Abstract
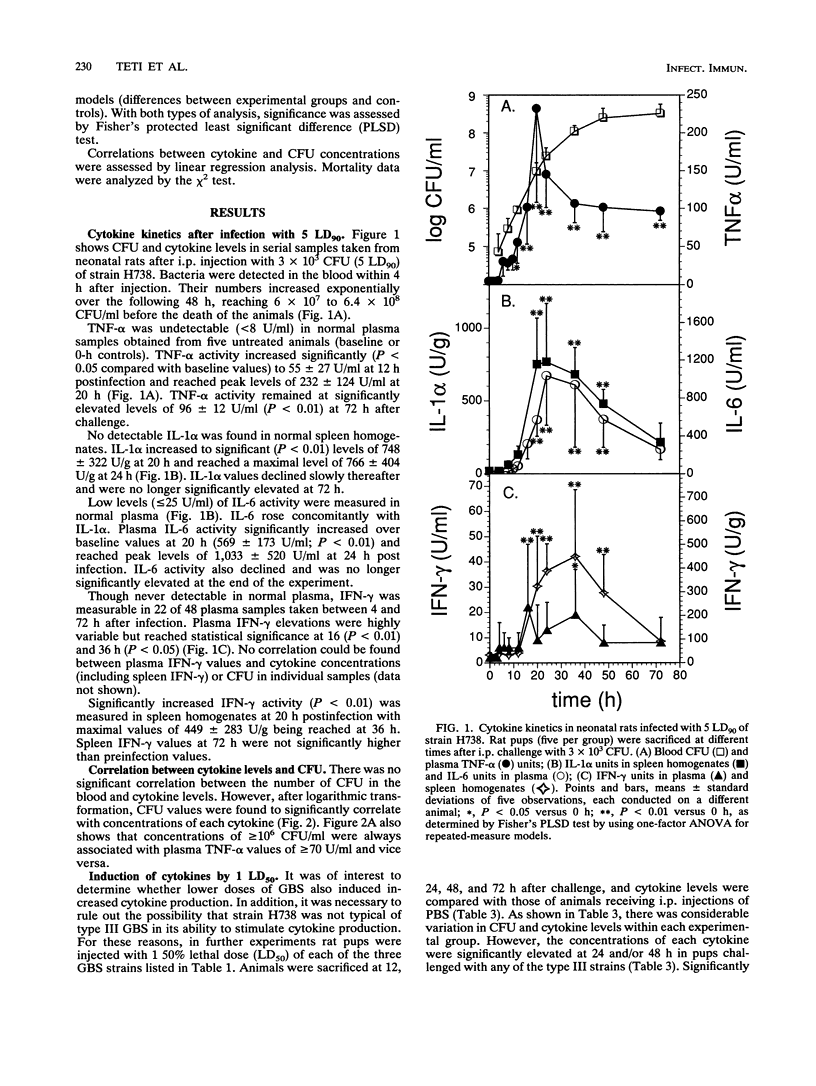
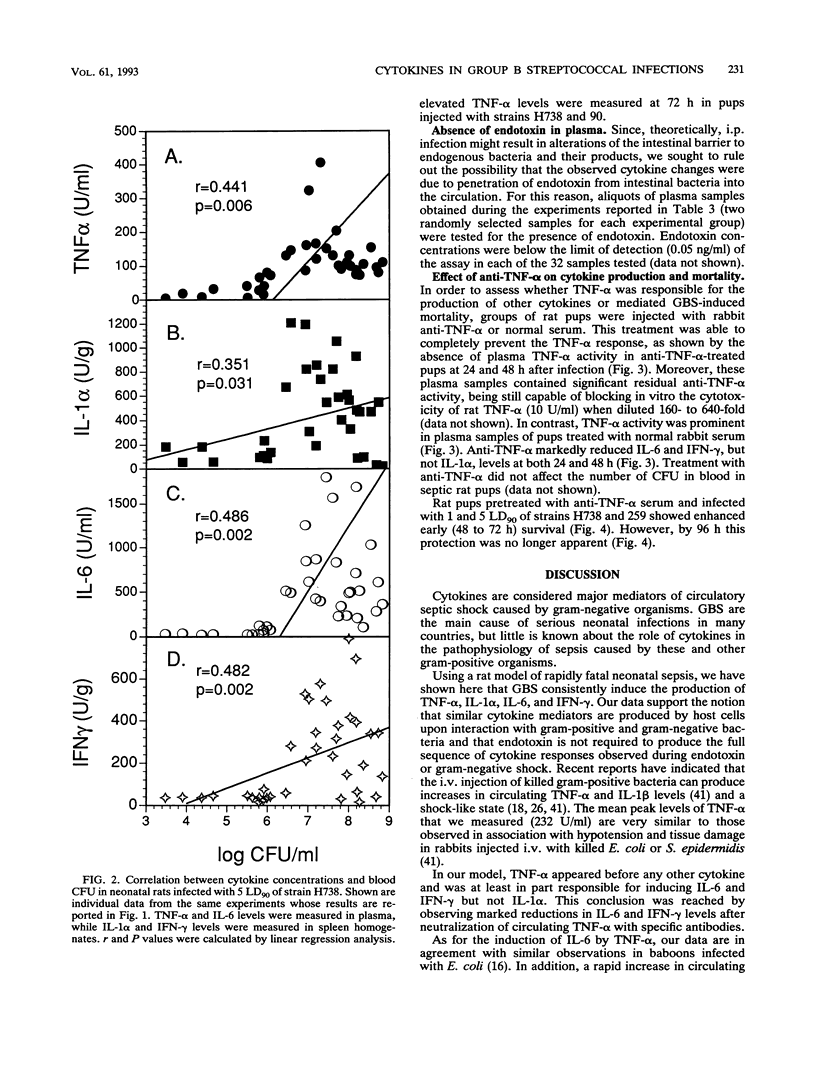
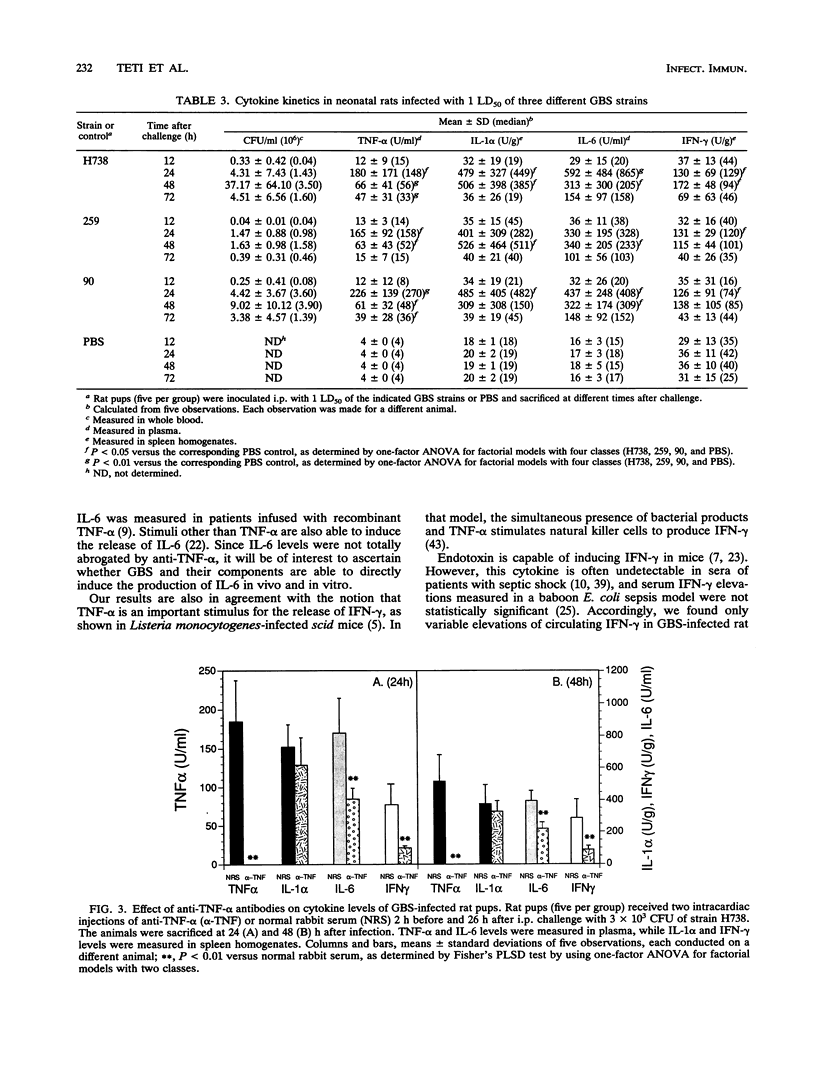
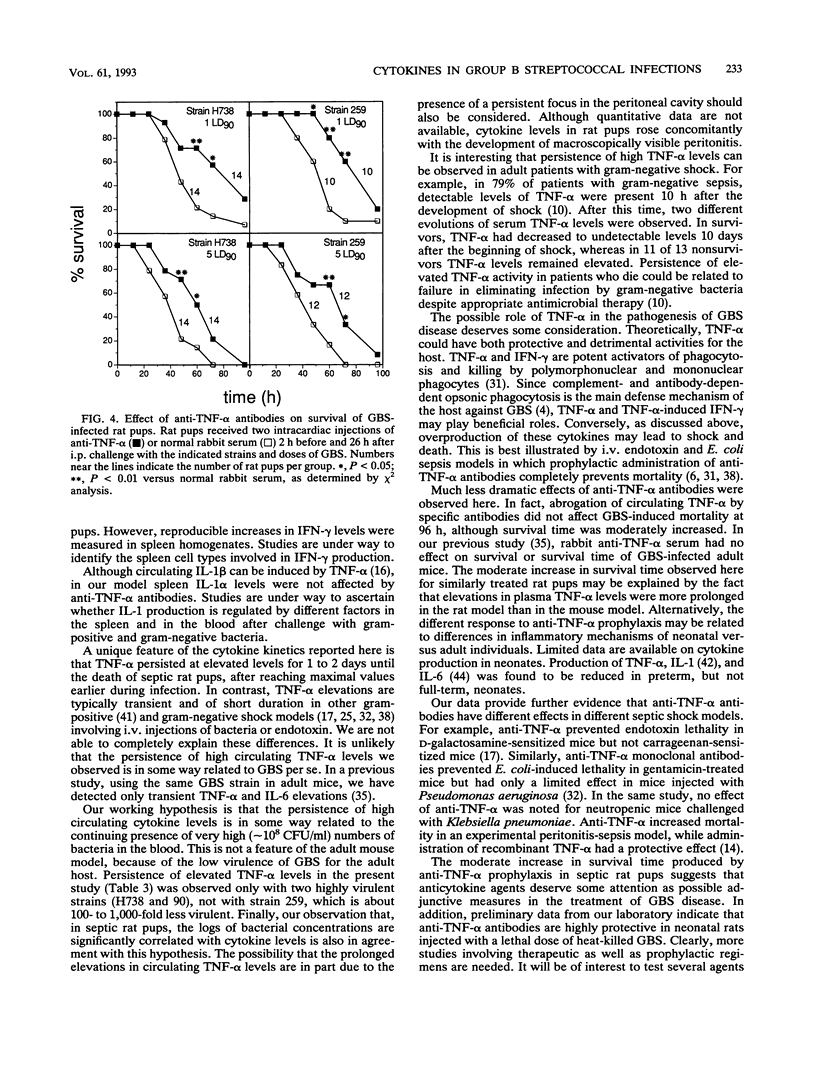
Cytokines are suspected of playing an important role in the pathophysiology of septic shock. This study was undertaken to determine whether tumor necrosis factor alpha (TNF-alpha) induces the production of other cytokines and mediates mortality in a neonatal rat model of sepsis caused by group B streptococci (GBS). We have measured TNF-alpha, interleukin-1 alpha (IL-1 alpha), interleukin-6 (IL-6), and gamma interferon (IFN-gamma) levels in neonatal rats infected with different strains (H738, 259, and 90) and doses (1 50% lethal dose [LD50] and 5 90% lethal doses [LD90]) of type III GBS. TNF-alpha and IL-6 were detected by the L929 cytotoxicity and the B9 proliferation assays, respectively, in serial plasma samples. IL-1 alpha and IFN-gamma were measured in spleen homogenates by enzyme-linked immunosorbent assay kits by using antibodies raised against the corresponding mouse cytokines. Plasma TNF-alpha levels significantly rose above baseline values within 12 h after intraperitoneal challenge with 5 LD90 of GBS strain H738, corresponding to 3 x 10(3) CFU. A mean peak TNF-alpha concentration of 232 +/- 124 U/ml was reached at 20 h. Peak IL-1 alpha and IL-6 levels of 766 +/- 404 U/g and 1,033 +/- 520 U/ml, respectively, were reached at 24 h after bacterial challenge. Maximal spleen concentrations of IFN-gamma (449 +/- 283 U/g) were measured at 36 h. Concentrations of TNF-alpha, but not other cytokines, remained significantly elevated at 72 h, a time when mortality approached 100%. Significant correlations were found between concentrations of each of the cytokines tested and the logs of CFU concentrations in the blood. In order to ascertain whether TNF-alpha influenced the production of other cytokines, rat pups received two injections of anti-murine TNF-alpha or normal rabbit serum at 2 h before and at 26 h after challenge with live GBS. Plasma TNF-alpha bioactivity was undetectable in anti-TNF-alpha-treated animals, while IL-6 and IFN-gamma, but not IL-1 alpha, levels were significantly reduced, compared with normal serum controls. Rat pups pretreated with anti-TNF-alpha serum and infected with 1 and 5 LD90 of strains H738 and 259 showed enhanced early (48 to 72 h) survival. However, by 96 h this protection was no longer apparent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Concepcion N. F., McGeary S. A., Ward J. I., Heiner D. C., Shapshak P., Insel R. A. Immunospecificity and quantitation of an enzyme-linked immunosorbent assay for group B streptococcal antibody. J Clin Microbiol. 1982 Aug;16(2):350–354. doi: 10.1128/jcm.16.2.350-354.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditi M., Manogue K. R., Caplan M., Yogev R. Cerebrospinal fluid cachectin/tumor necrosis factor-alpha and platelet-activating factor concentrations and severity of bacterial meningitis in children. J Infect Dis. 1990 Jul;162(1):139–147. doi: 10.1093/infdis/162.1.139. [DOI] [PubMed] [Google Scholar]
- Bagby G. J., Plessala K. J., Wilson L. A., Thompson J. J., Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991 Jan;163(1):83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- Baker C. J. Group B streptococcal infection in newborns: prevention at last? N Engl J Med. 1986 Jun 26;314(26):1702–1704. doi: 10.1056/NEJM198606263142609. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986 Feb 1;136(3):963–970. [PubMed] [Google Scholar]
- Brouckaert P., Spriggs D. R., Demetri G., Kufe D. W., Fiers W. Circulating interleukin 6 during a continuous infusion of tumor necrosis factor and interferon gamma. J Exp Med. 1989 Jun 1;169(6):2257–2262. doi: 10.1084/jem.169.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Carey R. B., Eisenstein T. K., Shockman G. D., Greber T. F., Swenson R. M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980 Apr;28(1):195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991 Jun;163(6):1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- Echtenacher B., Falk W., Männel D. N., Krammer P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990 Dec 1;145(11):3762–3766. [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks A. K., Kujawa K. I., Yaffe L. J. Experimental elimination of tumor necrosis factor in low-dose endotoxin models has variable effects on survival. Infect Immun. 1991 Aug;59(8):2609–2614. doi: 10.1128/iai.59.8.2609-2614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. L., Redding G. J., Henderson W. R., Truog W. E. Group B streptococcus induces tumor necrosis factor in neonatal piglets. Effect of the tumor necrosis factor inhibitor pentoxifylline on hemodynamics and gas exchange. Am Rev Respir Dis. 1991 Mar;143(3):598–604. doi: 10.1164/ajrccm/143.3.598. [DOI] [PubMed] [Google Scholar]
- Günther C., Röllinghoff M., Beuscher H. U. Proteolysis of the native murine IL 1 beta precursor is required to generate IL 1 beta bioactivity. Immunobiology. 1989 Feb;178(4-5):436–448. doi: 10.1016/s0171-2985(89)80064-6. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Sehgal P. B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991 Jan 15;146(2):756–761. [PubMed] [Google Scholar]
- Heinzel F. P. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990 Nov 1;145(9):2920–2924. [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Håkansson S., Holm S. Influence of polysaccaride capsule and ionic strength on buoyant density of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1986 Jun;94(3):139–143. doi: 10.1111/j.1699-0463.1986.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Natanson C., Danner R. L., Elin R. J., Hosseini J. M., Peart K. W., Banks S. M., MacVittie T. J., Walker R. I., Parrillo J. E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest. 1989 Jan;83(1):243–251. doi: 10.1172/JCI113866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S., Bagby G. J., Bainton B. G., Wilson L. A., Thompson J. J., Summer W. R. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989 Feb;159(2):189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Teti G., Calapai M., Calogero G., Tomasello F., Mancuso G., Galli A., Riggio G. Specificity and protective activity of murine monoclonal antibodies directed against the capsular polysaccharide of type III group B streptococci. Hybridoma. 1992 Feb;11(1):13–22. doi: 10.1089/hyb.1992.11.13. [DOI] [PubMed] [Google Scholar]
- Teti G., Mancuso G., Tomasello F., Chiofalo M. S. Production of tumor necrosis factor-alpha and interleukin-6 in mice infected with group B streptococci. Circ Shock. 1992 Oct;38(2):138–144. [PubMed] [Google Scholar]
- Teti G., Tomasello F., Chiofalo M. S., Orefici G., Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987 Dec;55(12):3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Jung W. K., Connolly R. J., Burke J. F., Dinarello C. A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Invest. 1991 Jun;87(6):1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherstone K. B., Rich E. A. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989 Apr;25(4):342–346. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A., Takano N., Ohta K., Uehara T., Fujita S., Miyawaki T., Taniguchi N. Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun. 1992 Mar;60(3):749–753. doi: 10.1128/iai.60.3.749-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



