Abstract
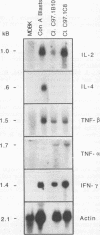
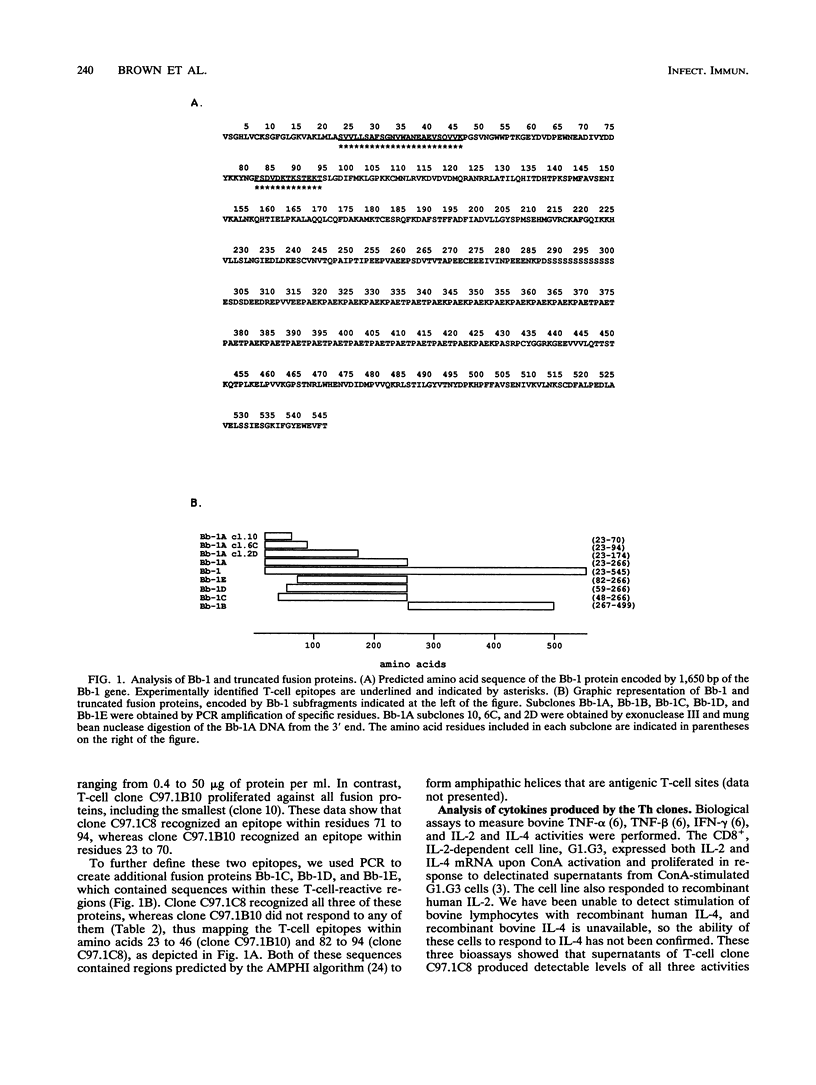
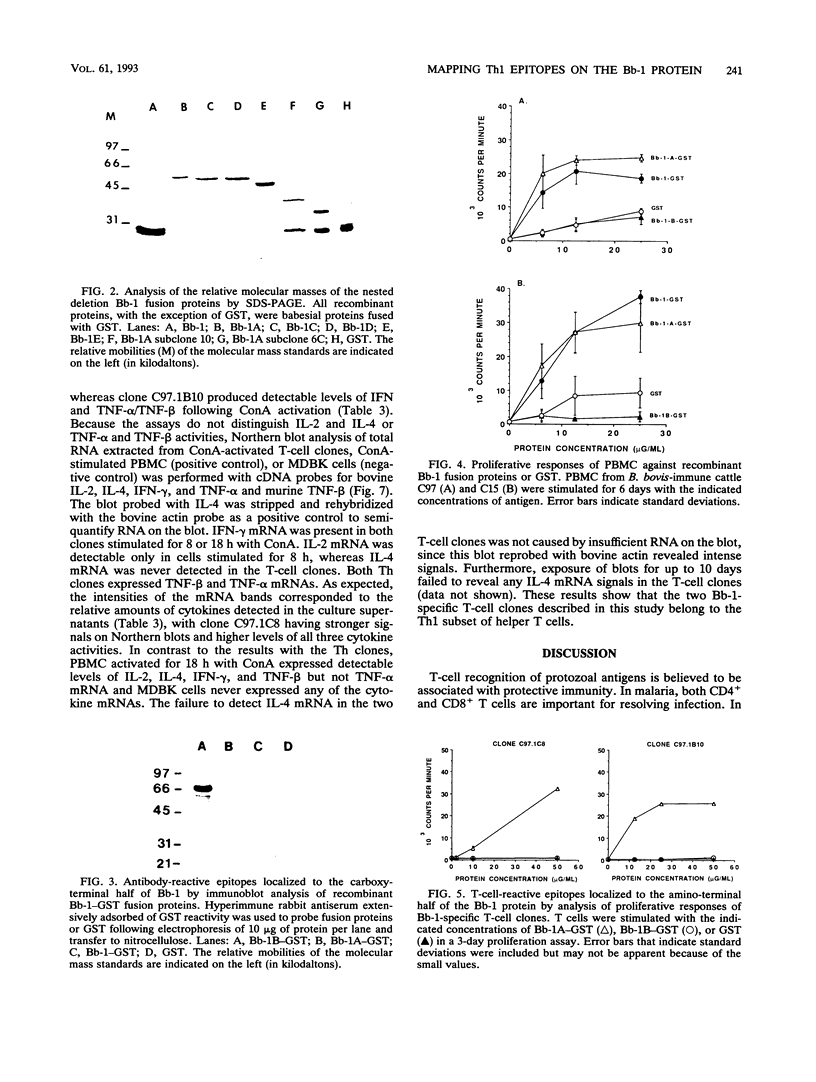
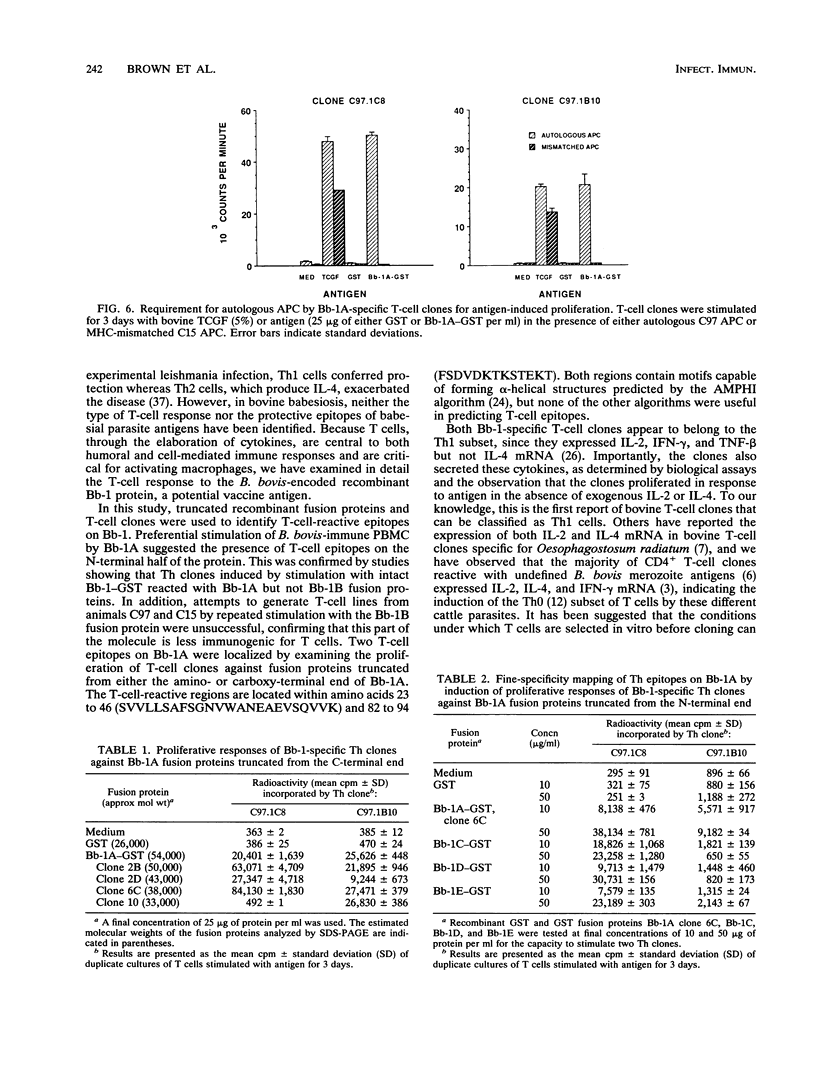
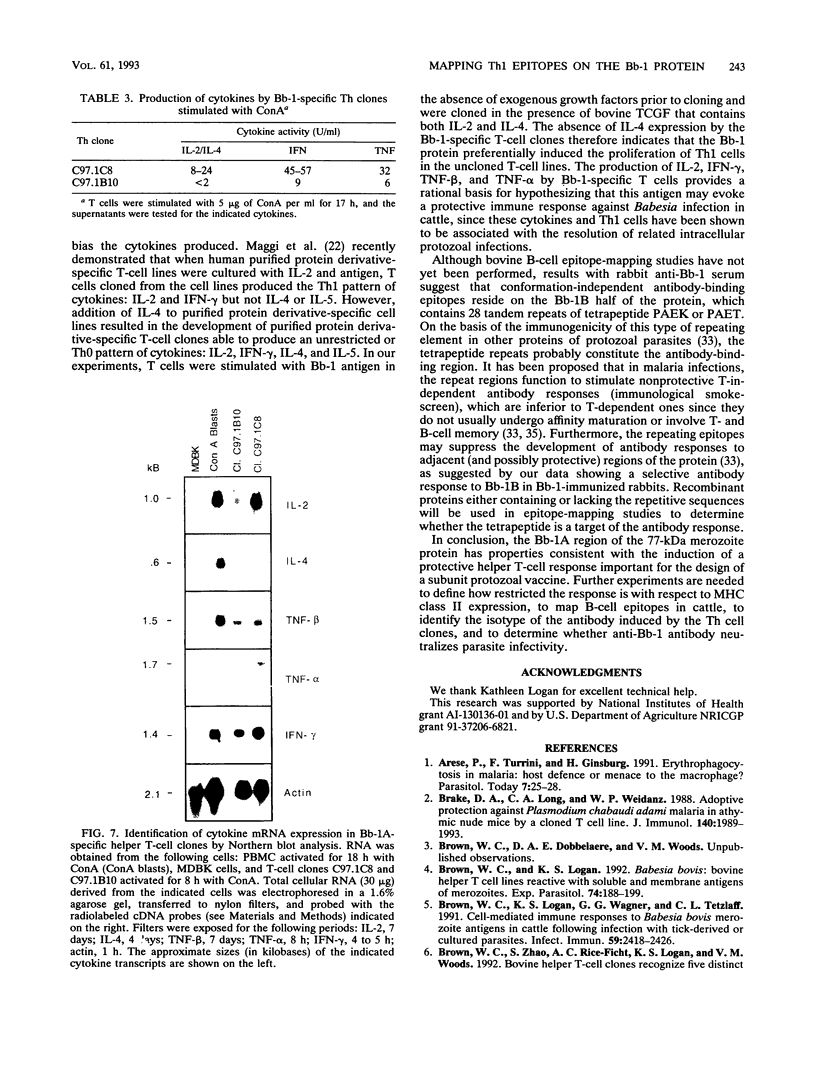
Previous studies have demonstrated the serologic and T-cell immunogenicity for cattle of a recombinant form of the apical complex-associated 77-kDa merozite protein of Babesia bovis, designated Bb-1. The present study characterizes the immunogenic epitopes of the Bb-1 protein. A series of recombinant truncated fusion proteins spanning the majority of the Bb-1 protein were expressed in Escherichia coli, and their reactivities with bovine peripheral blood mononuclear cells and T-cell clones derived from B. bovis-immune cattle and with rabbit antibodies were determined. Lymphocytes from two immune cattle were preferentially stimulated by the N-terminal half of the Bb-1 protein (amino acids 23 to 266, termed Bb-1A), localizing the T-cell epitopes to the Bb-1A portion of the molecule. CD4+ T-cell clones derived by stimulation with the intact Bb-1 fusion protein were used to identify two T-cell epitopes in the Bb-1A protein, consisting of amino acids SVVLLSAFSGN VWANEAEVSQVVK and FSDVDKTKSTEKT (residues 23 to 46 and 82 to 94). In contrast, rabbit antiserum raised against the intact fusion protein reacted only with the C-terminal half of the protein (amino acids 267 to 499, termed Bb-1B), which contained 28 tandem repeats of the tetrapeptide PAEK or PAET. Biological assays and Northern (RNA) blot analyses for cytokines revealed that following activation with concanavalin A, T-cell clones reactive against the two Bb-1A epitopes produced interleukin-2, gamma interferon, and tumor necrosis factors beta and alpha, but not interleukin-4, suggesting that the Bb-1 antigen preferentially stimulates the Th1 subset of CD4+ T cells in cattle. The studies described here report for the first time the characterization, by cytokine production, of the Th1 subset of bovine T cells and show that, as in mice, protozoal antigens can induce Th1 cells in ruminants. This first demonstration of B. bovis-encoded Th1 cell epitopes provides a rationale for incorporation of all or part of the Bb-1 protein into a recombinant vaccine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arese P., Turrini F., Ginsburg H. Erythrophagocytosis in malaria: Host defence or menace to the macrophage? Parasitol Today. 1991 Jan;7(1):25–28. doi: 10.1016/0169-4758(91)90082-y. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brown W. C., Logan K. S. Babesia bovis: bovine helper T cell lines reactive with soluble and membrane antigens of merozoites. Exp Parasitol. 1992 Mar;74(2):188–199. doi: 10.1016/0014-4894(92)90046-d. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Logan K. S., Wagner G. G., Tetzlaff C. L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991 Jul;59(7):2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Feller D. C., de la Cruz V. F. Identifying antigenic T-cell sites. Nature. 1991 Feb 21;349(6311):720–721. doi: 10.1038/349720a0. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Goeddel D. V., Aggarwal B. B., Gray P. W., Leung D. W., Nedwin G. E., Palladino M. A., Patton J. S., Pennica D., Shepard H. M., Sugarman B. J. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- Good M. F., Miller L. H. T-cell antigens and epitopes in malaria vaccine design. Curr Top Microbiol Immunol. 1990;155:65–78. doi: 10.1007/978-3-642-74983-4_5. [DOI] [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Heussler V. T., Eichhorn M., Dobbelaere D. A. Cloning of a full-length cDNA encoding bovine interleukin 4 by the polymerase chain reaction. Gene. 1992 May 15;114(2):273–278. doi: 10.1016/0378-1119(92)90587-f. [DOI] [PubMed] [Google Scholar]
- Hines S. A., Palmer G. H., Jasmer D. P., McGuire T. C., McElwain T. F. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol Biochem Parasitol. 1992 Oct;55(1-2):85–94. doi: 10.1016/0166-6851(92)90129-8. [DOI] [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A., Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991 Jan 15;146(2):762–767. [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Mirre G. B. Bovine babesiasis: the persistence of immunity to Babesia argentina and B. bigemina in calves (Bos taurus) after naturally acquired infection. Ann Trop Med Parasitol. 1973 Jun;67(2):197–203. doi: 10.1080/00034983.1973.11686877. [DOI] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Reeves R., Spies A. G., Nissen M. S., Buck C. D., Weinberg A. D., Barr P. J., Magnuson N. S., Magnuson J. A. Molecular cloning of a functional bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3228–3232. doi: 10.1073/pnas.83.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebush M. J., Hanson W. L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980 Jul;29(4):507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today. 1991 May;7(5):99–105. doi: 10.1016/0169-4758(91)90166-l. [DOI] [PubMed] [Google Scholar]
- Schofield L., Uadia P. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J Immunol. 1990 Apr 1;144(7):2781–2788. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Buus S., Appella E., Smith J. A., Chesnut R., Miles C., Colon S. M., Grey H. M. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989 May;86(9):3296–3300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Ghadirian E. Human recombinant tumor necrosis factor alpha protects susceptible A/J mice against lethal Plasmodium chabaudi AS infection. Infect Immun. 1989 Dec;57(12):3936–3939. doi: 10.1128/iai.57.12.3936-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Tavernier J., Fiers W., Playfair J. H. Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987 Jan;67(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff C. L., Rice-Ficht A. C., Woods V. M., Brown W. C. Induction of proliferative responses of T cells from Babesia bovis-immune cattle with a recombinant 77-kilodalton merozoite protein (Bb-1). Infect Immun. 1992 Feb;60(2):644–652. doi: 10.1128/iai.60.2.644-652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. A., Wagner G. G., Rice-Ficht A. C. Babesia bovis: gene isolation and characterization using a mung bean nuclease-derived expression library. Exp Parasitol. 1989 Oct;69(3):211–225. doi: 10.1016/0014-4894(89)90068-4. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Clark I. A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988 Aug;4(8):214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]