Abstract
The androgen receptor blocker flutamide and the 5α-reductase inhibitor finasteride have been used in a variety of species to investigate the ontogeny of sexual dimorphisms by treating pregnant females or neonates at critical periods of sexual differentiation. Likewise, we have used these drugs to study the profound masculinization of the external genitalia in female spotted hyenas. However, a potential pitfall of administering flutamide, either alone or in combination with finasteride, is that it maintains or even raises plasma concentrations of luteinizing hormone (LH) and testosterone (T), because negative feedback of the hypothalamic-pituitary-gonadal axis is disrupted. Contrary to expectations, when pregnant spotted hyenas were treated with flutamide and finasteride (F&F), the concentrations of T during late gestation were suppressed relative to values in untreated dams. Herein, we further investigate the paradoxical effects of F&F treatment on a battery of sex hormones in spotted hyenas. Beyond the effects on T, we found plasma concentrations of LH, estradiol, progesterone and androstenedione (A4) were also significantly lower in F&F-treated pregnant hyenas than in controls. Flutamide and finasteride did not have similar effects on LH, T, and A4 concentrations in male hyenas. The paradoxical effect of F&F treatment on LH and T concentrations in the maternal circulation suggests that negative feedback control of gonadotropin and androgen secretion may be modified in spotted hyenas during pregnancy.
Keywords: hyena, hyaena, androgens, endocrine, flutamide, finasteride
Introduction
The female spotted hyena, Crocuta crocuta, is renowned for having the most highly masculinized external genitalia among extant mammals. The spotted hyena clitoris approximates the size and length of the penis and is traversed by a single urogenital canal, through which the female receives the male during copulation, urinates, and gives birth. To test the hypothesis that prenatal androgens masculinize the external genitalia in female spotted hyenas, Drea et al. [4] treated dams with anti-androgen drugs from early gestation to parturition. The most commonly used regimen was a combination of an androgen receptor blocker (flutamide) and a 5α-reductase inhibitor (finasteride). Finasteride blocks the conversion of T to the more potent metabolite 5α-dihydrotestosterone (DHT), which is the androgen principally responsible for the masculinization of the genital tubercle in eutherian mammals [13, 27]. One potential drawback of using flutamide and finasteride (F&F) to prevent the masculinization of the external genitalia is that F&F treatment could disrupt negative feedback of the hypothalamic-pituitary-gonadal (HPG) axis and consequently increase the concentration of T [5, 14]. As a result, increased T could theoretically overcome the intended androgen blockade. When F&F-treated hyena dams failed to produce offspring with a diminutive phallus and a vaginal opening separate from the urethra [4], the disruption of negative feedback due to F&F treatment was considered a possible explanation. However, contrary to expectations, Drea et al. [4] found T levels were actually lower in three F&F-treated dams during late gestation than in the same dams during untreated pregnancies.
Because the preliminary investigation by Drea et al. [4] found no evidence of the anticipated negative feedback of F&F treatment on maternal T, in the present study we performed a comprehensive analysis of sex hormones using archived samples from the original F&F-treated dams. To more fully assess the effects of anti-androgens on the HPG axis in spotted hyenas, we analyzed plasma concentrations of luteinizing hormone (LH), estradiol (E2), androstenedione (A4), and progesterone (P4), in addition to measuring T. We also increased the sample size of subjects by collecting blood samples from two additional F&F-treated hyenas and nine untreated hyenas during late gestation. The hormone concentrations of F&F-treated and untreated dams were matched for gestational age.
In spotted hyenas, the concentrations of T in untreated pregnant females during late gestation were as high, if not higher, than in adult males [8, 15]. Therefore, we included a modest number of male hyenas in the present study as a preliminary investigation to determine if the hormonal changes in response to F&F treatment observed in pregnant females would be apparent in males. Moreover the inclusion of males provided an opportunity to evaluate the effects of administering flutamide and finasteride alone, as well as F&F combined, on circulating LH and androgen concentrations.
Materials and Methods
All experiments were completed at the Field Station for the Study of Behavior, Ecology, and Reproduction at the University of California, Berkeley, where a captive colony of spotted hyenas has been studied since 1985. All studies received prior approval from the Animal Care and Use Committee of the University of California at Berkeley and conformed to principles described in the NIH guide for the use and care for laboratory animals. All animals were adults when blood samples were collected for hormone analyses. Animals were immobilized with ketamine (4 - 6 mg kg-1) and xylazine (1 mg kg-1) administered by blow dart. Blood was collected from the external jugular vein, centrifuged, and the drawn-off plasma was aliquotted and frozen at -80°C until assayed.
Experiment 1: Pregnant hyenas
A single blood sample was collected from each of five F&F-treated and nine untreated, pregnant hyenas during the third trimester of an 110-d gestation. F&F-treated dams were given oral flutamide (17-25 mg/kg/d, Schering Corporation, Kenilworth, NJ) and finasteride (0.55-1 mg/kg/d, Merck and Co., Inc., West Point, PA) twice daily, starting in the first or second trimester and continuing until parturition [4]. All pregnancies derived from timed matings and fetal viability and growth were documented by serial ultrasound examinations [22].
Experiment 2: Treatment of males
The number of adult males that could be treated with anti-androgens was limited by the modest size of the Berkeley colony and the need to maintain stud males for breeding. Three adult male hyenas were sampled once before treatment and then again at the end of a four-week of treatment with flutamide alone (25 mg/kg/d) and then with finasteride alone (1 mg/kg/d), given orally twice daily. The hiatus between the flutamide and finasteride treatments was at least 3 mo. These three males received no further treatment so that they could resume breeding. Two different adult males were treated with F&F for 4 wk, at the doses given above. These latter males were also sampled once before and at the end of treatment.
Hormone assays
All hormones were analyzed by radioimmunoassay (RIA). Plasma concentrations of testosterone (T), androstenedione (A4), and luteinizing hormone (LH) were measured in nearly all hyenas studied, but the determination of progesterone (P4) and estradiol (E2) concentrations were limited to pregnant dams.
For measurement of T, A4, and E2 concentrations, plasma samples were analyzed using RIA's that have been validated for spotted hyenas and in use by the Berkeley Spotted Hyena Project for many years [4, 15, 21]. Intra- and inter-assay coefficients of variation (CV) were < 12 and 10%, respectively. The cross-reactivity of the T antiserum with DHT was 44%. Licht et al. (1992) found that a substantial proportion of “testosterone” measured in unchromatographed hyena samples was DHT (29.5% in males and 44.1% in pregnant females), and thus DHT accounts for some proportion of the T concentrations reported herein.
Plasma concentrations of LH were measured with a heterologous RIA validated for spotted hyenas [21] using reagents provided by Dr. A. F. Parlow at the National Hormone and Peptide Program (NHPP). The NHPP reagents were from a kit for measuring LH in rats. Samples were run in two batches. The first run included samples from pregnant females and had a minimum detectable limit (MDL) of 0.06 ng/mL. The second run included samples from males and had an MDL of 0.09 ng/mL. The intra-and inter-assay CVs for the LH assay were 7.4 and 9.0% respectively.
Plasma P4 concentration was measured by RIA as previously described Dahl et al. [3]. Briefly, P4 concentrations spanned a broad range and thus hyena plasma samples were variably diluted (1:10 to 1:600) in PBSG-gelatin (PBSG) to ensure that values fell on the standard curve (12.5 to 5000 pg/ml; P4 standards from Steraloids, Newport, RI). Diluted samples were extracted with diethyl ether and reconstituted in PBSG. Extracted samples were incubated overnight at 4°C with 3H-P4 (New England Nuclear, Waltham, MA) and antiserum (#8939, Stebenfeldt, University of California, Davis). Bound and unbound P4 were separated with charcoal dextrin. Samples were incubated for 20 min at 4°C, centrifuged at 4°C (1160 × g), and the supernatant was poured into vials with 4 ml of scintillation cocktail for counting. A serial dilution of pooled hyena plasma was parallel to the standard curve. The intra-and inter-assay CVs for the P4 assay were 4.2 and 6.9% respectively.
Statistics
Hormone data were analyzed with a commercial statistical program (JMP 8.0.1, SAS Institute, Cary, NC). All data were log-transformed to better approximate normal distributions and homogeneity of variances. Untransformed data were used for graphical purposes. Observed differences were considered statistically significant if p < 0.05, and these are reported as such regardless of the actual p-value.
In experiment 1, log-transformed hormone values from F&F-treated and untreated pregnant dams were compared using Student's t-test. In experiment 2, sample sizes for males were necessarily small because treatment of hyenas with anti-androgens removed them from the pool of breeders for other investigations and for maintenance of the colony. Therefore, statistical analyses were limited to situations when the sample sizes were at least three per group. Log-transformed hormone data pre and post anti-androgen treatments were analyzed using paired t-tests. Analysis of data from the two F&F-treated males was limited to descriptive representations. Note, however, that when the paradoxical effects of F&F treatment on T levels were originally detected in pregnant hyenas, the outcomes were pronounced and apparent in just three animals [4]. Therefore, we were looking for large effects that could be evident in a small number of males.
Results
Experiment 1: Pregnant hyenas
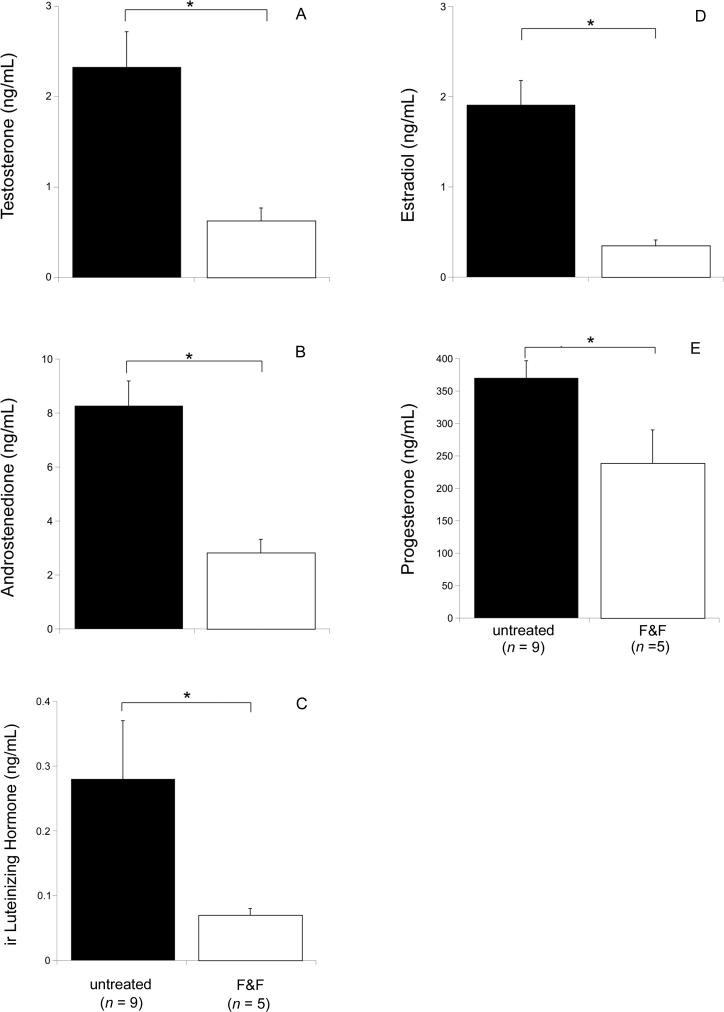
F&F treatment was associated with a generalized reduction in sex hormones during late gestation. Relative to untreated pregnant hyenas, F&F-treated dams had significantly lower T, A4, LH, E2, and P4 concentrations (p < 0.05, Fig. 1A-E).
Figure 1.
Mean (+ SEM) sex hormone concentrations in pregnant spotted hyenas measured during late gestation. Dams were either untreated (n = 9) or treated (n = 5) with flutamide and finasteride (F&F) in an attempt to block the masculinization of the external genitalia. Samples were taken before treatment and following a duration of F&F treatment that lasted at least 4 wk. * Denotes means that are statistically different (p < 0.05).
Experiment 2: Male hyenas
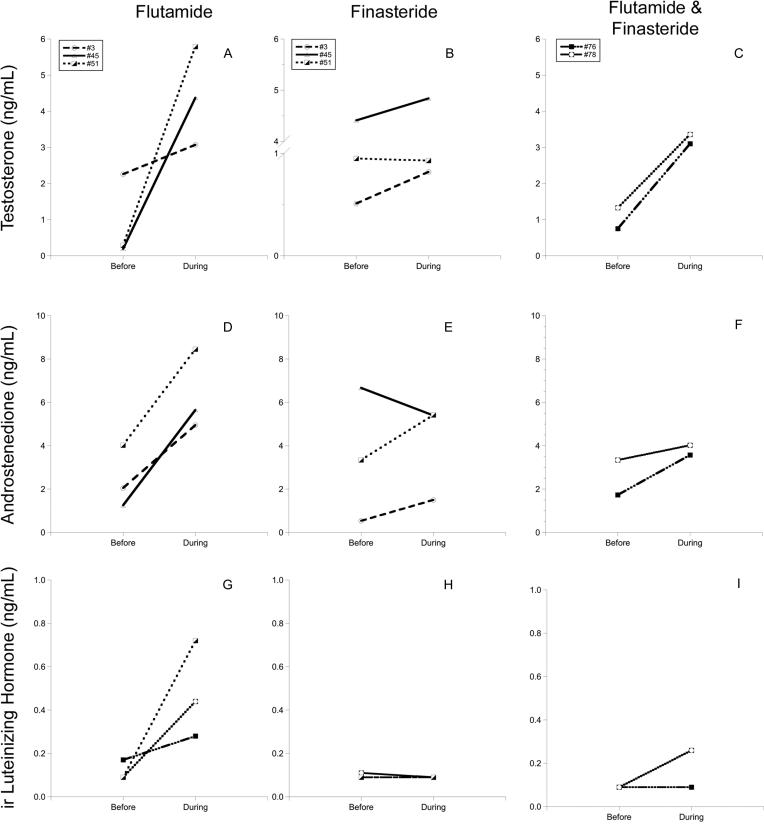
Each of the three males treated with flutamide alone showed a 1.4 to 29.5-fold increase in T (p > 0.05, range of increase 0.8 to 5.5 ng/mL; Fig. 2A). However, finasteride alone did not have a consistent effect on T concentrations (Fig. 2B). In contrast to the effect of combined F&F treatment on T in pregnant hyenas, the same regimen appeared to increase the concentration of T in the two males that were tested. After 4 wk of F&F, treated males showed a 2.7 to 5.2-fold increase in T above the pre-treatment concentrations (range of increase 2.0 to 2.4 ng/mL; Fig. 2C).
Figure 2.
Concentrations of testosterone (A-C), androstenedione (D-F), and luteinizing hormone (G-I) in individual male hyenas before and during treatment with three different anti-androgen regimens: flutamide alone (n = 3), finasteride alone (n = 3), and flutamide plus finasteride (n = 2). Note: the lines for hyenas #3 and #51 completely overlap in the LH panel for finasteride alone (H).
With respect to A4 concentrations, the effects of the anti-androgen treatments in males were similar to those seen for T, except that flutamide alone was associated with a statistically significant increase in A4 (p < 0.05, Fig. 2 D). Flutamide alone and F&F combined (Fig 2 F) were associated with a 1.2 to 3.9-fold increase in A4 in all treated males (range of increase 0.7 to 4.4 ng/mL), but the effects of finasteride alone were inconsistent (Fig. 2E).
With respect to LH, pre-treatment levels in males were relatively low and flutamide alone (Fig. 2 G) was associated with a 1.8 to 7.9-fold rise in LH concentration in all three males (p > 0.05, range of increase 0.11 to 0.63 ng/mL). Finasteride alone (Fig. 2 H) appeared to have no effect (p > 0.05). One of the two F&F-treated males had an increase in LH, but the other male maintained LH levels below the MDL of the assay Fig. (2 I).
Discussion
The results of the present study confirm the paradoxical effects that F&F treatment had on maternal T concentrations during late gestation in the spotted hyena, and extend the findings of Drea et al. [4] to a battery of additional sex hormones (LH, A4, E2, and P4) that also appear to be reduced in response to F&F. Interestingly, flutamide-alone and F&F treatment of male hyenas was associated with a rise in LH and and T, which is typical of other species, including human [5, 14, 24]. However, owing to small sample sizes, the outcomes of anti-androgen treatment of male hyenas should be considered preliminary until more animals can be tested.
Because F&F-treatment was associated with a suppression of plasma androgen concentrations in pregnant hyenas, the persistence of a large clitoris and pseudoscrotum and the lack of an external vaginal opening in the offspring of spotted hyenas treated with F&F throughout gestation does not appear to be secondary to a F&F-induced disruption of negative feedback, which is typically associated with a rise in T concentrations [5, 14]. Our analyses of plasma from pregnant hyenas were limited to samples collected during late gestation, when T levels in females of this species are at their highest [15]. By late gestation sexual differentiation of the external genitalia is nearly complete in the spotted hyena [2, 7] and the effects of F&F on maternal and more importantly, fetal hormone levels during the period of sexual differentiation remain to be determined. However, even if F&F-treatment was to cause an elevation in T during early to mid gestation in spotted hyenas, it would not be unreasonable to expect a pronounced effect of F&F on the development of the external genitalia. For example, in a study of the effectiveness of flutamide as an androgen blocker in sheep, the external genitalia of male and female offspring born to pregnant ewes treated concurrently with flutamide and testosterone were found to be phenotypically female [11].
Evidence of flutamide having androgen agonist, as well as antagonist, actions may be an alternate explanation for the persistence of the large clitoris in spotted hyenas following in utero treatment with F&F. The paradoxical effects of F&F on maternal LH and T concentrations point to this possibility. Wallen [26] reported that male offspring of rhesus monkeys treated with flutamide during late gestation were paradoxically “hypermasculinized” as compared to controls, in that flutamide-treated males displayed mounting behavior at a significantly higher rate than control males. However, flutamide increased the concentration of T in pregnant monkeys [9] and the higher T may have acted centrally in male offspring to eventually modulate their mounting behavior [26]. The agonist/antagonist actions of flutamide were also evident in an in vitro study of rat neurons [20]. Flutamide had neuroprotective effects on neuron cultures that mimicked the effects of DHT, while at the same time flutamide also acted like an antagonist by blocking androgen-mediated gene expression. Whereas these studies call into question the central/neuronal effects of flutamide, its ability to block androgenic effects in sensitive peripheral tissues has been well established in rodents and dogs [10, 18, 19].
Our preliminary investigation of the effects of anti-androgens in male spotted hyenas suggests that the paradoxical effects of F&F treatment may be a peculiarity of pregnant females of this species. Modulation of hormone negative feedback during pregnancy is not uncommon, and in other species it has been reported for prolactin [25] and the hypothalamic-pituitary-adrenal (HPA) axis [6, 12]. Therefore, the negative feedback qualities of the LH - androgen system may be altered during pregnancy in the spotted hyena, which may have led to the unexpected responses to F&F. The placenta contributes to the modulation of the HPA axis in other species [28], and the placenta is a significant source of testosterone in the spotted hyena [16, 29]. As such, the hyena placenta may respond to F&F treatment in unanticipated ways. Unfortunately, hyenas ingest their placentas post-partum before we can safely retrieve them, but if placentas were harvested at the time of Cesarean section, we could perform in vitro studies to determine if F&F treatment modulates the level and activity of 17β-hydroxysteroid dehydrogenase, the placental enzyme that converts A4 from the maternal ovaries to T [29].
In addition to the potential for F&F to modulate placental function, the paradoxical effects of F&F on sex hormone levels in pregnant hyenas may be a central phenomenon. The plasma concentration of LH was consistently lower in F&F-treated than in untreated pregnant hyenas, and the generalized suppression of sex steroids may be secondary to a F&F-induced decrease in gonadotropin levels. The plasma concentration of A4 appears to be modulated by LH in spotted hyenas, as suppression of LH by pharmaceutical means was associated with a decrease in A4 in non-pregnant hyenas [21] and administration of an LH analog (human chorionic gonadotropin) was associated with a rise in A4 [17, 21]. Because relatively high T concentrations during late gestation in spotted hyenas are thought to derive from placental 17β-HSD conversion of A4 from the maternal ovaries [15, 16, 29], a F&F-induced decline in LH could reduce the substrate for placental 17β-HSD and lower T. However, the impact of suppressed LH levels on P4 and E2 has not been investigated in spotted hyenas, and these non-androgen steroids were also suppressed in F&F-treated pregnant dams.
In conclusion, the present study highlights the potential for unanticipated outcomes that may result when trying to modulate the developmental effects of hormones by administering drugs to pregnant dams. Moreover, we have only assessed the impact of F&F on circulating sex hormone levels in the mother, but we cannot necessarily assume that the fetuses responded in kind. In few studies have investigators determined the impact of maternal hormonal manipulation on fetal hormone concentrations. However, in one notable exception, Resko et al. [23]) found treatment-induced changes in the maternal circulation were not reflected in the fetal circulation. The complexities and limitations associated with research on a large carnivore like the spotted hyena makes it challenging to elucidate the mechanism that underlies the paradoxical effects of F&F on maternal sex hormones. However, if the opportunity presents itself and placentas from F&F-treated hyenas can be collected, analyses of placental steroidogenic enzyme activity [1] may help differentiate between central and peripheral (placental) effects of F&F.
Acknowledgments
The authors would like to thank Laurence Frank for his role in establishing the spotted hyena colony at the University of California, Berkeley. We also thank Jane Venier and Karen Hubbard for technical assistance with the LH assay, the veterinary staff of the Office of Laboratory Animal Care at the University of California, Berkeley, and two anonymous reviewers for their comments.
Funding: This work was supported by the National Institutes of Health (grant numbers MH39917, HD07684, and HD08729) and the National Science Foundation (grant numbers IOB 0618022 and IOS 0809914).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Conley AJ, Corbin CJ, Browne P, Mapes SM, Place NJ, Hughes AL, Glickman SE. Placental expression and molecular characterization of aromatase cytochrome P450 in the spotted hyena (Crocuta crocuta). Placenta. 2007;28:668–675. doi: 10.1016/j.placenta.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Cunha GR, Place NJ, Baskin L, Conley A, Weldele M, Cunha TJ, Wang YZ, Cao M, Glickman SE. The ontogeny of the urogenital system of the spotted hyena (Crocuta crocuta Erxleben). Biol. Reprod. 2005;73:554–564. doi: 10.1095/biolreprod.105.041129. [DOI] [PubMed] [Google Scholar]
- 3.Dahl NJ, Olson D, Schmitt DL, Blasko DR, Kristipati R, Roser JF. Development of an enzyme-linked immunosorbent assay (ELISA) for Luteinizing Hormone (LH) in the elephant (Loxodonta africana and Elephas maximus). Zoo Biol. 2004;23:65–78. [Google Scholar]
- 4.Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. J. Reprod. Fertil. 1998;113:117–127. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- 5.Fleshner NE, Trachtenberg J. Combination finasteride and flutamide in advanced carcinoma of the prostate: effective therapy with minimal side effects. J Urol. 1995;154:1642–1645. discussion 1645-1646. [PubMed] [Google Scholar]
- 6.Giussani DA, Jenkins SL, Mecenas CA, Winter JA, Pedro JG, Farber DM, Goland RS, Nathanielsz PW. Effect of androstenedione administration on the maternal hypothalamo-pituitary-adreno-placental axis in the pregnant rhesus monkey. Endocrinology. 1996;137:608–614. doi: 10.1210/endo.137.2.8593809. [DOI] [PubMed] [Google Scholar]
- 7.Glickman SE, Cunha GR, Drea CM, Conley AJ, Place NJ. Mammalian sexual differentiation: lessons from the spotted hyena. Trends Endocrinol. Metab. 2006;17:349–356. doi: 10.1016/j.tem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Glickman SE, Frank LG, Pavgi S, Licht P. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J. Reprod. Fertil. 1992;95:451–462. doi: 10.1530/jrf.0.0950451. [DOI] [PubMed] [Google Scholar]
- 9.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm. Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- 10.Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED. Comparison of the effects of the 5 alpha-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation: dose-response studies. Endocrinology. 1992;131:1149–1156. doi: 10.1210/endo.131.3.1324152. [DOI] [PubMed] [Google Scholar]
- 11.Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J. Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- 13.Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 1953;8:379–418. [Google Scholar]
- 14.Kirby R, Robertson C, Turkes A, Griffiths K, Denis LJ, Boyle P, Altwein J, Schroder F. Finasteride in association with either flutamide or goserelin as combination hormonal therapy in patients with stage M1 carcinoma of the prostate gland. International Prostate Health Council (IPHC) Trial Study Group. The Prostate. 1999;40:105–114. doi: 10.1002/(sici)1097-0045(19990701)40:2<105::aid-pros6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Licht P, Frank LG, Pavgi S, Yalcinkaya TM, Siiteri PK, Glickman SE. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 2. Maternal and fetal steroids. J. Reprod. Fertil. 1992;95:463–474. doi: 10.1530/jrf.0.0950463. [DOI] [PubMed] [Google Scholar]
- 16.Licht P, Hayes T, Tsai P, Cunha G, Kim H, Golbus M, Hayward S, Martin MC, Jaffe RB, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 1. Urogenital morphology and placental androgen production during fetal life. J. Reprod Fertil. 1998;113:105–116. doi: 10.1530/jrf.0.1130105. [DOI] [PubMed] [Google Scholar]
- 17.Lindeque M, Skinner JD, Millar P. Adrenal and gonadal contribution to circulating androgens in spotted hyaenas (Crocuta crocuta) as revealed by LHRH, hCG and ACTH stimulation. J. Reprod. Fertil. 1986;78:211–217. doi: 10.1530/jrf.0.0780211. [DOI] [PubMed] [Google Scholar]
- 18.Neri RO. Studies on the biology and mechanism of action of nonsteroidal antiandrogens. In: Martini L, Motta L, editors. Androgens and antiandrogens. Raven Press; New York: 1977. pp. 179–189. [Google Scholar]
- 19.Neumann F, Elger W, Steinbeck H. Antiandrogens and reproductive development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970;259:179–184. doi: 10.1098/rstb.1970.0057. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T-VV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 21.Place NJ, Holekamp KE, Sisk CL, Weldele ML, Coscia EM, Drea CM, Glickman SE. Effects of prenatal treatment with antiandrogens on luteinizing hormone secretion and sex steroid concentrations in adult spotted hyenas, Crocuta crocuta. Biol. Reprod. 2002;67:1405–1413. doi: 10.1095/biolreprod.102.004226. [DOI] [PubMed] [Google Scholar]
- 22.Place NJ, Weldele ML, Wahaj SA. Ultrasonic measurements of second and third trimester fetuses to predict gestational age and date of parturition in captive and wild spotted hyenas Crocuta crocuta. Theriogenology. 2002;58:1047–1055. doi: 10.1016/s0093-691x(02)00937-8. [DOI] [PubMed] [Google Scholar]
- 23.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol. Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- 24.Södersten P, Gray G, Damassa DA, Smith ER, Davidson JM. Effects of a nonsteroidal antiandrogen on sexual behavior and pituitary-gonadal function in the male rat. Endocrinology. 1975;97:1468–1475. doi: 10.1210/endo-97-6-1468. [DOI] [PubMed] [Google Scholar]
- 25.Steyn FJ, Anderson GM, Grattan DR. Expression of ovarian steroid hormone receptors in tuberoinfundibular dopaminergic neurones during pregnancy and lactation. J. Neuroendocrinol. 2007;19:788–793. doi: 10.1111/j.1365-2826.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 26.Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JD, George FW, Griffin JE. The hormonal control of sexual development. Science. 1981;211:1278–1284. doi: 10.1126/science.7010602. [DOI] [PubMed] [Google Scholar]
- 28.Wood CE. Control of parturition in ruminants. J. Reprod. Fertil. 1999;54(Suppl.):115–126. [PubMed] [Google Scholar]
- 29.Yalcinkaya TM, Siiteri PK, Vigne JL, Licht P, Pavgi S, Frank LG, Glickman SE. A mechanism for virilization of female spotted hyenas in utero. Science. 1993;260:1929–1931. doi: 10.1126/science.8391165. [DOI] [PubMed] [Google Scholar]