor. Alkaloids: Chemical and Biological Perspective. Springer-Verlag; New York: 1992. pp. 151–196. [Google Scholar]
- Scott JG. Cytochromes P450 and insecticide resistance. Insect Biochemistry and Molecular Biology. 1999;29:757–777. doi: 10.1016/s0965-1748(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Seigler DS. Plant Secondary Metabolism. Kluwer Academic Publishers; Boston: 1998. pp. 506–512. [Google Scholar]
- Shields VDC, Broomell BP, Salako JOB. Host selection and acceptability of selected tree species by gypsy moth larvae, Lymantria dispar (L.) Annals of the Entomological Society of America. 2003;96:920–926. [Google Scholar]
- Shields VDC, Rodgers EJ, Arnold NS, Williams D. Feeding responses to selected alkaloids by gypsy moth larvae, Lymantria dispar (L.) Naturwissenschaften. 2006;93:127–130. doi: 10.1007/s00114-005-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GS, Slansky F, Jr, Yu SJ. Food consumption, utilization and detoxification enzyme activity of larvae of three polyphagous noctuid moth species when fed the botanical insecticide rotenone. Entomologia Experimentalis et Applicata. 2001;98:225–239. [Google Scholar]
- Willaman JJ, Schubert BG. Alkaloid-bearing plants and their contained alkaloids. USDA Techical Bulletin 1234 1961 [Google Scholar]
- Wrubel RP, Bernays EA. The relative insensitivity of Manduca sexta larvae to non-host plant secondary compounds. Entomologia Experimentalis et Applicata. 1990;54:117–124. [Google Scholar]