Abstract
A novel thermal 3-aza-Claisen rearrangement of N-allyl ynamides for the synthesis of α-allyl imidates is described. Also, a sequential aza-Claisen, Pd-catalyzed Overman rearrangement is described for the synthesis of azapine-2-ones.
Keywords: Ynamide, aza-Claisen, Imidate, Ketenimine, Overman rearrangement
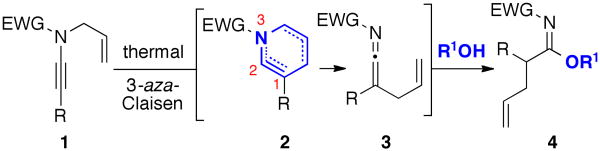
We recently reported the de novo synthesis of pharmacologically useful amidines from N-allyl ynamides1,2 featuring either a Pd-catalyzed3 N-to-C allyl transfer or unprecedented thermal3,4 3-aza-Claisen5 rearrangement followed by trapping of the in situ generated ketenimine6 with amine nucleophiles. There has been great interest within the synthetic community on preparing amidines and imidates,7 most commonly through interception of the ketenimine intermediate produced during a Cu-catalyzed Huisgen-[3+2] cycloaddition of azides and alkynes.8-10 Herein, we describe our efforts at the synthesis of imidates via trapping of ketenimines 3 formed via aza-Claisen rearrangement of ynamides 1 in the presence of alcoholic nucleophiles.
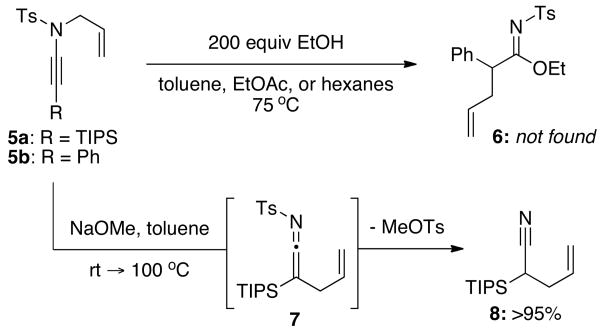
It was quickly discovered that the nucleophilicity of even simple alcohols such as methanol and ethanol was not sufficient to yield imidates 6 despite the alcohols being used in 200 fold excess [Scheme 2]! Furthermore, attempts to trap ketenimine 7 generated from ynamide 5a with more nucleophilic sodium methoxide led to cleavage of the N-toluenesulfonamide protecting group furnishing nitrile 8 in quantitative yield.
Scheme 2. Alkoxide Induced Detosylation of the Ketenimine.
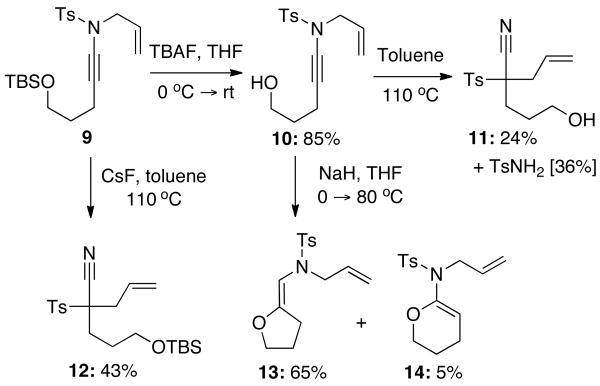
Our subsequent attempts to carry out this transformation intramolecular were also met with difficulty. Alcohol 10 could be prepared by simple TBAF mediated desilylation of 9. Upon heating of 10 in toluene, the only isolable products were nitrile 11 formed through a 1,3-sulfonyl4,11 transfer during the ketenimine intermediate and p-toluenesulfonamide, which implies that the aza-Claisen did in fact occur but was not productive towards imidate formation. Alternatively, when 10 was treated with sodium hydride to increase the nucleophilicity of the oxygen and then heated to 80 °C, only enamides 13 and 14 were formed through 5-exo-dig and 6-endo-dig cyclization onto the ynamide, respectively. Since this was too nucleophilic for the aza-Claisen to occur, we instead opted to cleave the silyl protecting group of ynamide 9 in situ to trigger the cyclization, however again only nitrile 12 was found, demonstrating that the 1,3-sulfonyl shift is quite facile.
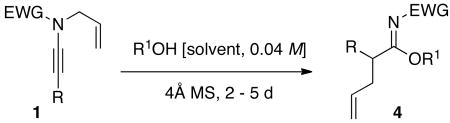
Finally, we found that heating of ynamides 1 in alcoholic solvents in the presence of 4Å molecular sieves led to imidates 4 in moderate to excellent yields. The reaction conditions tolerated both silyl and aryl-terminated ynamides and showed moderate sensitivity to the electron-withdrawing nature of N-sulfonyl protecting group with p-Ns providing the corresponding imidates in the highest yields due to increased electrophilicity of the ketenimine [entries 3 vs. 6 and 4 vs. 7]. Notably, there was no reaction observed with N-Boc or N-Ac ynamides even at temperatures of >140 °C, indicating the strong electronic effect on the initial aza-Claisen rearrangement. There was also a clear sensitivity to sterics with the use of more sterically hindered nucleophiles giving rise to nitriles through the competing intramolecular 1,3-sulfonyl shift in the ketenimine intermediate when R = Ph [entries 8 vs. 10]. Interestingly, no nitrile formation was observed in the cases where R = TIPS, likely due to the increased steric bulk preventing the necessary migration.
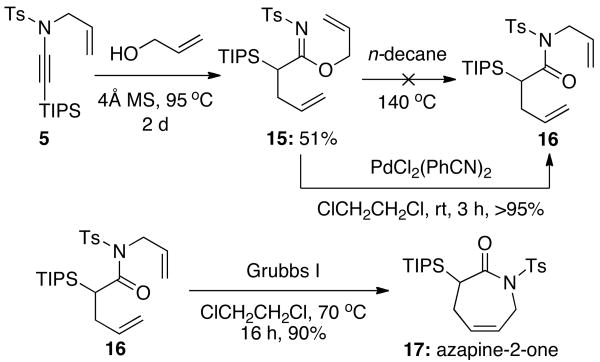
Next, we sought to develop a tandem aza-Claisen–Overman12 rearrangement, which followed by RCM may be used for the synthesis of useful azapine-2-one scaffolds 17. Ynamide 5a underwent reaction with allyl alcohol to yield diallyl imidate 15 in moderate yield. Unfortunately, our attempts at a thermal Overman rearrangement were unsuccessful, as heating of 15 in n-decane at 140 °C even for several days resulted in no formation of 16. However, to our delight, exposure of 15 to a catalytic amount of PdCl2(PhCN)2 at room temperature led to [3,3] rearrangement product 16 in >95% yield.9b With 16 in hand, efficient ring-closing metathesis13 was achieved using Grubbs' first generation catalyst to provide azapine-2-one 17 in 90% yield.
Herein, we have disclosed a novel synthesis of α-allyl imidates via a thermal 3-aza-Claisen rearrangement of N-allyl ynamides. We found that it is necessary to use the alcohol as solvent to avoid a competing 1,3-sulfonyl transfer forming nitriles. Also, the use of alkoxides intermolecularly led to efficient desulfonylation, while intramolecularly gave 5-exo-dig cyclization of the alkoxide onto the ynamide. In addition, we have demonstrated the use of a sequential 3-aza-Claisen–Overman rearrangement, which followed by ring-closing metathesis may be used to provide access to azapine-2-one scaffolds.
Scheme 1. In Situ Trapping of a Ketenimine Intermediate.
Scheme 3. Attempts at Intramolecular Imidate Formation.
Scheme 4. Construction of an Azapine-2-one Scaffold.
Table 1. Synthesis of α-Allyl Imidates.
 | ||||
|---|---|---|---|---|
| Entry | Alcohol | Temp/Time | Imidate | Yield [%]a |
| 1 | MeOH | 75 °C/2 d | 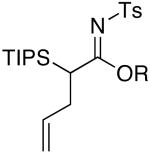 |
4a: R = Me [81%] |
| 2 | EtOH | 75 °C/2 d | 4b: R = Et [43%] | |
| 3 | i-PrOH | 90 °C/5 d | 4c: R = i-Pr [39%] | |
| 4 | c-pentanol | 110 °C/5 d | 4d: R = c-pent [45%] | |
| 5 | EtOH | 85 °C/3 d | 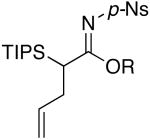 |
4e: R = Et [76%] |
| 6 | i-PrOH | 90 °C/4 d | 4f: R = i-Pr [76%] | |
| 7 | c-pentanol | 115 °C/4 d | 4g: R = c-pent [71%] | |
| 8 | MeOH | 75 °C/2 d | 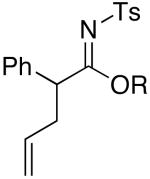 |
4h: R = Me [95%]c |
| 9 | EtOH | 75 °C/2 d | 4i: R = Et [75%] | |
| 10 | i-PrOH | 75 °C/5 d | 4j: R = i-Pr [47%]d | |
| 11 | EtOH | 75 °C/2 d |
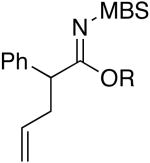 b b
|
4k: R = Et [82%] |
| 12 | c-pentanol | 75 °C/2 d | 4l: R = c-pent [38%] | |
Isolated Yields.
MBS = p-methoxybenzenesulfonyl.
No nitrile observed in crude 1H NMR.
Est. nitrile yield by crude 1H NMR = 20%.
Acknowledgments
Authors thank NIH [GM066055] for financial support.
Selected Experimental Procedures and Characterizations
Synthesis of Nitrile 8
To a stirring solution of ynamide 5a (75.0 mg, 0.19 mmol) in toluene (2 mL) at rt was slowly added freshly prepared NaOMe (31.0 mg, 0.58 mmol). After addition, the reaction mixture was sealed under dry nitrogen and heated to 100 °C for 1 h. Over the course of the reaction, TsOMe was observed to precipitate out of solution and was subsequently removed by filtration of the crude reaction mixture through a plug of Celite™ to afford the pure nitrile 8 (45.6 mg, 0.19 mmol, >95% yield) as a colorless oil.
Rf = 0.45 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.00 – 1.41 (m, 21H), 2.05 (dd, 1H, J = 7.5, 4.0 Hz), 2.28 – 2.34 (m, 1H), 2.35 – 2.44 (m, 1H), 5.14 (d, 1H, J = 10.5 Hz), 5.17 (d, 1H, J = 18.0 Hz), 5.88 – 5.98 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 11.3, 14.4, 18.9, 31.8, 117.2, 122.7, 136.2; IR (film) cm-1 2946m, 2869m, 2222w, 1595m, 1377m; mass spectrum (APCI): m/e (% relative intensity) 238 (100) (M+H)+.
Synthesis of Alcohol 10
To a stirring solution of ynamide 9 (315.0 mg, 0.77 mmol) in THF (2 mL) at rt was slowly added TBAF (0.85 mL, 1.0 M in THF). After 2 h, the solvent was removed via rotary evaporation and the crude oil was purified by flash silica gel column chromatography [isocratic eluent: 1:1 hexanes/EtOAc] to afford the alcohol 10 (191.0 mg, 0.65 mmol, 85% yield) as a pale yellow oil.
Rf = 0.09 [2:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.52 (brs, 1H), 1.73 (pent, 2H, J = 6.5 Hz), 2.38 (t, 2H, J = 7.0 Hz), 3.71 (d, 2H, J = 7.0 Hz), 3.91 (d, 2H, J = 7.0 Hz), 5.19 (d, 1H, J = 10.5 Hz), 5.24 (d, 1H, J = 17.5 Hz), 5.72 (ddt, 1H, J = 17.5, 10.5, 7.0 Hz), 7.34 (d, 2H, J = 8.0 Hz), 7.78 (d, 2H, J = 8.0 Hz); 13C NMR (125 MHz, CDCl3) δ 15.4, 21.9, 31.8, 54.4, 61.9, 69.9, 73.8, 120.0, 128.0, 130.0, 131.4, 135.0, 144.8; IR (film) cm-1 3200brs, 2981m, 2878m, 1644m; mass spectrum (APCI): m/e (% relative intensity) 294 (100) (M+H)+.
Nitrile 12. Rf = 0.41 [8:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 0.02 (s, 6H), 0.86 (s, 9H), 1.66 – 1.85 (m, 2H), 2.05 (dd, 2H, J = 8.4, 6.8 Hz), 2.48 (s, 3H), 2.67 – 2.79 (m, 2H), 3.59 (t, 2H, J = 6.0 Hz), 5.24 (d, 1H, J =16.8 Hz), 5.26 (d, 1H, J = 10.0 Hz), 5.82 (ddt, 1H, J = 17.6, 10.0 Hz, 7.2 Hz), 7.40 (d, 2H, J = 8.0 Hz), 7.88 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 5.2, 18.4, 22.0, 26.1, 28.3, 28.7, 36.8, 62.1, 65.8, 116.9, 121.9, 129.8, 130.2, 131.0, 131.7; IR (film) cm-1 2929m, 2857m, 2238w, 1596m, 1331s, 1150s; mass spectrum (APCI): m/e (% relative intensity) 408 (100) (M+H)+; HRMS (ESI): m/e calcd for C21H33NO3SSiNa 430.1843, found 430.1860.
Synthesis of 13
To a solution of ynamide 10 (75.0 mg, 0.26 mmol) in THF (5 mL) at 0 °C was added NaH (19.0 mg, 0.46 mmol, 60% wt/wt in mineral oil). The reaction mixture was warmed to rt and stirred for 20 min to allow for complete deprotonation and then sealed under dry nitrogen and heated to 85 °C for 3 h. The reaction mixture was quenched with water and the organic phase was extracted with EtOAc, and then dried over Na2SO4. Removal of the solvent by rotary evaporation and purification by flash silica gel column chromatography [isocratic eluent: 4:1 hexanes/EtOAc] afforded 13 (50.0 mg, 0.17 mmol, 65% yield) as a colorless oil.
13: Rf = 0.35 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.90 (pent, 2H, J = 9.5 Hz), 2.40 (s, 3H), 2.49 (td, 2H, J = 9.5, 2.0 Hz), 3.96 (t, 2H, J = 8.0 Hz), 3.97 (d, 2H, J = 8.5 Hz), 4.86 (s, 1H), 5.06 (dd, 1H, J = 13.0, 2.0 Hz), 5.14 (dd, 1H, J = 20.0, 2.0 Hz), 5.76 (ddt, 1H, J = 20.0, 13.0, 8.5 Hz), 7.26 (d, 2H, J = 9.5 Hz), 7.70 (d, 2H, J = 9.5 Hz); 13C NMR (125 MHz, CDCl3) δ 21.8, 24.6, 28.4, 52.3, 72.0, 94.9, 117.6, 127.7, 129.4, 134.1, 136.9, 143.1, 157.4; IR (film) cm-1 3055m, 2980m, 1597s, 1337s; mass spectrum (APCI): m/e (% relative intensity) 294 (100) (M+H)+; HRMS (ESI): m/e calcd for C15H19NO3SNa 316.0978, found 316.0986.
14: Rf = 0.38 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.99 (pent, 2H, J = 7.0 Hz), 2.43 (s, 3H), 2.80 (td, 2H, J = 8.0, 2.0 Hz), 3.66 (d, 2H, J = 6.5 Hz), 4.16 (t, 2H, J = 6.5 Hz), 4.76 (t, 1H, J = 2.0 Hz), 5.08 – 5.13 (m, 2H), 5.70 (ddt, 1H, J = 17.0, 10.0, 6.5 Hz), 7.30 (d, 2H, J = 8.0 Hz), 7.67 (d, 2H, J = 8.5 Hz); 13C NMR (125 MHz, CDCl3) δ 21.8, 24.4, 28.1, 54.8, 72.1, 97.8, 119.1, 127.9, 129.8, 133.1, 135.0, 143.5, 166.8; mass spectrum (APCI): m/e (% relative intensity) 294 (20) (M+H)+.
General Procedure for the Synthesis of Imidates 4a-4l
To a flame-dried vial containing 4Å MS was added the appropriate ynamide and dry alcohol solvent (0.04 M in ynamide). The reaction mixture was sealed under dry nitrogen and heated to 75 – 95 °C for 2 – 5 d. Upon cooling to rt, the mixture was filtered through a plug of Celite™. Removal of the alcohol solvent in vacuo followed by flash silica gel column chromatography afforded the respective imidate.
Imidate 4a
Rf = 0.45 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.15 (d, 9H, J = 7.5 Hz), 1.20 (d, 9H, J = 7.5 Hz), 1.35 (sept, 3H, J = 7.5 Hz), 2.46 (s, 3H), 2.50 (m, 1H), 2.62 (ddd, 1H, J = 20.5, 12.5, 8.5 Hz), 3.71 (dd, 1H, J = 3.0, 12.0 Hz), 3.73 (s, 3H), 4.92 (dd, 1H, J = 10.0, 1.0 Hz), 5.00 (ddt, 1H, J = 17.0, 3.0, 1.5 Hz), 5.73 (dddd, 1H, J = 22.5, 14.0, 8.5, 5.5 Hz), 7.31 (d, 2H, J = 8.0 Hz), 7.86 (d, 2H, J = 8.0 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 19.1, 19.1, 21.8, 32.6, 34.2, 54.0, 115.8, 126.9, 129.4, 137.5, 140.2, 143.0, 178.4; IR (film) cm-1 2948m, 2868m, 1582s, 1288s; mass spectrum (APCI): m/e (% relative intensity) 424 (100) (M+H)+; HRMS (ESI): m/e calcd for C22H37NO3SSiNa 446.2156, found 446.2161.
Imidate 4b
Rf = 0.38 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.11 (d, 9H, J = 7.5 Hz), 1.16 (d, 9H, J = 7.5 Hz), 1.24 (t, 3H, J = 7.0 Hz), 1.31 (sept, 3H, J = 7.5 Hz), 2.41 (s, 3H), 2.59 (dt, 1H, J = 13.5, 9.0 Hz), 3.64 (dd, 1H, J = 12.5, 3.0 Hz), 4.04 – 4.17 (m, 2H), 4.87 (d, 1H, J = 10.0 Hz), 4.95 (d, 1H, J = 16.5 Hz), 5.63 – 5.73 (m, 1H), 7.26 (d, 2H, J = 7.5 Hz), 7.80 (d, 2H, J = 8.5 Hz); 13C NMR (125 MHz, CDCl3) δ 11.7, 13.9, 19.1, 19.1, 21.7, 32.4, 34.2, 64.2, 115.7, 126.8, 129.3, 137.5, 140.2, 142.8, 177.8; IR (film) cm-1 2948m, 2871m, 1965w, 1575s, 1289s, 1155s; mass spectrum (APCI): m/e (% relative intensity) 438 (100) (M+H)+; HRMS (ESI): m/e calcd for C23H39NO3SSiNa 460.2313, found 460.2295.
Imidate 4c
Rf = 0.19 [15:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.13 (d, 9H, J = 7.5 Hz), 1.14 (d, 3H, J = 6.0 Hz), 1.15 (d, 9H, J = 7.5 Hz), 1.25 (d, 3H, J = 6.0 Hz), 1.31 (sept, 3H, J = 7.5 Hz), 2.41 (s, 3H), 2.40 – 2.48 (m, 1H), 2.57 (dt, 1H, J = 14.0, 8.5 Hz), 2.84 (dd, 1H, J = 12.0, 3.0 Hz), 4.86 (d, 1H, J = 10.0 Hz), 4.96 (dd, 1H, J = 17.0, 1.0 Hz), 5.03 (sept, 1H, J = 6.0 Hz), 5.68 (dddd, 1H, J = 17.0, 10.0, 8.5, 5.5 Hz), 7.26 (d, 2H, J = 8.0 Hz), 7.80 (d, 2H, J = 8.0 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 19.2, 21.6, 21.7, 21.8, 32.4, 34.4, 71.9, 115.8, 126.8, 129.3, 137.4, 140.4, 142.7, 177.2; IR (film) cm-1 2946m, 2868m, 1570s, 1464m, 1287m, 1153s; mass spectrum (APCI): m/e (% relative intensity) 410 (100) (M–propene+H)+; HRMS (ESI): m/e calcd for C24H41NO3SSiNa 474.2469, found 474.2446.
Imidate 4d
Rf = 0.41 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.12 (d, 9H, J = 9.5 Hz), 1.15 (d, 9H, J = 9.5 Hz), 1.31 (sept, 3H, J = 9.5 Hz), 1.50 – 1.82 (m, 8H), 2.41 (s, 3H), 2.40 – 2.47 (m, 1H), 2.55 (dt, 1H, J =18.0, 10.5 Hz), 3.64 (dd, 1H, J = 15.5, 4.0 Hz), 4.86 (d, 1H, J = 12.5 Hz), 4.94 (d, 1H, J = 21.0 Hz), 5.12 – 5.16 (m, 1H), 5.62 – 5.74 (m, 1H), 7.25 (d, 2H, J = 10.0 Hz), 7.80 (d, 2H, J = 10.0 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 19.2, 21.7, 23.8, 24.2, 32.2, 32.9, 34.4, 81.4, 115.6, 126.8, 129.3, 137.6, 140.5, 142.7, 117.5; IR (film) cm-1 2946m, 2869m, 1571s, 1463w, 1287m, 1153s; mass spectrum (APCI): m/e (% relative intensity) 410 (100) (M-cyclopentene+H)+; HRMS (ESI): m/e calcd for C26H43NO3SSiNa 500.2626, found 500.2618.
Imidate 4e
Rf = 0.42 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.12 (d, 9H, J = 9.0 Hz), 1.16 (d, 9H, J = 9.5 Hz), 1.26 (t, 3H, J = 9.0 Hz), 1.32 (sept, 3H, J = 9.5 Hz), 2.44 – 2.52 (m, 1H), 2.61 (dt, 1H, J = 17.5, 11.0 Hz), 3.59 (dd, 1H, J = 15.5, 4.0 Hz), 4.05 – 4.15 (m, 2H), 4.93 (d, 1H, J = 12.5 Hz), 4.99 (d, 1H, J = 21.0 Hz), 5.71 – 5.82 (m, 1H), 8.09 (d, 2H, J = 11.5 Hz), 8.32 (d, 2H, J = 11.5 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 13.9, 19.0, 19.1, 33.5, 34.3, 64.8, 116.1, 124.2, 128.0, 137.3, 148.6, 179.5; IR (film) cm-1 2943w, 2868w, 1566s, 1531s, 1295s, 1159s; mass spectrum (APCI): m/e (% relative intensity) 469 (100) (M+H)+; HRMS (ESI): m/e calcd for C22H36N2O5SSiNa 491.2007, found 491.2007.
Imidate 4f
Rf = 0.27 [15:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 1.14 (d, 9H, J = 7.2 Hz), 1.15 (d, 3H, J = 6.4 Hz), 1.17 (d, 9H, J = 7.6 Hz), 1.28 (d, 3H, J = 6.4 Hz), 1.32 (sept, 3H, J = 7.6 Hz), 2.46 – 2.52 (m, 1H), 2.60 (dt, 1H, J = 14.4, 8.8 Hz), 3.61 (dd, 1H, J = 12.0, 3.6 Hz), 4.93 (d, 1H, J = 10.0 Hz), 4.96 – 5.04 (m, 2H), 5.76 (dddd, 1H, J = 17.0, 10.0, 8.8, 5.2 Hz), 8.10 (d, 2H, J = 9.2 Hz), 8.33 (d, 2H, J = 8.8 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 19.1, 19.1, 21.5, 21.8, 33.4, 34.4, 72.9, 116.1, 124.2, 128.0, 137.3, 148.7, 149.9, 178.9; IR (film) cm-1 2946m, 2869m, 1562s, 1531s, 1349s, 1296s, 1156s; mass spectrum (APCI): m/e (% relative intensity) 441 (30) [M-propene+H]+; HRMS (ESI): m/e calcd for C23H38N2O5SSiNa 505.2163, found 505.2164.
Imidate 4g
Rf = 0.36 [10:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 1.13 (d, 9H, J = 7.2 Hz), 1.16 (d, 9H, J = 7.6 Hz), 1.33 (sept, 3H, J = 7.6 Hz), 1.50 – 1.88 (m, 8H), 2.46 – 2.51 (m, 1H), 2.58 (dt, 1H, J = 14.0, 8.4 Hz), 3.60 (dd, 1H, J = 12.0, 3.2 Hz), 4.92 (d, 1H, J = 10.0 Hz), 5.00 (d, 1H, J = 16.8 Hz), 5.09 – 5.14 (m, 1H), 5.70 – 5.82 (m, 1H), 8.11 (d, 2H, J = 8.4 Hz), 8.33 (d, 2H, J = 8.8 Hz); 13C NMR (125 MHz, CDCl3) δ 11.8, 19.1, 23.8, 24.1, 32.2, 32.9, 33.2, 34.4, 82.2, 116.0, 124.1, 128.0, 137.4, 148.7, 149.9, 179.1; IR (film) cm-1 2947m, 2871m, 1565s, 1531s, 1349s, 1303m, 1157s; mass spectrum (APCI): m/e (% relative intensity) 441 (30) [M-cyclopentene+H]+; HRMS (ESI): m/e calcd for C25H40N2O5SSiNa 531.2320, found 530.2333.
Imidate 4h
Rf = 0.30 [4:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 2.43 (s, 3H), 3.59 (tt, 2H, J = 6.0, 1.2 Hz), 3.69 (s, 3H), 4.49 (t, 1H, 6.0 Hz), 5.10 (ddt, 1H, J = 10.4, 2.8, 1.6 Hz), 5.16 (ddt, 1H, J = 17.2, 2.8, 1.6 Hz), 5.72 (ddt, 1H, J = 17.2, 10.4, 6.0), 7.24 – 7.34 (m, 7H), 7.76 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 21.6, 41.3, 45.8, 52.2, 117.7, 127.2, 127.3, 128.7, 129.4, 129.8, 133.2, 134.1, 137.1, 143.6, 172.2; IR (film) cm-1 3034m, 2951m, 1735s, 1597m, 1325s; mass spectrum (APCI): m/e (% relative intensity) 344 (100) [M+H]+; HRMS (ESI): m/e calcd for C19H21NO3SNa 366.1135, found 366.1150.
Imidate 4i
Rf = 0.48 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.26 (t, 3H, J = 7.0 Hz), 2.41 (s, 3H), 2.63 (dt, 1H, J = 13.5, 6.5 Hz) 2.83 (dt, 1H, J = 16.5, 8.0 Hz), 4.09 (dq, 1H, J = 11.0, 7.0 Hz), 4.19 (dq, 1H, J = 11.0, 7.0 Hz), 4.98 – 5.04 (m, 2H), 5.10 (dd, 1H, J = 17.0, 1.5 Hz), 5.70 – 5.80 (m, 1H), 7.23 – 7.28 (m, 3H), 7.32 (t, 2H, J = 8.0 Hz), 7.46 (d, 2H, J = 7.5 Hz), 7.77 (d, 2H, J = 8.0 Hz); 13C NMR (125 MHz, CDCl3) δ 13.7, 21.7, 36.0, 37.9, 48.9, 64.6, 117.7, 126.9, 127.8, 128.8, 129.0, 129.5, 134.9, 137.8, 143.3, 174.6; IR (film) cm-1 2985w, 1592s, 1301s, 1152s; mass spectrum (APCI): m/e (% relative intensity) 358 (100) [M+H]+; HRMS (ESI): m/e calcd for C20H23NO3SNa 380.1291, found 380.1287.
Imidate 4j
Rf = 0.32 [6:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.14 (d, 3H, J = 6.0 Hz), 1.25 (d, 3H, J = 6.5 Hz), 2.40 (s, 3H), 2.59 (dt, 1H, J = 12.5, 5.5 Hz), 2.80 (dt, 1H, J = 14.5, 8.5 Hz), 4.97 (m, 3H), 5.10 (d, 1H, J = 17.5 Hz), 5.70 – 5.79 (m, 1H), 7.22 – 7.27 (m, 3H), 7.31 (t, 2H, J = 7.5 Hz), 7.44 (d, 2H, J = 8.0 Hz), 7.75 (d, 2H, J = 8.5 Hz); 13C NMR (125 MHz, CDCl3) δ 21.3, 21.4, 21.7, 38.1, 48.9, 72.5, 117.7, 126.8, 127.7, 128.7, 128.8, 129.5, 134.8, 137.9, 139.7, 143.1, 174.0; IR (film) cm-1 2984w, 1592s, 1302s, 1156s; mass spectrum (APCI): m/e (% relative intensity) 330 (100) [M-propene+H]+; HRMS (ESI): m/e calcd for C21H25NO3SNa 394.1448, found 394.1440.
Imidate 4k
Rf = 0.33 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.25 (t, 3H, J = 7.0 Hz), 2.62 (dt, 1H, J = 13.5, 7.0 Hz), 2.83 (dt, 1H, J = 15.5, 8.5 Hz), 3.84 (s, 3H), 4.09 (dq, 1H, J = 11.0, 7.0 Hz), 4.18 (dq, 1H, J = 11.0, 7.0 Hz), 4.99 – 5.04 (m, 2H), 5.09 (d, 1H, J = 17.0 Hz), 5.70 – 5.59 (m, 1H), 6.91 (d, 2H, J = 9.0 Hz), 7.26 (t, 1H, J = 7.5 Hz), 7.32 (d, 2H, J = 7.5 Hz), 7.45 (d, 2H, J = 7.5 Hz), 7.81 (d, 2H, J = 9.0 Hz); 13C NMR (100 MHz, CDCl3) δ 13.7, 37.9, 48.8, 55.7, 64.8, 114.0, 117.7, 127.7, 128.8, 128.9, 128.9, 133.1, 134.9, 137.8, 162.9, 174.4; IR (film) cm-1 2986w, 1593s, 1499m, 1298s, 1150s; mass spectrum (APCI): m/e (% relative intensity) 374 (100) [M+H]+; HRMS (ESI): m/e calcd for C20H23NO4SNa 396.1231, found 396.1240.
Imidate 4l
Rf = 0.20 [4:1 hexanes/EtOAc]; 1H NMR (500 MHz, CDCl3) δ 1.55 – 1.83 (m, 8H), 2.59 (dt, 1H, J = 12.5, 6.5 Hz), 2.79 (dt, 1H, J = 17.0, 8.0 Hz), 3.85 (s, 3H), 4.99 (dd, 1H, J = 9.0, 7.0 Hz), 5.02 (d, 1H, J = 11.0 Hz), 5.09 (dd, 1H, J = 17.0, 1.5 Hz), 5.12 – 5.17 (m, 1H), 5.74 (dddd, 1H, J = 17.0, 10.0, 7.5, 5.5 Hz), 6.92 (d, 2H, J = 9.0 Hz), 7.24 – 7.28 (m, 1H), 7.31 (t, 2H, J = 7.0 Hz), 7.43 (d, 2H, J = 7.5 Hz), 7.81 (d, 2H, J = 9.0 Hz); 13C NMR (125 MHz, CDCl3) δ 24.0, 32.4, 32.6, 38.0, 48.8, 55.8, 81.8, 114.0, 117.6, 127.7, 128.7, 128.8 128.8, 134.6, 134.9, 137.9, 162.7, 173.9; IR (film) cm-1 2968w, 2850w, 1579s, 1294s, 1257s, 1150s; mass spectrum (APCI): m/e (% relative intensity) 346 (100) (M-cyclopentene+H)+; HRMS (ESI): m/e calcd for C23H27NO4SNa 436.1553, found 436.1552.
Imidate 15
Rf = 0.50 [4:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 1.11 (d, 9H, J = 7.6 Hz), 1.17 (d, 9H, J = 7.6 Hz), 1.31 (sept, 3H, J = 7.6 Hz), 2.41 (s, 3H), 2.43 – 2.48 (m, 1H), 2.61 (td, 1H, J = 13.6, 8.8 Hz), 3.67 (dd, 1H, J = 12.0, 3.2 Hz), 4.47 (dd, 1H, J = 12.8, 6.0 Hz), 4.59 (dd, J = 12.8, 6.0 Hz), 4.87 (d, 1H, J = 10.0 Hz), 4.95 (d, 1H, J = 17.2 Hz), 5.22 (d, 1H, J = 10.4 Hz), 5.28 (dd, 1H, J = 16.0, 1.2 Hz), 5.63 – 5.74 (m, 1H), 5.87 (ddt, 1H, J = 16.8, 10.0, 6.0 Hz), 7.26 (d, 2H, J = 8.0 Hz), 7.80 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 11.8, 19.1, 21.7, 32.5, 34.2, 69.1, 115.9, 119.8, 126.8, 126.9, 129.4, 131.6, 137.4, 140.1, 142.9, 177.4; IR (film) cm-1 2943m, 2869m, 1584s, 1302m, 1155s; mass spectrum (APCI): m/e (% relative intensity) 450 (100) [M+H]+; HRMS (ESI): m/e calcd for C24H39NO3SSiNa 472.2313, found 472.2305.
Synthesis of Amide 16
To a stirring solution of imidate 15 (35.0 mg, 0.078 mmol) in 1,2-dichloroethane (0.4 mL) was added PdCl2(PhCN)2 (1.5 mg, 0.004 mol). The reaction was stirred under a nitrogen atmosphere for 3 h at room temperature and then the solvent was removed by rotary evaporation. The crude residue was purified by flash silica gel column chromatography [isocratic eluent: 20:1 hexanes/EtOAc] to afford the amide 16 (35.0 mg, 0.078 mmol, >95% yield) as a colorless oil.
Rf = 0.50 [4:1 hexanes/EtOAc]; 1H NMR (400 MHz, CDCl3) δ 1.05 (d, 9H, J = 6.8 Hz), 1.11 (d, 9H, J = 6.8 Hz), 1.13 – 1.25 (m, 3H), 2.26 (dd, 1H, J = 11.6, 6.4 Hz), 2.43 (s, 3H), 2.66 (td, 1H, J = 13.2, 7.2 Hz), 3.00 (brs, 1H), 4.34 (dd, 1H, J = 16.4, 6.8 Hz), 4.47 – 4.54 (m, 1H), 4.69 (d, 1H, J = 10.0 Hz), 4.82 (d, 1H, J = 16.8 Hz), 5.21 (d, 1H, J = 10.0 Hz), 5.27 (d, 1H, J = 17.2 Hz), 5.21 – 5.37 (m, 1H), 7.29 (d, 2H, J = 8.8 Hz), 7.83 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 11.8, 18.8, 19.2, 21.8, 34.8, 49.5, 116.0, 119.0, 128.0, 128.8, 129.5, 133.6, 137.4, 144.6, 176.0; IR (film) cm-1 2950m, 2870m, 1678s, 1352s; mass spectrum (APCI): m/e (% relative intensity) 450 (100) [M+H]+; HRMS (ESI): m/e calcd for C24H39NO3SSiNa 472.2313, found 472.2317.
Synthesis of Azapine-2-one 17
To a solution of amide 16 (35.0 mg, 0.078 mmol) in 1,2-dichloroethane was added Grubbs I catalyst (3.2 mg, 0.004 mmol). The reaction vial was flushed with dry nitrogen, sealed, and heated to 70 °C for 16 hours. The solvent was removed by rotary evaporation and the crude residue was purified by flash silica gel column chromatography [isocratic eluent: 10:1 hexanes/EtOAc] to afford azapine-2-one 17 (29.5 mg, 0.070 mmol, 90% yield) as a white solid.
Rf = 0.30 [8:1 hexanes/EtOAc]; m.p. = 129 – 130 °C; 1H NMR (500 MHz, CDCl3) δ 0.95 (d, 9H, J = 7.5 Hz), 0.99 (d, 9H, J = 7.5 Hz), 1.18 (sept, 3H, J = 7.5 Hz), 2.28 – 2.40 (m, 1H), 2.41 (s, 3H), 2.41 – 2.48 (m, 1H), 2.90 (dd, 1H, J = 13.0, 2.5 Hz), 4.49 (dt, 1H, J = 18.0, 3.0 Hz), 4.81 (dd, 1H, J = 18.0, 8.0 Hz), 5.75 – 5.79 (m, 1H), 5.83 – 5.88 (m, 1H), 7.26 (d, 2H, J = 8.0 Hz), 7.83 (d, 2H, J = 8.0 Hz); 13C NMR (120 MHz, CDCl3) δ 11.2, 19.3, 21.8, 28.1, 31.0, 43.1, 123.9, 128.5, 129.3, 133.6, 136.8, 144.3, 175.4; IR (film) cm-1 2943m, 2866m, 1963s, 1597w, 1350s; mass spectrum (APCI): m/e (% relative intensity) 422 (100) [M+H]+; HRMS (ESI): m/e calcd for C22H35NO3SSiNa 444.2000, found 444.2000.
References
- 1.For reviews, see: DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem Rev. 2010 doi: 10.1021/cr100003s. ASAP.Evano G, Coste A, Jouvin K. Angew Chem Int Ed. 2010;49:2840. doi: 10.1002/anie.200905817.
- 2.For examples in 2010, see: Li H, Antoline JE, Yang JH, Al-Rashid ZF, Hsung RP. New J Chem. 2010;34 doi: 10.1039/c0nj00063a. ASAP.Kramer S, Madsen JLH, Rottländer M, Skrydstrup T. Org Lett. 2010;12 doi: 10.1021/ol1008685. ASAP.Banerjee B, Litvinov DN, Kang J, Bettale JD, Castle SL. Org Lett. 2010;12:2650. doi: 10.1021/ol1008679.Gourdet B, Rudkin ME, Lam HW. Org Lett. 2010;12:2554. doi: 10.1021/ol100769p.Jia W, Jiao N. Org Lett. 2010;12:2000. doi: 10.1021/ol1004615.Burley GA, Davies DL, Griffith GA, Lee M, Singh K. J Org Chem. 2010;75:980. doi: 10.1021/jo902466f.Yamasaki R, Terashima N, Sotome I, Komagawa S, Saito S. J Org Chem. 2010;75:480. doi: 10.1021/jo902251m.
- 3.Zhang Y, DeKorver KA, Lohse AG, Zhang YS, Huang J, Hsung RP. Org Lett. 2009;11:899. doi: 10.1021/ol802844z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org Lett. 2010;12:1840. doi: 10.1021/ol100446p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For leading reviews on aza-Claisen rearrangements, see: Majumdar KC, Bhayyacharyya T, Chattopadhyay B, Nandi RK. Synthesis. 2009:2117.Nubbemeyer U. Top Curr Chem. 2005;244:149.. For some examples of aza-Claisen rearrangements, see: Majumdar KC, Samanta S, Chattopadhyay B, Nandi RK. Synthesis. 2010:863.Cheung LLW, Yudin AK. Org Lett. 2009;11:1281. doi: 10.1021/ol900118d.Cant AA, Bertrand GHV, Henderson JL, Roberts L, Greaney MF. Angew Chem Int Ed. 2009;48:5199. doi: 10.1002/anie.200901410.
- 6.For reviews on the chemistry of ketenimines, see: Krow GR. Angew Chem, Int Ed Engl. 1971;10:435.Gambaryan NP. Usp Khim. 1976;45:1251.Dondoni A. Heterocycles. 1980;14:1547.Barker MW, McHenry WE. In: In The Chemistry of Ketenes, Allenes and Related Compounds. Patai S, editor. Part 2. Wiley-Interscience; Chichester, UK: 1980. pp. 701–720.Alajarin M, Vidal A, Tovar F. Targets Heterocycl Syst. 2000;4:293.
- 7.For examples of imidates serving as prodrugs, see: Maryanoff BE, McComsey DF, Costanzo MJ, Yabut SC, Lu T, Player MR, Giardino EC, Damiano BP. Chem Biol Drug Des. 2006;68:29. doi: 10.1111/j.1747-0285.2006.00408.x.Poon SF, Stock N, Payne JE, McGuire AR, Stearns B, Yang X, Chen W, Munoz B, Smith ND. Bioorg Med Chem Lett. 2005;15:2259. doi: 10.1016/j.bmcl.2005.03.009.Bundgaard H, Drustrup Larsen J. J Med Chem. 1988;31:2066. doi: 10.1021/jm00119a002.
- 8.For recent examples of amidine formation, see: Shang Y, He X, Hu J, Wu J, Zhang M, Yu S, Zhang Q. Adv Synth Catal. 2009;351:2709.She J, Jiang Z, Wang Y. Synlett. 2009;12:2023.Yu RT, Rovis T. J Am Chem Soc. 2008;130:3262. doi: 10.1021/ja710065h.Yoo EJ, Ahlquist M, Bae I, Sharpless B, Fokin V, Chang S. J Org Chem. 2008;73:5520. doi: 10.1021/jo800733p.
- 9.For recent examples of imidate formation, see: Song W, Lu W, Wang J, Lu P, Wang Y. J Org Chem. 2010;75:3481. doi: 10.1021/jo100354h.Yoo EJ, Park SH, Lee SH, Chang S. Org Lett. 2009;11:1155. doi: 10.1021/ol900023t.Yoo EJ, Bae I, Cho SH, Han H, Chang S. Org Lett. 2006;8:1347–1350. doi: 10.1021/ol060056j.
- 10.For a recent example of α-functionalization of imidates, see: Matsubara R, Berthiol F, Kobayashi S. J Am Chem Soc. 2008;130:1804–1805. doi: 10.1021/ja077054u.
- 11.For a related 1,3-shift, see: Bendikov M, Duong HM, Bolanos E, Wudl F. Org Lett. 2005;7:783. doi: 10.1021/ol0477327.
- 12.(a) For leading references, see: Anderson CE, Overman LE. J Am Chem Soc. 2003;125:12412. doi: 10.1021/ja037086r.. (b) For a review, see: Overman LE. Acc Chem Res. 1980;13:218. Also see: (c) Overman LE. J Am Chem Soc. 1974;96:597.. (d) Overman LE. J Am Chem Soc. 1976;98:2901.
- 13.Tomooka K, Suzuki M, Uehara K, Shimada M, Akiyama T. Synlett. 2008;16:2518. [Google Scholar]