Abstract
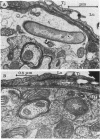
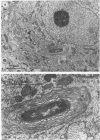
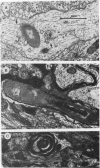
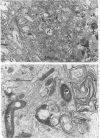
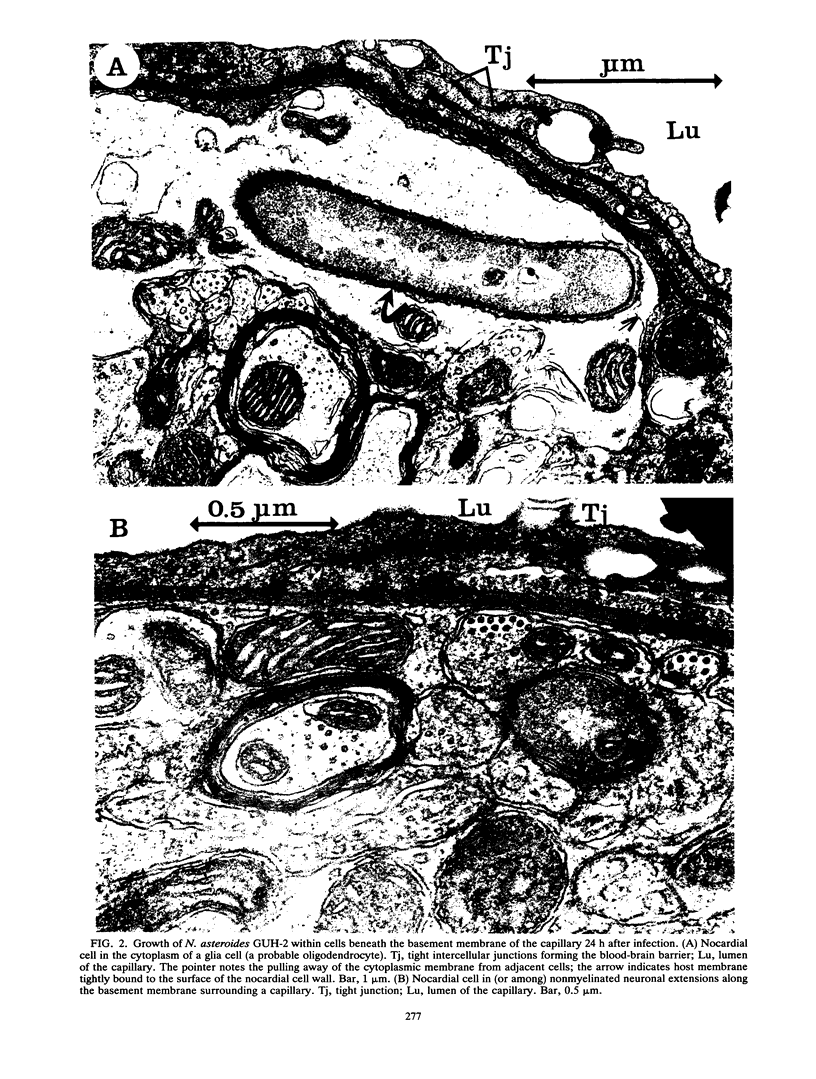
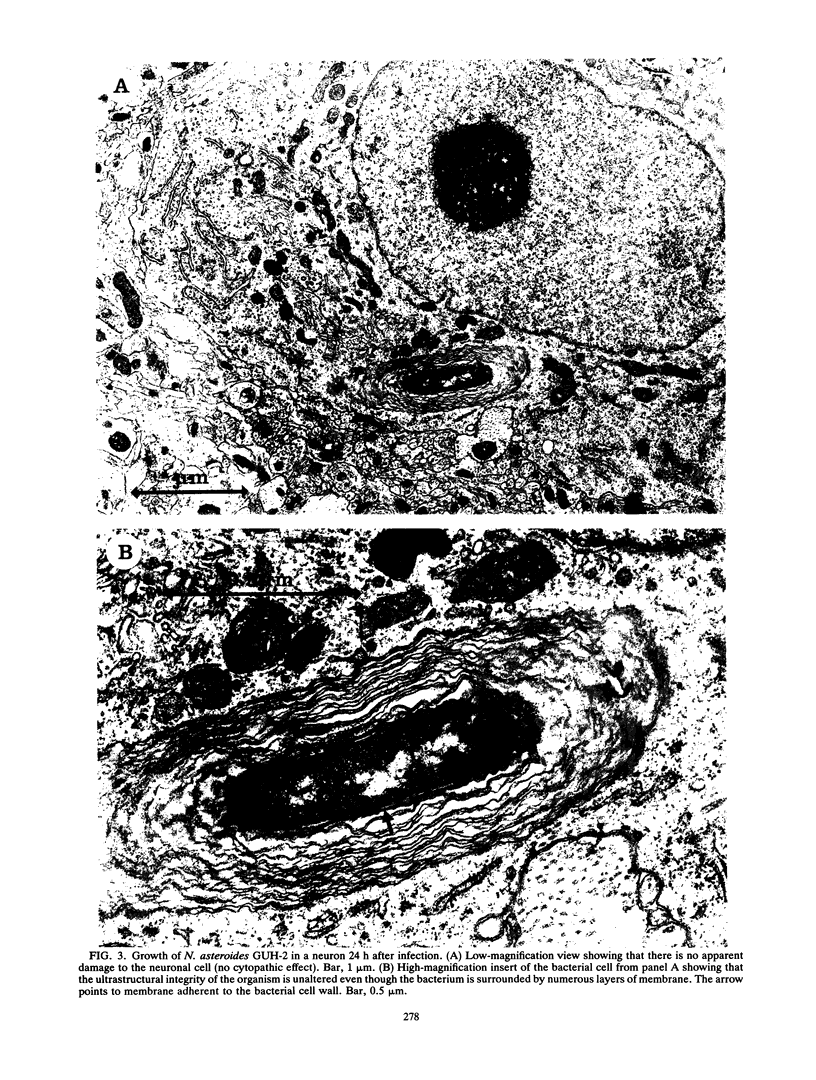
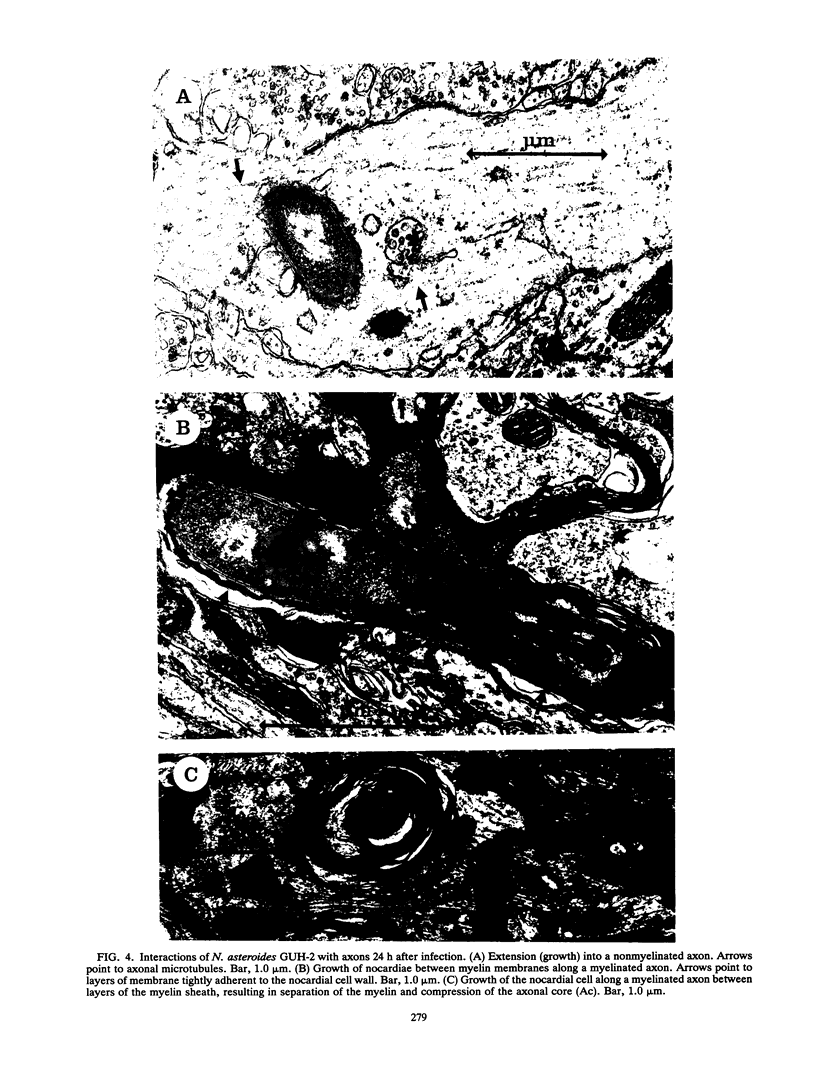
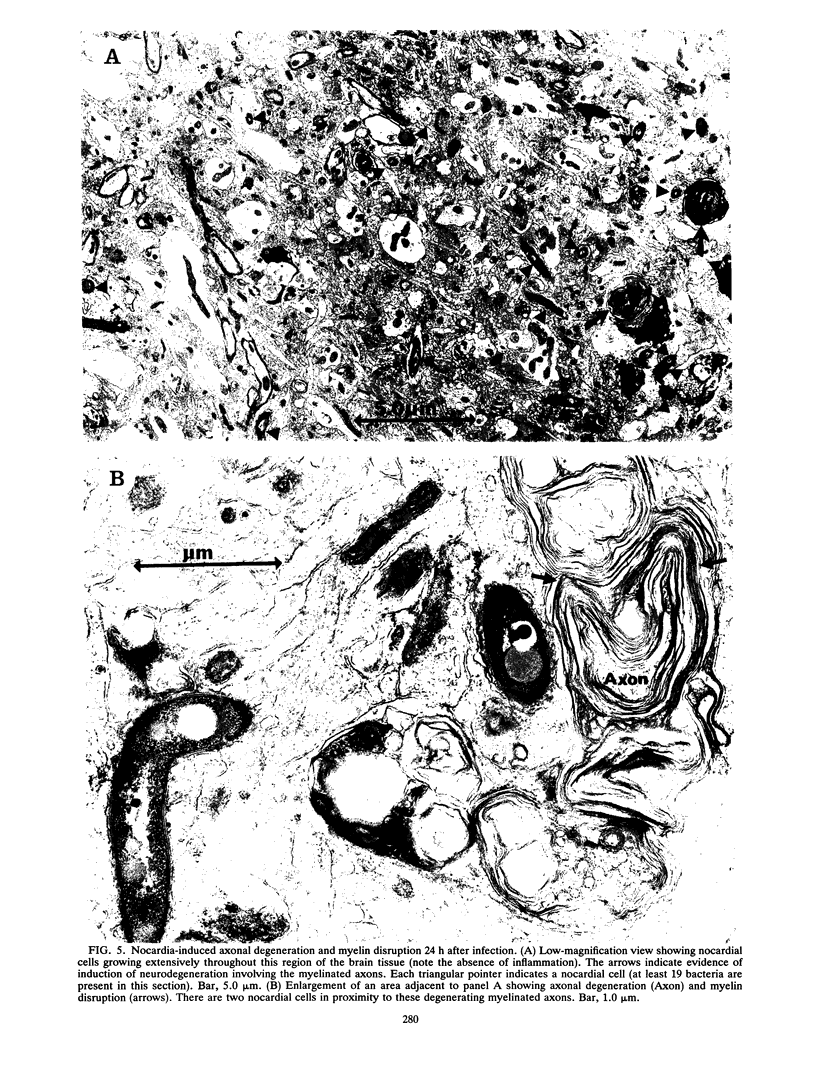
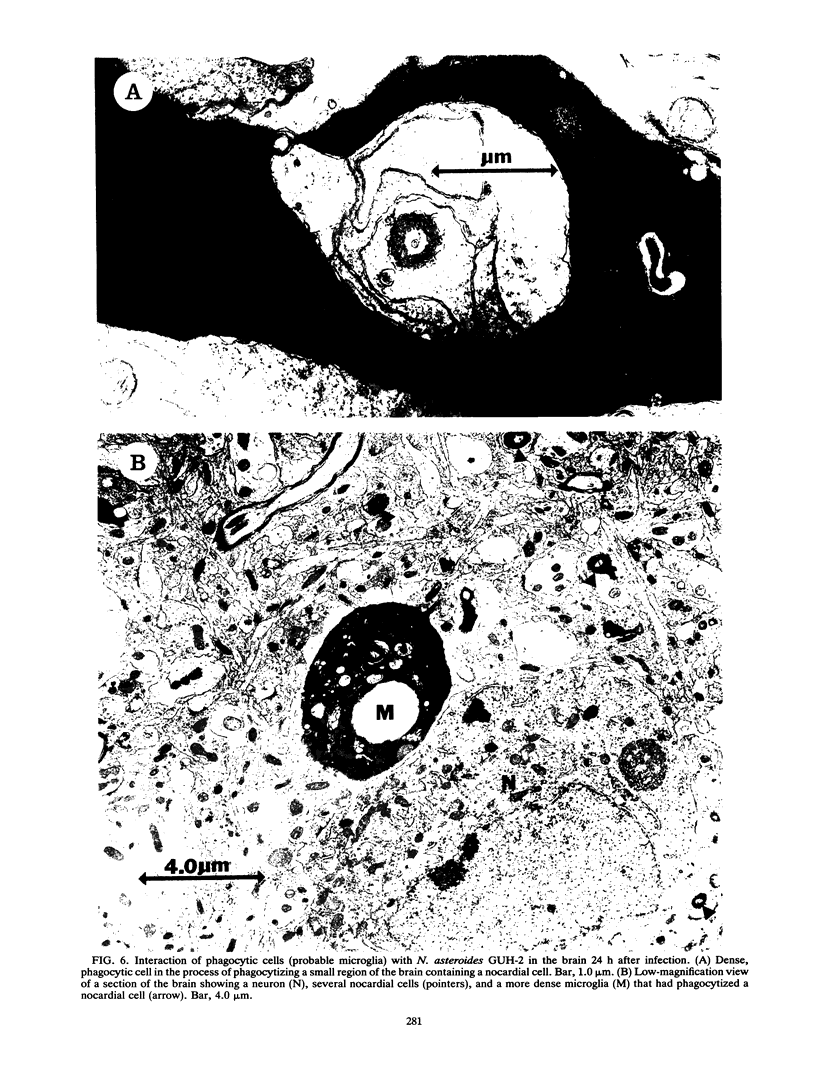
BALB/c mice were infected with 10(6) CFU of log-phase cells of Nocardia asteroides GUH-2 by tail vein injection (at this lethal inoculum dose, approximately 800 to 1,000 CFU becomes deposited in the brain). At 24 h after infection, the ultrastructural interactions of the nocardiae during growth within the murine brain were investigated. The nocardiae grew perivascularly in the pons, substantia nigra, hypothalamus, and thalamus portions of the brain, where they were either within or associated with most brain cell types. There appeared to be a propensity for growth within the soma of neurons and their axonal extensions. The nocardial cells were surrounded by 1 to 30 layers of membrane, and the innermost membrane was usually tightly adherent to the cell wall. This compartmentalization of nocardiae within brain cells could contribute to their failure to induce an inflammatory response or a cytopathic effect. Nevertheless, the bacteria were able to obtain adequate nutrients from the host to grow within the brain. The nocardiae were not completely inert, since some of the brain cells showed signs of degeneration. The myelin sheaths of axons were the most strongly affected, and there was evidence of demyelinization and axonal degeneration. Nocardiae growing within brain cells were phagocytized by compact, dense cells that were probably microglia. There was no ultrastructural evidence that the nocardiae were damaged by these phagocytes 24 h after infection; nevertheless, these cells may be important for the elimination of nocardiae from the brain during a nonlethal infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Scates S. S., Ohsugi Y. Immunobiology of germfree mice infected with Nocardia asteroides. Infect Immun. 1980 Aug;29(2):733–743. doi: 10.1128/iai.29.2.733-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Interaction of Nocardia asteroides at different phases of growth with in vitro-maintained macrophages obtained from the lungs of normal and immunized rabbits. Infect Immun. 1979 Oct;26(1):355–361. doi: 10.1128/iai.26.1.355-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Moring S. E. Relationship among cell wall composition, stage of growth, and virulence of Nocardia asteroides GUH-2. Infect Immun. 1988 Mar;56(3):557–563. doi: 10.1128/iai.56.3.557-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Nocardia as a pathogen of the brain: mechanisms of interactions in the murine brain--a review. Gene. 1992 Jun 15;115(1-2):213–217. doi: 10.1016/0378-1119(92)90561-3. [DOI] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Interactions of Nocardia asteroides with murine glia cells in culture. Infect Immun. 1993 Jan;61(1):343–347. doi: 10.1128/iai.61.1.343-347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. The timing of exposure of mononuclear phagocytes to recombinant interferon gamma and recombinant tumor necrosis factor alpha alters interactions with Nocardia asteroides. J Leukoc Biol. 1992 Mar;51(3):276–281. doi: 10.1002/jlb.51.3.276. [DOI] [PubMed] [Google Scholar]
- Beaman L., Paliescheskey M., Beaman B. L. Acid phosphatase stimulation of the growth of Nocardia asteroides and its possible relationship to the modification of lysosomal enzymes in macrophages. Infect Immun. 1988 Jun;56(6):1652–1654. doi: 10.1128/iai.56.6.1652-1654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Beaman B. L., Donovan R. M., Goldstein E. Intracellular acid phosphatase content and ability of different macrophage populations to kill Nocardia asteroides. Infect Immun. 1985 Feb;47(2):375–383. doi: 10.1128/iai.47.2.375-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Paliescheskey M., Beaman B. L., Donovan R. M., Goldstein E. Acidification of phagosomes in murine macrophages: blockage by Nocardia asteroides. J Infect Dis. 1986 Dec;154(6):952–958. doi: 10.1093/infdis/154.6.952. [DOI] [PubMed] [Google Scholar]
- Black C. M., Paliescheskey M., Beaman B. L., Donovan R. M., Goldstein E. Modulation of lysosomal protease-esterase and lysozyme in Kupffer cells and peritoneal macrophages infected with Nocardia asteroides. Infect Immun. 1986 Dec;54(3):917–919. doi: 10.1128/iai.54.3.917-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Krick J. A., Remington J. S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980 Sep;142(3):432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- Kohbata S., Beaman B. L. L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991 Jan;59(1):181–191. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S. A., Beaman B. L. Adherence of Nocardia asteroides within the murine brain. Infect Immun. 1992 May;60(5):1800–1805. doi: 10.1128/iai.60.5.1800-1805.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S. A., Beaman B. L. Site-specific growth of Nocardia asteroides in the murine brain. Infect Immun. 1992 Aug;60(8):3262–3267. doi: 10.1128/iai.60.8.3262-3267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi J. S., Tanwar R. K. Comparative distribution of acid phosphatase and simple esterase in the mouse neocortex and hippocampal formation. Acta Anat (Basel) 1989;135(4):323–329. doi: 10.1159/000146776. [DOI] [PubMed] [Google Scholar]