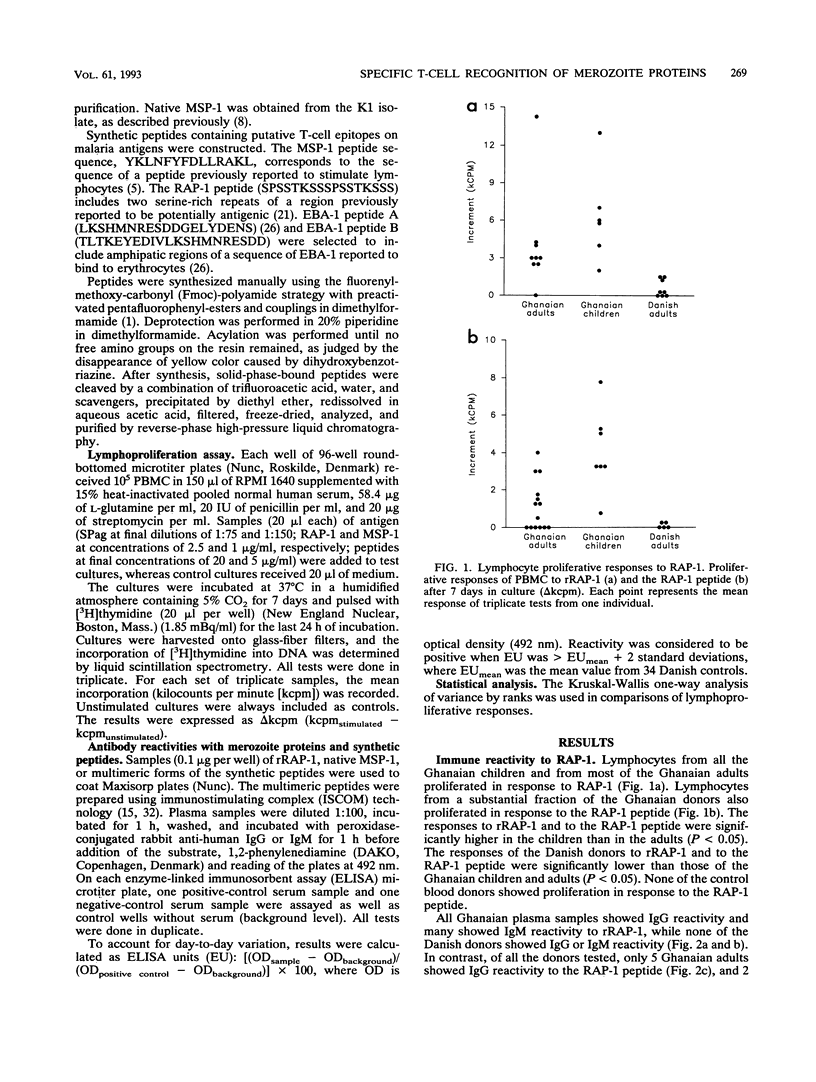
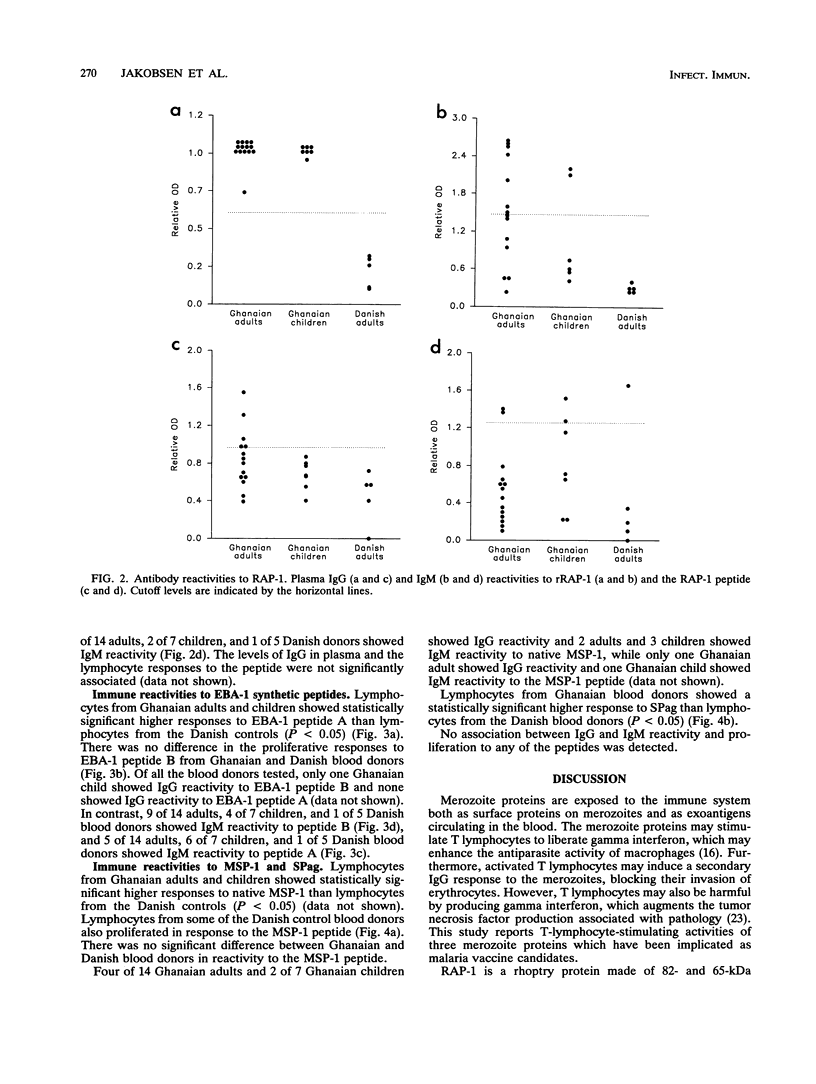
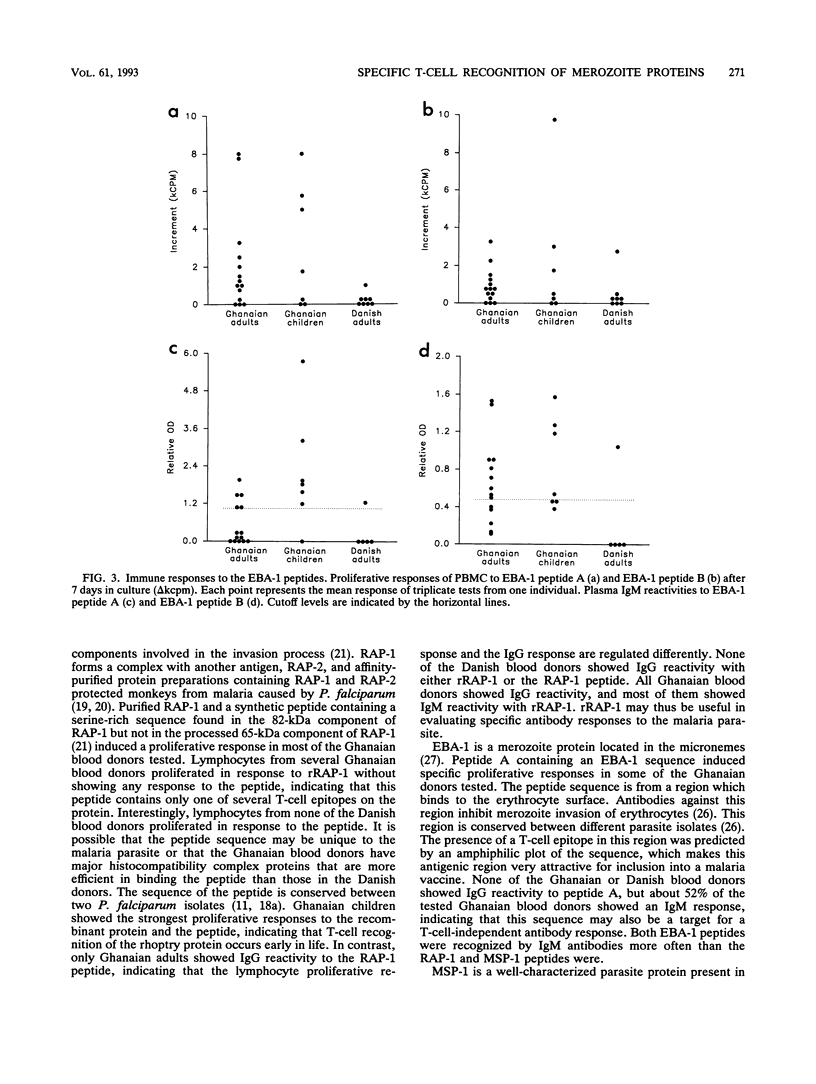
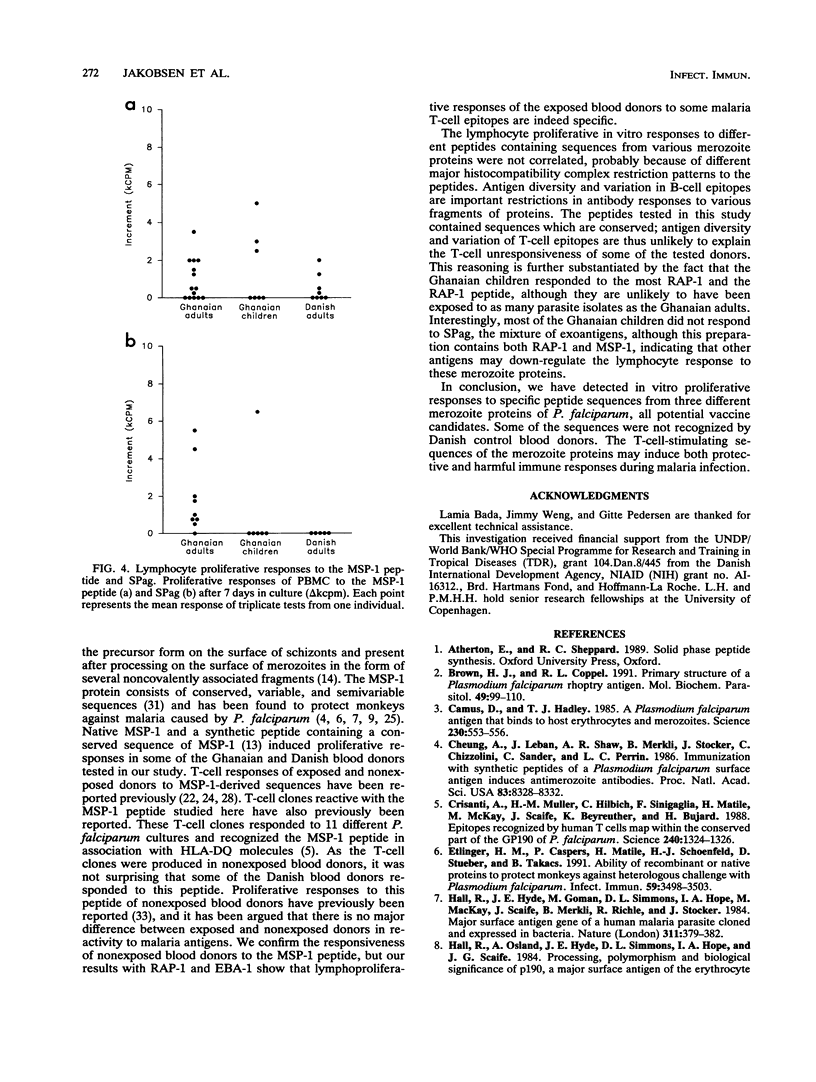
Abstract
The merozoite proteins merozoite surface protein 1 (MSP-1) and rhoptry-associated protein 1 (RAP-1) and synthetic peptides containing sequences of MSP-1, RAP-1, and erythrocyte-binding antigen 1, induced in vitro proliferative responses of lymphocytes collected from Ghanaian blood donors living in an area with a high rate of transmission of malaria. Lymphocytes from a large proportion of the Ghanaian blood donors proliferated in response to the RAP-1 peptide, unlike those of Danish control blood donors, indicating that this sequence contains a malaria-specific T-cell epitope broadly recognized by individuals living in an area with a high transmission rate of malaria. Most of the donor plasma samples tested contained immunoglobulin G (IgG) and IgM antibodies recognizing the merozoite proteins, while only a minority showed high IgG reactivity to the synthetic peptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. J., Coppel R. L. Primary structure of a Plasmodium falciparum rhoptry antigen. Mol Biochem Parasitol. 1991 Nov;49(1):99–110. doi: 10.1016/0166-6851(91)90133-q. [DOI] [PubMed] [Google Scholar]
- Camus D., Hadley T. J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985 Nov 1;230(4725):553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- Cheung A., Leban J., Shaw A. R., Merkli B., Stocker J., Chizzolini C., Sander C., Perrin L. H. Immunization with synthetic peptides of a Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8328–8332. doi: 10.1073/pnas.83.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Caspers P., Matile H., Schoenfeld H. J., Stueber D., Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991 Oct;59(10):3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Rosero F., Herrera S., Caspers P., Rotmann D., Sinigaglia F., Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992 Jan;60(1):154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Howard R. F. The sequence of the p82 rhoptry protein is highly conserved between two Plasmodium falciparum isolates. Mol Biochem Parasitol. 1992 Apr;51(2):327–330. doi: 10.1016/0166-6851(92)90083-v. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Theander T. G., Jensen J. B., Mølbak K., Jepsen S. Soluble Plasmodium falciparum antigens contain carbohydrate moieties important for immune reactivity. J Clin Microbiol. 1987 Nov;25(11):2075–2079. doi: 10.1128/jcm.25.11.2075-2079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S., Tanabe K., Nakazawa S., Yanagi T., Kanbara H. Sequence variation in the tripeptide repeats and T cell epitopes in P190 (MSA-1) of Plasmodium falciparum from field isolates. Mol Biochem Parasitol. 1992 Mar;51(1):81–89. doi: 10.1016/0166-6851(92)90203-v. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Heidrich H. G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987 Feb;23(1):71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Orlandi P. A., Sim B. K., Chulay J. D., Haynes J. D. Characterization of the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990 May;40(2):285–294. doi: 10.1016/0166-6851(90)90050-v. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Marshall V. M., Smythe J. A., Crewther P. E., Lew A., Silva A., Anders R. F., Kemp D. J. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989 Jul;9(7):3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley R. G., Lahm H. W., Takács B., Scaife J. G. Genetic and structural relationships between components of a protective rhoptry antigen complex from Plasmodium falciparum. Mol Biochem Parasitol. 1991 Aug;47(2):245–246. doi: 10.1016/0166-6851(91)90184-8. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Etlinger H., Scaife J. G. A rhoptry antigen of Plasmodium falciparum is protective in Saimiri monkeys. Parasitology. 1990 Oct;101(Pt 2):187–192. doi: 10.1017/s0031182000063228. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Lahm H. W., Delves C. J., Goman M., Certa U., Matile H., Woollett G. R., Scaife J. G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990 Jun;41(1):125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Wheeler J. G., Blackman M. J., Bennett S., Takacs B., Schönfeld H. J., Holder A. A., Greenwood B. M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992 May;14(3):321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jakobsen P. H., Allen S. J., Wheeler J. G., Bennett S., Jepsen S., Greenwood B. M. Immune response to soluble exoantigens of Plasmodium falciparum may contribute to both pathogenesis and protection in clinical malaria: evidence from a longitudinal, prospective study of semi-immune African children. Eur J Immunol. 1991 Apr;21(4):1019–1025. doi: 10.1002/eji.1830210424. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Ramasamy R., Mutch D. A., Ho P. C., Battistutta D., Anderson K. L., Parkinson D., Doran T. J., Honeyman M. Analysis of human T cell response to two Plasmodium falciparum merozoite surface antigens. Eur J Immunol. 1989 Oct;19(10):1797–1802. doi: 10.1002/eji.1830191006. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim B. K. Sequence conservation of a functional domain of erythrocyte binding antigen 175 in Plasmodium falciparum. Mol Biochem Parasitol. 1990 Jun;41(2):293–295. doi: 10.1016/0166-6851(90)90193-p. [DOI] [PubMed] [Google Scholar]
- Sim B. K., Toyoshima T., Haynes J. D., Aikawa M. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1992 Mar;51(1):157–159. doi: 10.1016/0166-6851(92)90211-2. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Takacs B., Jacot H., Matile H., Pink J. R., Crisanti A., Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contain both T and B cell epitopes. J Immunol. 1988 May 15;140(10):3568–3572. [PubMed] [Google Scholar]
- Smythe J. A., Peterson M. G., Coppel R. L., Saul A. J., Kemp D. J., Anders R. F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Mar;39(2):227–234. doi: 10.1016/0166-6851(90)90061-p. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Uggla A., Araujo F. G., Lunden A., Lövgren K., Remington J. S., Morein B. Immunizing effects in mice of two Toxoplasma gondii iscom preparations. Zentralbl Veterinarmed B. 1988 May;35(4):311–314. doi: 10.1111/j.1439-0450.1988.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Zevering Y., Amante F., Smillie A., Currier J., Smith G., Houghten R. A., Good M. F. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992 Mar;22(3):689–696. doi: 10.1002/eji.1830220311. [DOI] [PubMed] [Google Scholar]


