Abstract
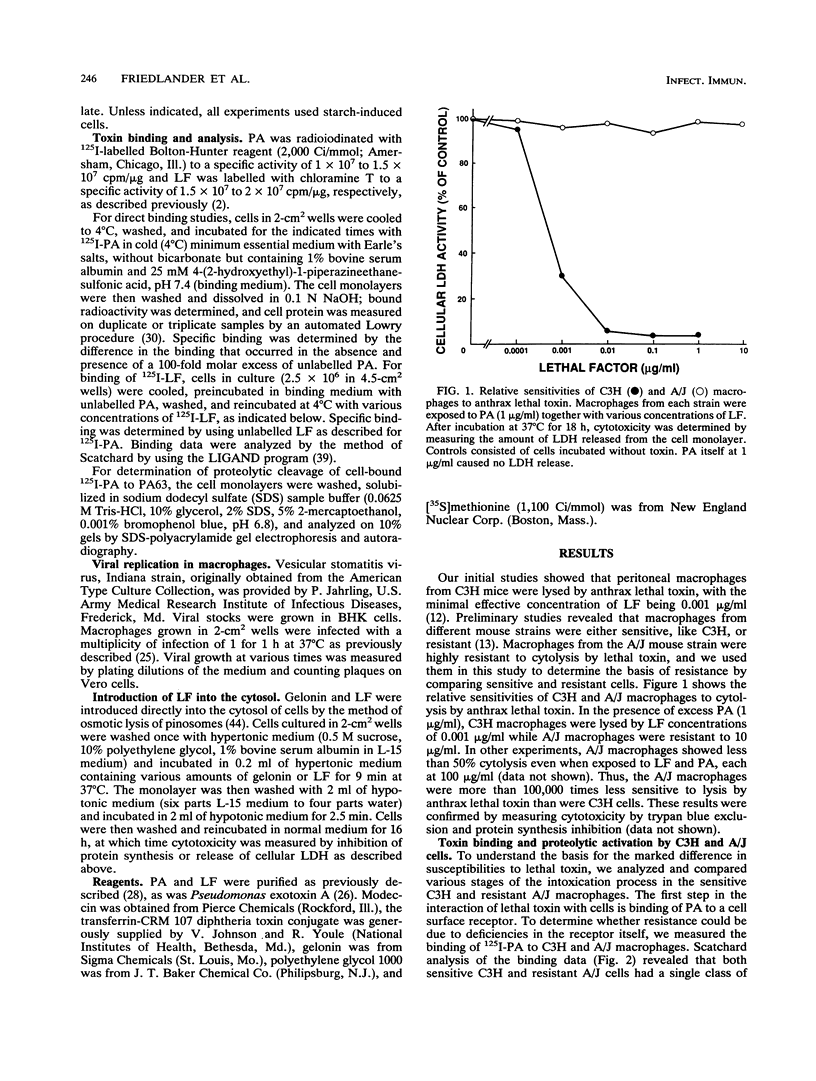
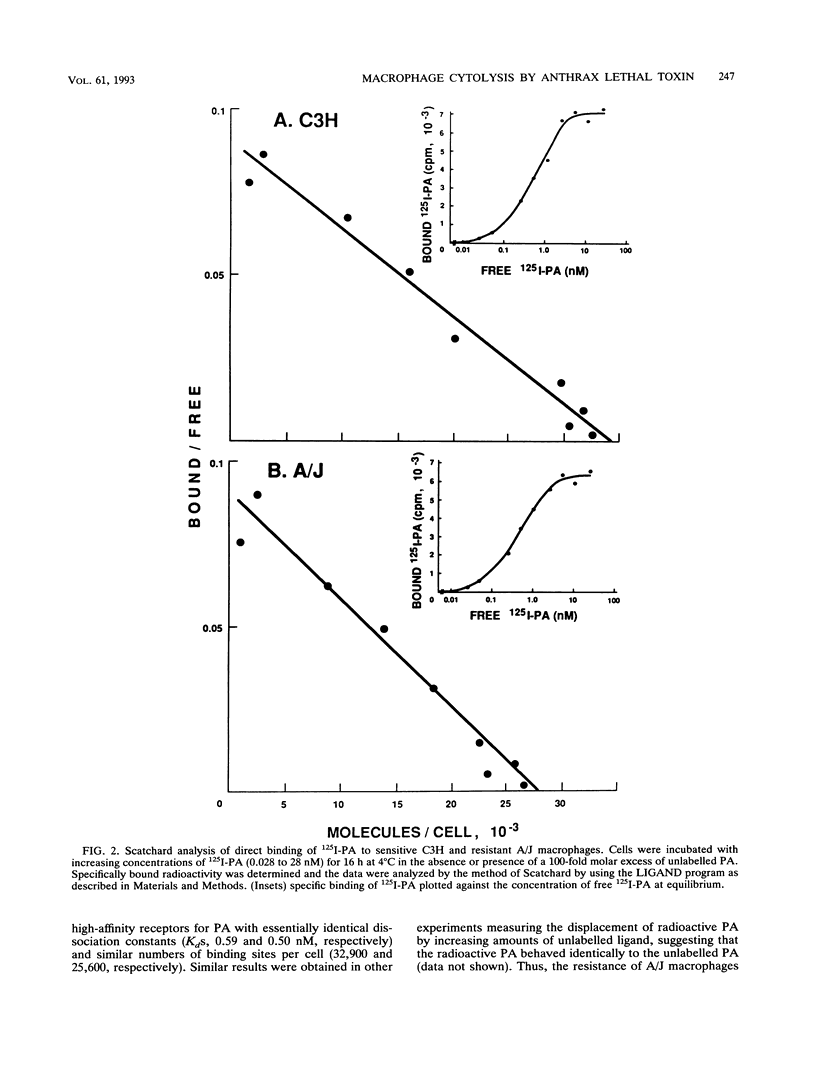
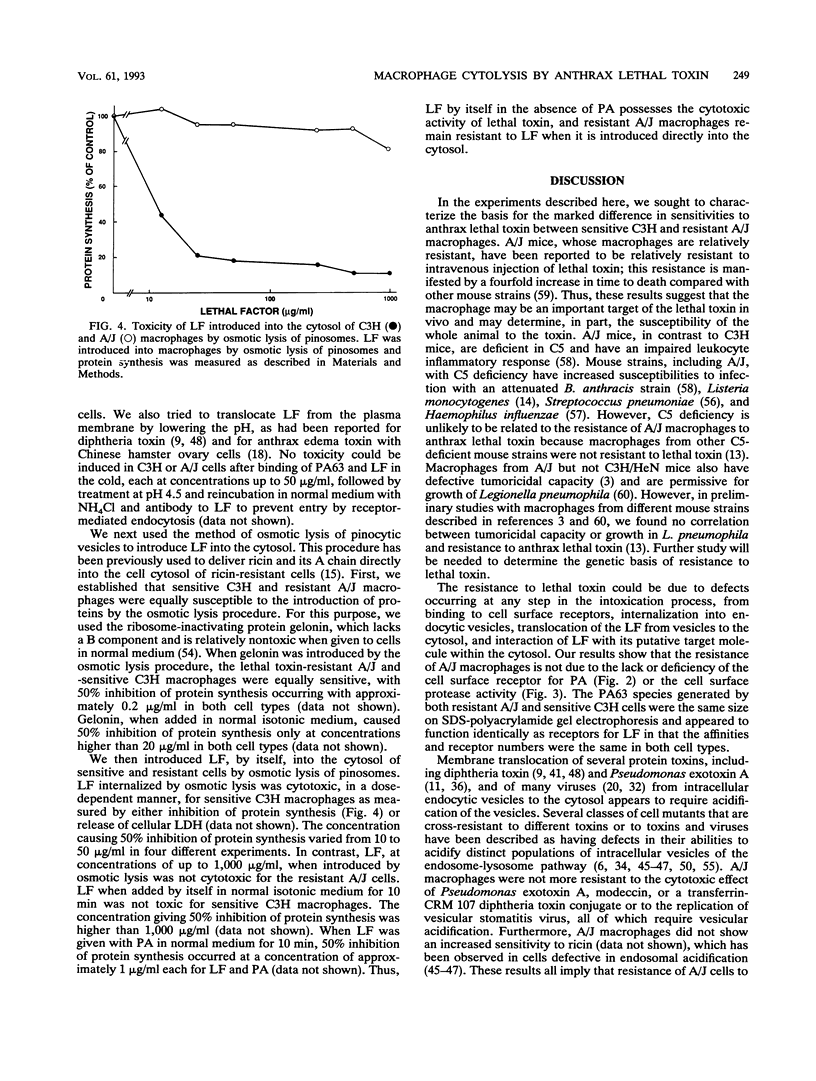
Anthrax lethal toxin, which consists of two proteins, protective antigen and lethal factor, is cytolytic for macrophages. Macrophages from different mouse strains were found to vary in their sensitivities to toxin. C3H mouse macrophages lysed by lethal factor concentrations of 0.001 micrograms/ml were 100,000 times more sensitive than those from resistant A/J mice. We analyzed various stages of the intoxication process to determine the basis for this resistance. Direct binding studies with radioiodinated protective antigen revealed that the affinity (Kd, approximately 0.5 nM) and number of receptors per cell (25,000 to 33,000) were the same in sensitive and resistant cells. Proteolytic activation of protective antigen by a cell surface protease and subsequent binding of lethal factor were also the same in both sensitive and resistant macrophages. Resistant A/J macrophages were not cross-resistant to other toxins and a virus which, like lethal toxin, require vesicular acidification for activity, implying that resistance is not due to a defect in vesicular acidification. When introduced into the cytosol by osmotic lysis of pinosomes, lethal factor in the absence of protective antigen was cytolytic for the sensitive macrophages while resistant cells were unaffected. Thus, lethal factor by itself possesses the toxic activity of lethal toxin. These results suggest that macrophage resistance is due to a defect at a stage occurring after toxin internalization. A/J macrophages may lack the putative lethal factor target in the cytosol or be defective in the further processing or activation of lethal factor in the cytosol or in endocytic vesicles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALL F. A., TAYLOR M. J., THORNE C. B. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J Bacteriol. 1962 Jun;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Singh Y., Leppla S. H., Friedlander A. M. Calcium is required for the expression of anthrax lethal toxin activity in the macrophagelike cell line J774A.1. Infect Immun. 1989 Jul;57(7):2107–2114. doi: 10.1128/iai.57.7.2107-2114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Macrophage activation for tumor cytotoxicity: genetic variation in macrophage tumoricidal capacity among mouse strains. Cell Immunol. 1979 Jun;45(1):188–194. doi: 10.1016/0008-8749(79)90375-7. [DOI] [PubMed] [Google Scholar]
- Canfield W. M., Johnson K. F., Ye R. D., Gregory W., Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24-29 of the cytoplasmic tail. J Biol Chem. 1991 Mar 25;266(9):5682–5688. [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Colbaugh P. A., Stookey M., Draper R. K. Impaired lysosomes in a temperature-sensitive mutant of Chinese hamster ovary cells. J Cell Biol. 1989 Jun;108(6):2211–2219. doi: 10.1083/jcb.108.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J Biol Chem. 1989 Aug 15;264(23):13403–13406. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuyer V., Collier R. J. Anthrax protective antigen interacts with a specific receptor on the surface of CHO-K1 cells. Infect Immun. 1991 Oct;59(10):3381–3386. doi: 10.1128/iai.59.10.3381-3386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Baldwin R. L., Wisnieski B. J. Pseudomonas exotoxin A. Membrane binding, insertion, and traversal. J Biol Chem. 1986 Aug 25;261(24):11404–11408. [PubMed] [Google Scholar]
- Friedlander A. M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986 Jun 5;261(16):7123–7126. [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Ghosh P. C., Wellner R. B., Wu H. C. Osmotically induced microinjection of ricin bypasses a ricin internalization defect in a Chinese hamster ovary mutant cell line. Mol Cell Biol. 1984 Jul;4(7):1320–1325. doi: 10.1128/mcb.4.7.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Ron A., Rogel A., Hanski E. Bordetella pertussis adenylate cyclase inactivation by the host cell. Biochem J. 1989 Aug 15;262(1):25–31. doi: 10.1042/bj2620025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Leppla S. H., Hewlett E. L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56(5):1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel F. G., Posner B. I., Bergeron J. J., Frank B. H., Duckworth W. C. Isolation of insulin degradation products from endosomes derived from intact rat liver. J Biol Chem. 1988 May 15;263(14):6703–6708. [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. G., Wilson D., Greenfield L., Youle R. J. The role of the diphtheria toxin receptor in cytosol translocation. J Biol Chem. 1988 Jan 25;263(3):1295–1300. [PubMed] [Google Scholar]
- Koehler T. M., Collier R. J. Anthrax toxin protective antigen: low-pH-induced hydrophobicity and channel formation in liposomes. Mol Microbiol. 1991 Jun;5(6):1501–1506. doi: 10.1111/j.1365-2958.1991.tb00796.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee M. T., Warren M. K. CSF-1-induced resistance to viral infection in murine macrophages. J Immunol. 1987 May 1;138(9):3019–3022. [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- Marnell M. H., Mathis L. S., Stookey M., Shia S. P., Stone D. K., Draper R. K. A Chinese hamster ovary cell mutant with a heat-sensitive, conditional-lethal defect in vacuolar function. J Cell Biol. 1984 Dec;99(6):1907–1916. doi: 10.1083/jcb.99.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Planck S. R., Magun B. E. Intracellular processing of epidermal growth factor. I. Acidification of 125I-epidermal growth factor in intracellular organelles. J Biol Chem. 1984 Mar 10;259(5):3047–3052. [PubMed] [Google Scholar]
- Merion M., Schlesinger P., Brooks R. M., Moehring J. M., Moehring T. J., Sly W. S. Defective acidification of endosomes in Chinese hamster ovary cell mutants "cross-resistant" to toxins and viruses. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5315–5319. doi: 10.1073/pnas.80.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H. M., Rose J. K., Mellman I. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell. 1989 Jul 28;58(2):317–327. doi: 10.1016/0092-8674(89)90846-5. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983 Sep;41(3):998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Cell-mediated reduction of the interfragment disulfide in nicked diphtheria toxin. A new system to study toxin entry at low pH. J Biol Chem. 1987 Jul 25;262(21):10339–10345. [PubMed] [Google Scholar]
- Mostov K. E., de Bruyn Kops A., Deitcher D. L. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986 Nov 7;47(3):359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. O., Draper R. K. Characterization of a transferrin-diphtheria toxin conjugate. J Biol Chem. 1985 Jan 25;260(2):932–937. [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Pease R. J., Smith G. D., Peters T. J. Degradation of endocytosed insulin in rat liver is mediated by low-density vesicles. Biochem J. 1985 May 15;228(1):137–146. doi: 10.1042/bj2280137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Livneh E., Ullrich A., Schlessinger J. Mutations in the cytoplasmic domain of EGF receptor affect EGF binding and receptor internalization. EMBO J. 1986 Sep;5(9):2179–2190. doi: 10.1002/j.1460-2075.1986.tb04482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M. Osmotic lysis of pinosomes. Methods Enzymol. 1987;149:42–48. doi: 10.1016/0076-6879(87)49042-3. [DOI] [PubMed] [Google Scholar]
- Robbins A. R., Oliver C., Bateman J. L., Krag S. S., Galloway C. J., Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984 Oct;99(4 Pt 1):1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Fuchs R., Mellman I., Robbins A. R. Chinese hamster ovary cell mutants with temperature-sensitive defects in endocytosis. I. Loss of function on shifting to the nonpermissive temperature. J Cell Biol. 1986 Dec;103(6 Pt 1):2283–2297. doi: 10.1083/jcb.103.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J., Mandel R., Hacobian A., Shen W. C. Methotrexate-poly(lysine) as a selective agent for mutants of Chinese hamster ovary cells defective in endocytosis. J Cell Physiol. 1988 May;135(2):277–284. doi: 10.1002/jcp.1041350215. [DOI] [PubMed] [Google Scholar]
- STANLEY J. L., SMITH H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961 Sep;26:49–63. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaudies R. P., Gorman R. M., Savage C. R., Jr, Poretz R. D. Proteolytic processing of epidermal growth factor within endosomes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):710–715. doi: 10.1016/0006-291x(87)91412-4. [DOI] [PubMed] [Google Scholar]
- Schmid S., Fuchs R., Kielian M., Helenius A., Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989 Apr;108(4):1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Chaudhary V. K., Leppla S. H. A deleted variant of Bacillus anthracis protective antigen is non-toxic and blocks anthrax toxin action in vivo. J Biol Chem. 1989 Nov 15;264(32):19103–19107. [PubMed] [Google Scholar]
- Singh Y., Leppla S. H., Bhatnagar R., Friedlander A. M. Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J Biol Chem. 1989 Jul 5;264(19):11099–11102. [PubMed] [Google Scholar]
- Stirpe F., Olsnes S., Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980 Jul 25;255(14):6947–6953. [PubMed] [Google Scholar]
- Timchak L. M., Kruse F., Marnell M. H., Draper R. K. A thermosensitive lesion in a Chinese hamster cell mutant causing differential effects on the acidification of endosomes and lysosomes. J Biol Chem. 1986 Oct 25;261(30):14154–14159. [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Hansen E. J. Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect Immun. 1985 Oct;50(1):207–212. doi: 10.1128/iai.50.1.207-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C. The role of C5 in polymorphonuclear leukocyte recruitment in response to Streptococcus pneumoniae. Am Rev Respir Dis. 1984 Jan;129(1):82–86. doi: 10.1164/arrd.1984.129.1.82. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Friedlander A. M. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb Pathog. 1988 Jan;4(1):53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Keener T. J., Gibbs P. H. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986 Mar;51(3):795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988 Feb;56(2):370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



