Two interconvertible phosphoinositides, PI(4,5)P2 and PI4P, function in different steps of clathrin-dependent endocytosis in pollen tubes. Whereas accumulation of PI(4,5)P2 is required for the initial steps of membrane invagination, PI4P is required for the later steps of closing and/or fission of invaginated membrane.
Abstract
Using the tip-growing pollen tube of Arabidopsis thaliana and Nicotiana tabacum as a model to investigate endocytosis mechanisms, we show that phosphatidylinositol-4-phosphate 5-kinase 6 (PIP5K6) regulates clathrin-dependent endocytosis in pollen tubes. Green fluorescent protein–tagged PIP5K6 was preferentially localized to the subapical plasma membrane (PM) in pollen tubes where it apparently converts phosphatidylinositol 4-phosphate (PI4P) to phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. RNA interference–induced suppression of PIP5K6 expression impaired tip growth and inhibited clathrin-dependent endocytosis in pollen tubes. By contrast, PIP5K6 overexpression induced massive aggregation of the PM in pollen tube tips. This PM abnormality was apparently due to excessive clathrin-dependent membrane invagination because this defect was suppressed by the expression of a dominant-negative mutant of clathrin heavy chain. These results support a role for PI(4,5)P2 in promoting early stages of clathrin-dependent endocytosis (i.e., membrane invagination). Interestingly, the PIP5K6 overexpression-induced PM abnormality was partially suppressed not only by the overexpression of PLC2, which breaks down PI(4,5)P2, but also by that of PI4Kβ1, which increases the pool of PI4P. Based on these observations, we propose that a proper balance between PI4P and PI(4,5)P2 is required for clathrin-dependent endocytosis in the tip of pollen tubes.
INTRODUCTION
Endocytosis internalizes extracellular materials and retrieves excessive components from the plasma membrane (PM), which can then be either degraded in lysosomes/vacuoles or recycled back to the PM. An increasing number of fundamental cellular processes, such as cell polarity establishment, cytokinesis, polarized cell growth, and cellular signaling, have been shown to require endocytosis. In plant cells, endocytosis creates the polar localization of PIN auxin efflux proteins (Dhonukshe et al., 2008; Kleine-Vehn and Friml, 2008; Yang, 2008), enhances brassinosteroid signaling (Geldner et al., 2007), and appears to regulate polarized cell growth (Helling et al., 2006; Moscatelli et al., 2007; Zonia and Munnik, 2008). Multiple pathways of endocytosis are known, but clathrin-dependent endocytosis is most extensively studied in yeast and in animal cells (Conner and Schmid, 2003; Mousavi et al., 2004). Clathrin-dependent endocytosis involves several stages, including assembly of clathrin and its adaptor proteins (such as AP-2 complex and AP-3) onto the inner leaflet of the PM to form coated pits, invagination of coated pits, dynamin-mediated pinching of coated vesicles, and uncoating of clathrin from endocytic vesicles (Conner and Schmid, 2003; Mousavi et al., 2004). Evidence suggests that phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] localized in the inner leaflet of the PM promotes the formation and invagination of coated pits by binding to the AP-2 complex, AP-3, Dab2, and Epsin in yeast and animal cells (Mousavi et al., 2004; Di Paolo and De Camilli, 2006). Acute depletion of PI(4,5)P2 caused loss of coated pits in mammalian COS-7 cells (Zoncu et al., 2007). PI(4,5)P2 may also be important for membrane fission, as it also binds dynamin. Several recent studies show that PIP phosphatase-dependent metabolism of PI(4,5)P2 is required for late stages of endocytosis in mammalian and yeast cells (Sun et al., 2005; Perera et al., 2006; Sun et al., 2007).
In contrast with yeast and mammalian cells, the molecular mechanism for clathrin-dependent endocytosis in plant cells remains poorly characterized, although the existence of clathrin-dependent endocytosis has been well documented and implicated in the polar localization of PIN proteins to the PM and polarized cell growth in plants. As a single cell system, pollen tubes are particularly well suited for investigating the mechanism for endocytosis and its role in polarized cell growth (Cheung and Wu, 2008; Lee and Yang, 2008; Yalovsky et al., 2008; Yang, 2008). Pollen tubes rapidly expand via tip growth that is dependent upon massive tip-targeted exocytosis. Thus, it is conceivable that tip-localized endocytosis must be coordinated with exocytosis to regulate rapid tip growth. Indeed, evidence suggests that clathrin is preferentially present at the subapical PM of pollen tubes and that disruption of clathrin-dependent endocytosis in tobacco (Nicotiana tabacum) pollen tubes causes growth inhibition (Derksen et al., 1995; Blackbourn and Jackson, 1996; Moscatelli et al., 2007). Signaling mechanisms regulating polarized pollen tube growth, including tip-localized ROP GTPases (Lin et al., 1996; Kost et al., 1999; Li et al., 1999; Fu et al., 2001; Gu et al., 2005; Hwang et al., 2008; Lee et al., 2008), calcium gradients (Holdaway-Clarke et al., 1997), calcium-dependent protein kinases (Yoon et al., 2006; Myers et al., 2009), F-actin dynamics (Fu et al., 2001; Vidali et al., 2001), Rab GTPases (de Graaf et al., 2005), and PI(4,5)P2 (Dowd et al., 2006; Helling et al., 2006), have been extensively studied. However, the mechanisms regulating clathrin-dependent endocytosis in pollen tubes are yet to be characterized.
PI(4,5)P2 has been reported to localize to the apical PM of pollen tubes and root hairs using a green fluorescent protein (GFP)-tagged pleckstrin-homology (PH) domain from phospholipase C (PLC), which is an in vivo PI(4,5)P2 marker and has been implicated in the regulation of tip growth (Kost et al., 1999; Dowd et al., 2006; Helling et al., 2006). Recent studies suggest that the PM accumulation of PI(4,5)P2 in pollen tubes may require one or more phosphatidylinositol-4-phosphate 5-kinase (PIP5K), which phosphorylates the D-5 position of the inositol ring of phosphatidylinositol-4-phosphate (PI4P). The Arabidopsis thaliana genome encodes 11 PIP5Ks divided into two groups: type B (PIP5K1-9) containing membrane occupation and recognition nexus (MORN) repeats in the N terminus and type A (PIP5K10 and 11) lacking MORN repeats (Mueller-Roeber and Pical, 2002; Im et al., 2007a). In Arabidopsis root hairs, the production of PI(4,5)P2 is through a type B PIP5K, PIP5K3 (Kusano et al., 2008; Stenzel et al., 2008). Overexpression of PIP5K3 causes abnormal root hair morphology, and disruption of PIP5K3 inhibits root hair growth. In pollen tubes, alteration of pollen-expressed PIP5K4 and PIP5K5 similarly affected pollen tube growth (Ischebeck et al., 2008; Sousa et al., 2008). It was proposed that these PIP5Ks affect either pectin exocytosis or recycling of endocytic vesicles (Ischebeck et al., 2008; Sousa et al., 2008). Although several studies have implicated phosphoinositides in the regulation of endocytosis in pollen tubes (Monteiro et al., 2005a, 2005b; Helling et al., 2006; Sousa et al., 2008), the role and the mode of action for phosphoinositides in endocytosis remain unclear.
In this research, we investigated the role of PI4P and PI(4,5)P2 in the regulation of clathrin-mediated endocytosis in the tip of pollen tubes. We found that a pollen-enriched Arabidopsis PIP5K, PIP5K6, was preferentially localized to the subapical PM in pollen tubes, as was clathrin. Importantly, downregulation of PIP5K6 inhibited the assembly of clathrin onto the apical PM and endocytosis. By contrast, overaccumulation of PIP5K6 overactivated the early stage of clathrin-dependent endocytosis and was accompanied by a defect in the late stage of endocytosis, resulting in excessive aggregates of invaginated PM. The alteration of the apical PI(4,5)P2 by inhibiting or overexpressing PLC did not cause this membrane deformation phenotype. Furthermore, PIP5K6-induced membrane deformation was suppressed by overexpression of either PLC2 or PI4Kβ1. Taken together, these findings support the hypothesis that PI(4,5)P2 promotes the early stages of clathrin-dependent endocytosis (formation and invagination of clathrin-coated pits), whereas PI4P is required for the completion of clathrin-dependent endocytosis.
RESULTS
PIP5K6 Encodes a PIP5K Localized to the Apical PM in Pollen Tubes
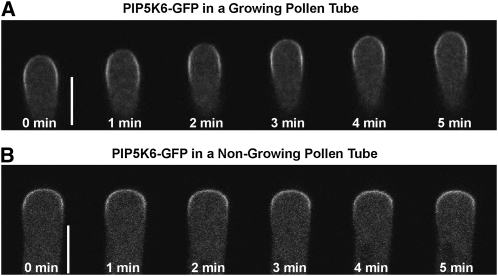
To investigate the role of the tip-localized PI(4,5)P2 in the regulation of pollen tube tip growth, we sought to identify a PIP5K responsible for the synthesis of PI(4,5)P2 for this particular PI(4,5)P2 pool. Three Arabidopsis PIP5K genes, PIP5K4, 5, and 6, are specifically expressed in pollen (Zimmermann et al., 2004). Recent reports suggest that PIP5K4 and PIP5K5 are preferentially localized to the subapical PM of pollen tubes (Ischebeck et al., 2008; Sousa et al., 2008). We found that PIP5K6-GFP was also enriched at the subapical PM, while GFP alone was only found in the cytosol (Figure 1A; see Supplemental Figure 1A online). In growing pollen tubes, PIP5K6-GFP was preferentially associated with the subapical PM (Figure 1A). By contrast, in tubes that had ceased elongation, PIP5K6-GFP was preferentially localized to the tip of the apical PM (Figure 1B).
Figure 1.
PIP5K6-GFP Is Localized to Subapical PM in Growing Tobacco Pollen Tubes.
Localization of PIP5K6-GFP in tobacco pollen tubes was examined using a bombardment-mediated transient expression method. Median images were taken using a Leica SP2 confocal microscope 4 to 5 h after bombardment.
(A) Time course (5 min) of a growing tobacco pollen tube expressing PIP5K6-GFP. Time 0 min refers to an arbitrary starting point. Note the strong PIP5K6-GFP signal at subapical PM. Bar = 10 μm.
(B) Time course (5 min) of a nongrowing tobacco pollen tube expressing PIP5K6-GFP. Time 0 min refers to an arbitrary starting point. Note the strong PIP5K6-GFP signal at the apex of the pollen tube. Bar = 10 μm.
To assess whether PIP5K6 is a functional PIP5K kinase in vivo, we visualized pollen PI(4,5)P2 and PI4P using GFP-based markers, GFP-PLCδ1 PH and GFP-FAPP1 PH (GFP-tagged PH domains of the human PLCδ1 and phosphatidylinositol-4-phosphate adaptor protein-1 [FAPP1]), respectively (Furutani et al., 2006). PI(4,5)P2, marked by the GFP-PLCδ1 PH domain, accumulated at the apical PM of pollen tubes (see Supplemental Figure 1B online), consistent with previous reports in pollen tubes (Kost et al., 1999; Dowd et al., 2006). PI4P, marked by the GFP-FAPP1 PH domain, accumulated at the apical PM and in a vesicle-like structure (presumably derived from the trans-Golgi network) in control pollen tubes (see Supplemental Figure 2A online), consistent with the PI4P localization pattern in root hair cells (Thole et al., 2008). Although considered as specific binding proteins for PI4P and PI(4,5)P2 in vitro, the phosphoinositide markers used in this study may compete with native proteins (including downstream effectors and native enzymes) for binding with PI4P and PI(4,5)P2. As a result, overexpression of these two markers might introduce some nonspecific effects on pollen tube growth (Sousa et al., 2008). To minimize the unspecific effects caused by marker overexpression, we only used low-dosage DNA (0.1 μg per shot) for transient expression in tobacco pollen tubes. In addition, we avoided pollen tubes expressing high levels of markers in our analyses. Using these two markers, we examined the effect of overexpressing (OX) PIP5K6 on PI(4,5)P2 and PI4P localization pattern in tobacco pollen tubes. Overexpression of the full-length PIP5K6 significantly increased GFP-PLCδ1 PH distribution to the apical PM (see Supplemental Figure 1C online) and decreased GFP-FAPP1 PH distribution to the apical PM (see Supplemental Figure 2B online). Overexpression of a PIP5K-dead mutant PIP5K6 (K443S), in which the conserved K443 residue required for the catalytic activity of PIP5K is mutated, seemed to have no significant effect on the distribution of PI(4,5)P2 and PI4P markers to the apical PM (see Supplemental Figures 1D and 2C online). These observations are consistent with the hypothesis that PIP5K6 is an active enzyme locally keeping the balance between PI(4,5)P2 and PI4P on the PM of pollen tubes.
PIP5K6 Overexpression Causes PM Deformation in Pollen Tube Tips
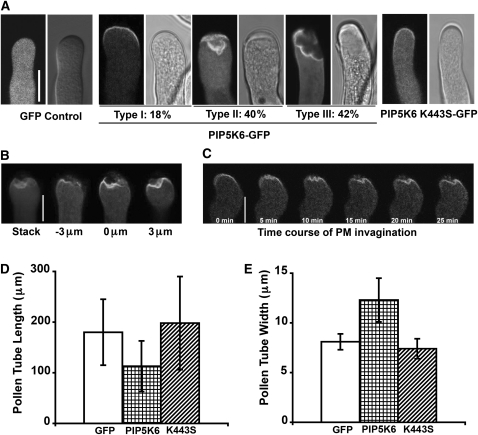
In the course of examining PIP5K6-GFP localization, we found that many PIP5K6-GFP–overexpressing pollen tubes exhibited PIP5K6-GFP aggregates at the tip (Figure 2A). Similar phenotypes were observed in tobacco pollen tubes overexpressing PIP5K4-GFP and PIP5K5-GFP (Ischebeck et al., 2008; Sousa et al., 2008). To assess whether these aggregates were due to PM deformation, multiple scanning at different focal planes was conducted in the tip of PIP5K6-GFP OX tubes. As shown in Figures 2A and 2B, in these abnormal tips PIP5K6-GFP appeared to be associated with the PM, which apparently retracted from the cell wall as a result of invagination, causing the overall deformation of the cytoplasm in the tip region. To confirm whether the aggregates were indeed due to invagination of the PM, we coexpressed untagged PIP5K6 with the PM marker RLK-GFP (Lee et al., 2008) and found that the apical PM containing RLK-GFP displayed a similar invagination, as did PIP5K6-GFP (see Supplemental Figure 3 online).
Figure 2.
PIP5K6 OX Phenotype in Tobacco Pollen Tubes.
Phenotype of tobacco pollen tubes transiently overexpressing GFP, PIP5K6-GFP, and PIP5K6 K443S-GFP. Pairs of panels represent fluorescence images (left) and bright-field images (right).
(A) Effect of PIP5K6-GFP overexpression on PM deformation at the tip of tobacco pollen tubes. Left two panels: GFP control is not PM associated and does not cause any defect of PM. Middle six panels: Overexpression of PIP5K6-GFP causes PM deformation at the apical region of pollen tube. At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP–overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM deformation was 18, 40, and 42%, respectively (n = 100). Bar = 10 μm. Right two panels: PIP5K6 K443S-GFP–overexpressing tobacco pollen tubes display no PM deformation. PIP5K6 K443S-GFP signal is strong at apical PM of pollen tube.
(B) Extensive PM deformation at different focal planes in a PIP5K6-GFP–overexpressing tobacco pollen tube. Stack image is shown on the left, and selected different images at different focal planes are shown on the right. Bar = 10 μm.
(C) Time course (25 min) of a PM invagination event in a tobacco pollen tube expressing PIP5K6-GFP. Note that the pollen tube is not growing during PM invagination. Bar = 10 μm.
(D) Effect of PIP5K6-GFP overexpression on length of tobacco pollen tubes. Length of tobacco pollen tubes was measured 4 to 5 h after bombardment. Overexpression of PIP5K6-GFP inhibited pollen tube length compared with the GFP OX control (n = 30, error bars indicate sd, Student’s t test value P < 0.05). PIP5K6 K443S-GFP OX does not have a significant effect on pollen tube length (n = 30, error bars indicate sd, Student’s t test value P > 0.05).
(E) Effect of PIP5K6-GFP overexpression on width of tobacco pollen tubes. Width of tobacco pollen tubes was measured 4 to 5 h after bombardment. Overexpression of PIP5K6-GFP increased pollen tube width compared with GFP OX control (n = 30, error bars indicate sd, Student’s t test value P < 0.05). PIP5K6 K443S-GFP OX does not have significant effect on pollen tube width (n = 30, error bars indicate sd, Student’s t test value P > 0.05).
PIP5K6-GFP OX pollen tubes exhibited different degrees of PM deformation. To better describe the severity of this phenotype, we categorized PIP5K6-GFP tubes into three groups: (1) those with the normal smooth PM, (2) those with moderate apical PM deformation in which the apical PM is still a distinguishable intact membrane compartment but is retracted from the apical cell wall, and (3) those with severe apical PM deformation in which the apical PM becomes fragmented membrane patches. At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP tubes falling into these three groups was 18, 40, and 42%, respectively (n = 100; Figure 2A). However, PM morphology was not altered by the overexpression of the GFP-tagged PIP5K6 K443S mutant (n = 30; shown in Figure 2A). Time-lapse imaging showed that the PM invagination started after PIP5K6-GFP localization invaded the extreme tip and became more severe over time (Figure 2C). These results suggest that excessive amount of PIP5K6 activity at the tip of pollen tubes caused the deformation of the PM.
The PM deformation occurred in PIP5K6-GFP OX tubes that had already stopped elongating (Figure 2C). At 4 to 5 h after bombardment, PIP5K6-GFP OX tubes were shorter in length and wider in tips compared with control tubes expressing GFP alone (Figures 2D and 2E). The mean length decreased from 180 μm in control tubes to 113 μm in PIP5K6-GFP OX tubes (n = 30, Student’s t test value P < 0.05). The mean width increased from 8.1 μm in control tubes to 12.4 μm in pollen tubes overexpressing PIP5K6-GFP (n = 30, Student’s t test value P < 0.05). However, pollen tube growth arrest induced by other treatments, such as overexpression of a dominant-negative form of ROP1, did not cause PM deformation (data not shown), suggesting that the PM deformation is a specific effect of PIP5K6 overexpression, which is further supported by the suppression of the PM deformation by the inhibition of the early stage of clathrin-dependent endocytosis (see below).
Overexpression of PIP5K6 Causes Overinitiated but Aborted Clathrin-Dependent Endocytosis
We hypothesized that one or both of the following membrane trafficking defects could contribute to the PM deformation induced by PIP5K6 OX: (1) an increase in exocytosis that was not coupled with an increase in endocytosis or cell wall expansion and (2) a defect in a late stage of endocytosis, in which the apical PM was invaginated but not pinched off, as observed in the dysfunction of dynamin (Hill et al., 2001; Kang et al., 2003).
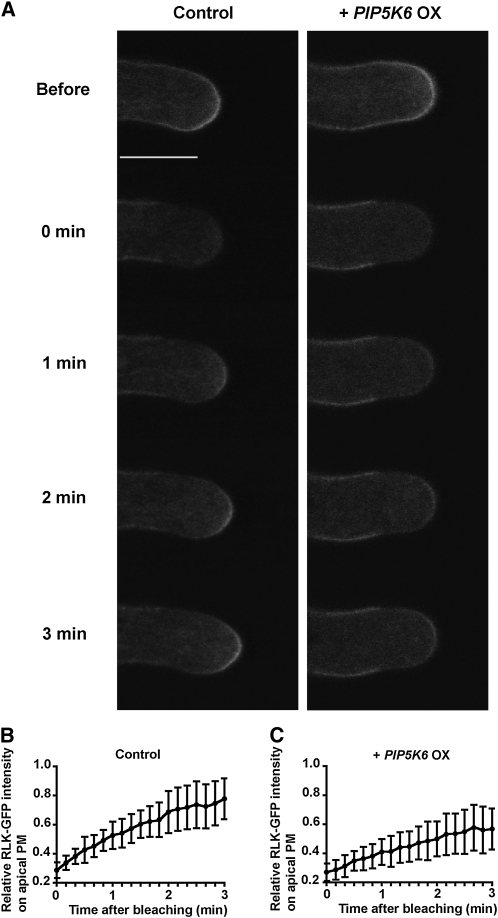
To investigate the effect of PIP5K6 OX on exocytosis, we visualized exocytosis at the tip of tobacco pollen tubes using the fluorescence recovery after photobleaching (FRAP) method (Lee et al., 2008). In pollen tubes expressing the GFP-RLK PM marker, we photobleached the apical PM region and tracked the recovery of the GFP signal. In control pollen tubes expressing GFP-RLK alone, the PM-associated GFP signal quickly recovered after photobleaching (n = 10; average curve shown in Figures 3A and 3B). The PM signal recovered to ~70% of the original signal intensity within 3 min after photobleaching. In pollen tubes coexpressing GFP-RLK and PIP5K6, even at the early times when PIP5K6 OX had not caused PM deformation, the recovery of the apical PM-associated GFP signal was slower than in control tubes (Figures 3A and 3C; Student’s t test value P < 0.05). At 3 min after photobleaching, the PM signal recovered to ~50% of the original signal intensity. This observation suggested that PIP5K6 OX did not enhance but might suppress exocytosis in pollen tubes. In further support of this conclusion, we found that PIP5K6 OX did not change the localization of a marker for exocytic vesicles yellow fluorescent protein-RabA4d (Lee et al., 2008), as shown in Supplemental Figure 4 online.
Figure 3.
PIP5K6 OX Does Not Enhance Exocytosis in Tobacco Pollen Tubes.
To test whether PIP5K6 OX increased exocytosis in tobacco pollen tubes, the GFP-RLK FRAP method was used (Lee et al., 2008).
(A) Effect of PIP5K6 OX on FRAP time course of GFP-RLK in tobacco pollen tubes. Left: A time course of FRAP in a tobacco pollen tube expressing GFP-RLK alone. Bar = 10 μm. Right: A time course of FRAP in a tobacco pollen tube coexpressing GFP-RLK and PIP5K6. Right panel is at the same magnification as the left panel. Note that PIP5K6 OX pollen tubes analyzed were growing but elongated at a slower rate than control tubes. The mean elongation rates for control pollen tubes and PIP5K6 OX tubes were 0.9 and 0.3 μm/min, respectively. Refer to Supplemental Movies 1 and 2 online for these time courses.
(B) Quantitative analysis of FRAP time courses of pollen tubes expressing GFP-RLK alone. Relative intensity of membrane-localized RLK-GFP compared with fluorescence before photobleaching (1.0) was used to quantify the speed of fluorescence recovery. Mean values of FRAP signals are shown (n = 10, error bars indicate sd).
(C) Quantitative analysis of FRAP time courses of pollen tubes expressing GFP-RLK and PIP5K6. Relative intensity of membrane-localized RLK-GFP compared with fluorescence before photobleaching was used to quantify the speed of fluorescence recovery. Mean values of FRAP signals are shown (n = 10, error bars indicate sd). Recovery of GFP-RLK signal on the PM was significantly slower than that in control pollen tubes (Student’s t test value P < 0.05).
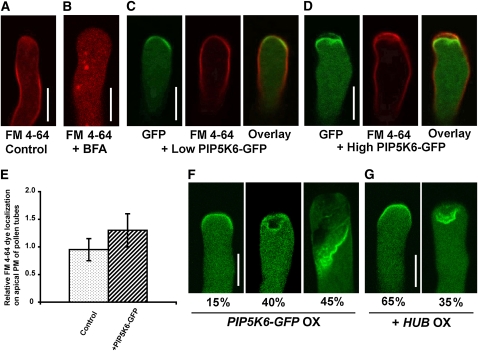
We then investigated whether PIP5K6 OX altered endocytosis. FM 4-64 dye is commonly used as a marker for endocytosis because it is incorporated in the PM and enters the cytoplasm only through endocytosis when applied to culture media. In normal elongating tobacco pollen tubes, FM 4-64 dye labeled the PM, particular structures (likely endosomes), and the apical cytoplasm as an inverted cone pattern that is thought to contain both recycling and secretory vesicles (Figure 4A). Ten to fifteen minutes after 2.5 μM FM 4-64 was applied to PIP5K6-GFP tubes, FM 4-64 strongly stained the PM region and weakly stained the cytoplasm (Figures 4C to 4E). PIP5K6-GFP overexpression inhibited pollen tube elongation (Figure 2). However, inhibition of FM 4-64 uptake in PIP5K6-GFP–overexpressing tubes was the direct specific effect of PIP5K6-GFP overaccumulation because treatments with brefeldin A (BFA), which also induced the arrest of pollen tube elongation, did not inhibit FM 4-64 entry into the pollen tubes (Figure 4B). These observations are consistent with a previous report that PIP5K4 OX inhibited FM dye uptake (Sousa et al., 2008) and support the hypothesis that PIP5K6 OX overactivates the early stages (i.e., membrane invagination) but inhibits the later stage (i.e., fission of invaginated membrane) of endocytosis, resulting in excessive accumulation of deformed membranes at the tip of pollen tubes.
Figure 4.
PIP5K6 OX Induces Clathrin-Dependent Abortive Endocytic Compartments in Pollen Tubes.
(A) FM 4-64 dye uptake in a control tobacco pollen tube. Tobacco pollen tubes were treated with 2.5 μM FM 4-64 for 15 min before imaging under a Leica SP2 confocal microscope. Bar = 10 μm.
(B) Effect of BFA treatment on FM 4-64 dye uptake in a tobacco pollen tube. Tobacco pollen tubes were treated with 10 μM BFA and 2.5 μM FM 4-64 for 15 min before imaging under a Leica SP2 confocal microscope. Bar = 10 μm.
(C) FM 4-64 dye uptake in tobacco pollen tubes with a low level of PIP5K6-GFP overexpression. Tobacco pollen tubes were treated with 2.5 μM FM 4-64 for 15 min before imaging under a Leica SP2 confocal microscope. Bar = 10 μm.
(D) FM 4-64 dye uptake in tobacco pollen tubes with a high level of PIP5K6-GFP overexpression. Tobacco pollen tubes were treated with 2.5 μM FM 4-64 for 15 min before imaging under a Leica SP2 confocal microscope. Bar = 10 μm.
(E) Quantitative analysis of PIP5K6-GFP OX’s effect on FM 4-64 uptake in tobacco pollen tubes. FM dye uptake is significantly suppressed by PIP5K6-GFP OX, as revealed by the fact that FM 4-64 relative localization on the PM is significantly increased by PIP5K6-GFP OX (n = 20, Student’s t test value P < 0.05, error bars indicate sd).
(F) PM invagination phenotype of PIP5K6-GFP OX in tobacco pollen tubes. Five to six hours after bombardment, the percentage of PIP5K6-GFP–overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM invagination was 15, 40, and 45%, respectively (n = 60). Bar = 10 μm.
(G) HUB OX suppresses the PM invagination phenotype induced by PIP5K6-GFP OX in tobacco pollen tubes. Five to six hours after bombardment, 65% of pollen tubes overexpressing both PIP5K6-GFP and HUB showed normal smooth PM, while 35% exhibited moderate PM invagination, and none had severe PM invagination (n = 60, Fisher’s exact probability test value P < 0.05). Bar = 10 μm.
PI(4,5)P2 is involved in recruiting clathrin and associated proteins that are required for the formation and invagination of coated pits in yeast and animal cells (Mousavi et al., 2004; Di Paolo and De Camilli, 2006). Thus, a simple explanation for the PIP5K6-induced membrane deformation phenotype is that overaccumulation of PI(4,5)P2 at the tip overactivated membrane invagination but not closure and pinching of endocytic vesicles during clathrin-dependent endocytosis. We tested this hypothesis using a dominant-negative form of Arabidopsis clathrin heavy chain (called Clathrin Hub) (Liu et al., 1995; Dhonukshe et al., 2007), which contains the C-terminal part of the clathrin heavy chain that can bind to and trap the clathrin light chain. Overexpression of HUB alone in tobacco pollen tubes slightly inhibited growth (see Supplemental Figure 5 online). When PIP5K6-GFP was co-overexpressed with HUB, the PM invagination phenotype was greatly suppressed (Figures 4F and 4G). Five to six hours after bombardment, the percentage of PIP5K6-GFP–overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM invagination was 15, 40, and 45%, respectively (Figure 4F; n = 60). Under the same condition, 65% of pollen tubes overexpressing both PIP5K6-GFP and HUB showed normal smooth PM, while 35% exhibited moderate PM invagination and none had severe PM invagination (Figure 4G; n = 60).
To further test this hypothesis, we used a marker for early stage of clathrin-dependent endocytosis, Arabidopsis AP180 protein. AP180 is an adaptor protein responsible for clathrin cage assembly and thus participates in the initiation of clathrin-mediated endocytosis (Barth and Holstein, 2004). In control tobacco pollen tubes, GFP-AP180 was localized to the subapical PM, similar to clathrin localization pattern (see Supplemental Figures 6A and 6B and Supplemental Movie 3 online). In pollen tubes co-overexpressing GFP-AP180 and PIP5K6, the GFP-AP180 accumulated in both of the apex and the subapical PM, especially the invaginated sites of PM (see Supplemental Figures 6C to 6E online). Taken together, these results strongly support the hypothesis that overinitiated but aborted clathrin-dependent endocytosis accounted for the PM deformation in PIP5K6-GFP OX pollen tubes.
PIP5K6 RNA Interference Inhibits Endocytosis by Blocking the PM Recruitment of Clathrin Heavy Chain
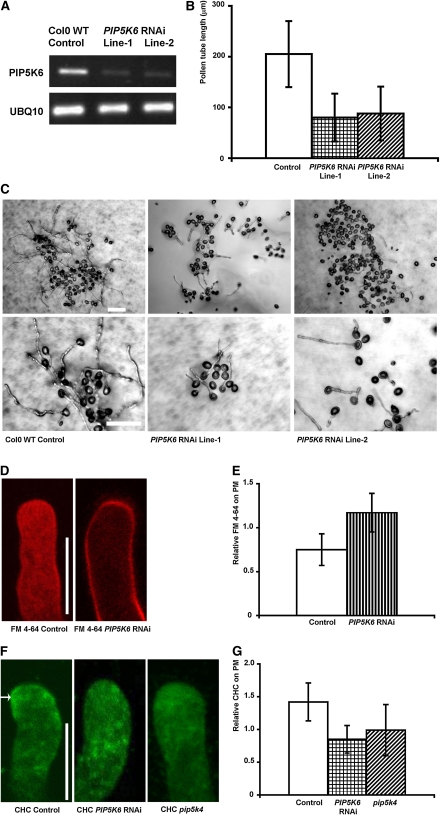
If the PM-localized PI(4,5)P2 indeed regulates the formation of coated pits by recruiting clathrin in pollen tubes as in yeast and animal cells, the suppression of PM-localized PIP5Ks is expected to inhibit clathrin-dependent endocytosis. We generated transgenic Arabidopsis plants expressing a PIP5K6 RNA interference (RNAi) construct under the control of LAT52 promoter. PIP5K6 RNAi lines exhibit reduced PIP5K6 mRNA levels in pollen compared with wild-type Arabidopsis plants (Figure 5A). Two representative lines examined showed similar phenotypes that are correlated with the reduction of mRNA levels in these lines. The PIP5K6 RNAi pollen tubes were greatly shorter (~60% reduction) than wild-type Columbia-0 (Col-0) pollen tubes (Figures 5B and 5C), similar to the previous findings with the pip5k4 mutant (Sousa et al., 2008). We also found that FM dye uptake was greatly inhibited in PIP5K6 RNAi pollen tubes (Figures 5D and 5E).
Figure 5.
PIP5K6 RNAi Inhibits Clathrin-Dependent Endocytosis in Arabidopsis Pollen Tubes.
(A) RT-PCR analysis of PIP5K6 expression in PIP5K6 RNAi pollen. Pollen RNA was extracted for RT-PCR analysis of PIP5K6 mRNA expression, and UBQ10 was used as a loading control. WT, wild type.
(B) PIP5K6 RNAi inhibits pollen tube growth by ~60%. Lengths of wild-type Col-0 and PIP5K6 RNAi pollen tubes were measured 4 h after germination (n = 100, error bars indicate sd, Student’s t test value P < 0.05.).
(C) Effect of PIP5K6 RNAi on pollen tube growth. Wild-type Col-0 and PIP5K6 RNAi pollen tubes were cultured for 4 h in solid germination medium. Note that PIP5K6 RNAi pollen tubes are shorter than wild-type Col-0 pollen tubes. Bars = 100 μm.
(D) PIP5K6 RNAi suppresses FM 4-64 uptake in Arabidopsis pollen tubes. Bar = 10 μm.
(E) PIP5K6 RNAi increases relative FM 4-64 localization on the PM. The mean value of this relative PM distribution of FM 4-64 dye at 15 min after incubation increased from 0.75 in control pollen tubes to 1.18 in PIP5K6 RNAi pollen tubes (n = 30, error bars indicate sd, Student’s t test value P < 0.05).
(F) PIP5K6 RNAi and pip5k4 mutant decreases CHC localization in Arabidopsis pollen tubes. Median planes of pollen tubes were scanned to show the subcellular localization pattern of CHC. Arrow indicates PM localization of CHC antibody. Bar = 10 μm.
(G) Both PIP5K6 RNAi and pip5k4 inhibit recruitment of clathrin to PM of Arabidopsis pollen tubes. Relative clathrin localization on PM is decreased in PIP5K6 RNAi and pip5k4 pollen tubes than in wild-type Col-0 pollen tubes (n = 20, error bars indicate sd, Student’s t test value P < 0.05).
We then analyzed the localization of clathrin heavy chain (CHC) in PIP5K6 RNAi pollen tubes using an immunostaining method (Blackbourn and Jackson, 1996; Kim et al., 2001). In control pollen tubes, anti-CHC antibody staining was mainly localized to the subapical PM (Figure 5F). In PIP5K6 RNAi pollen tubes, CHC antibody stained weakly at the PM (Figure 5F). In pip5k4 knockout pollen tubes (Sousa et al., 2008), CHC localization to the apical PM was also greatly reduced (Figure 5F). The relative PM-associated CHC signal was significantly decreased in both PIP5K6 RNAi and pip5k4 mutant tubes (Figure 5G; n = 20, error bar indicates sd, Student’s t test value P < 0.05). Thus, the apparent deficiency in PIP5K-dependent conversion of PI4P to PI(4,5)P2 led to a defect in clathrin-dependent endocytosis. These results, together with PIP5K6 OX–induced excessive clathrin-dependent PM invagination, suggest that PI(4,5)P2 production via type B PIP5Ks is important for the activation of the early stages (i.e., formation and invagination of coated pits) of clathrin-mediated endocytosis in the apical PM of pollen tubes.
Both Overaccumulation of PI(4,5)P2 and Depletion of PI4P in the Apical PM Induced PM Deformation in Pollen Tubes
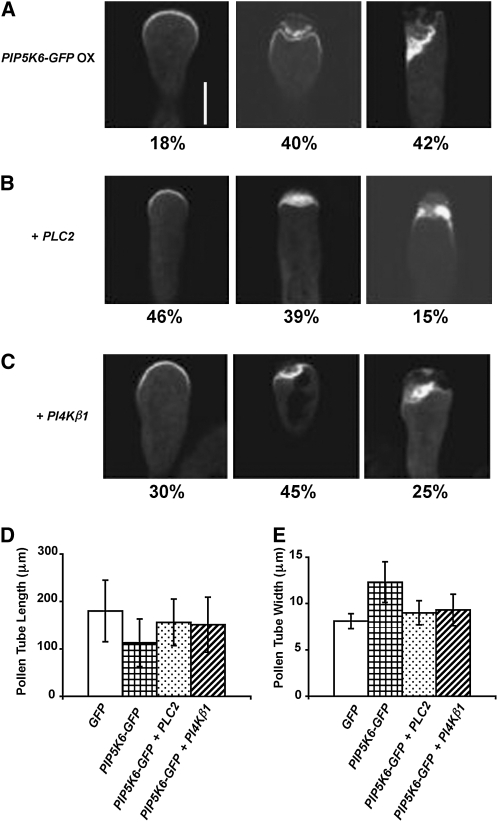
Since PIP5K6 OX not only caused apparent overaccumulation of its product PI(4,5)P2 but also depletion of its substrate PI4P in the apical PM (see Supplemental Figures 1 and 2 online), PI4P depletion and/or PI(4,5)P2 overaccumulation could contribute to the observed PM invagination induced by PIP5K6 overexpression. To assess which of the PIP5K6 OX effects was responsible for the PIP5K6 OX phenotype, we first co-overexpressed PIP5K6-GFP with Arabidopsis PLC2 in tobacco pollen tubes. PLC hydrolyzes PI(4,5)P2 into the second messengers inositol-3-phosphate and diacylglycerol. Arabidopsis PLC2 mRNA accumulates in pollen, and GFP-tagged PLC2 was localized to both apical and subapical PM of tobacco pollen tubes (see Supplemental Figure 7 online). Overexpression of PLC2 suppressed the overaccumulation of PI(4,5)P2 marker caused by PIP5K6 overexpression (see Supplemental Figure 8 online). When PIP5K6-GFP was coexpressed with PLC2 in tobacco pollen tubes, the PM invagination phenotype was partially suppressed. At 4 to 5 h after bombardment, 18, 40, and 42% of tobacco pollen tubes overexpressing PIP5K6-GFP displayed smooth normal PM, moderate PM invagination, and severe PM invagination, respectively (Figure 6A; n = 100). By contrast, 46, 39, and 15% of pollen tubes overexpressing both PIP5K6-GFP and PLC2 exhibited smooth PM, moderate PM invagination, and severe PM invagination (Figure 6B; n = 100, Fisher’s exact probability test value P < 0.05), respectively. Thus, the apparent removal of PI(4,5)P2 by PLC2 overexpression greatly but did not completely suppress PIP5K6 OX–induced PM invagination. This result supports the notion that overaccumulation of PI(4,5)P2 contributed to, but was not solely responsible for, the PM invagination phenotype. Consistent with this finding, we found that PLC2 OX partially suppressed the growth phenotype of PIP5K6 OX (Figures 6D and 6E; n = 30, Student’s t test value P < 0.05).
Figure 6.
PLC2 OX and PI4Kβ1 OX Suppress PIP5K6 OX Phenotype.
(A) PM invagination phenotype induced by PIP5K6-GFP OX in tobacco pollen tubes. At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM deformation was 18, 40, and 42%, respectively (n = 100). Bar = 10 μm.
(B) PLC2 OX suppresses the PM invagination phenotype induced by PIP5K6-GFP OX in tobacco pollen tubes. At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP and PLC2 cooverexpressing tobacco pollen tubes that displayed little, moderate, and severe PM deformation was 46, 39, and 15%, respectively (n = 100, Fisher’s exact probability test value P < 0.05). (B) is at the same magnification as (A).
(C) PI4Kβ1 OX suppresses the PM invagination phenotype induced by PIP5K6-GFP OX in tobacco pollen tubes. At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP and PI4Kβ1 co-overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM deformation was 30, 45, and 25%, respectively (n = 100, Fisher’s exact probability test value P < 0.05). (C) is at the same magnification as (A).
(D) PI4Kβ1 OX or PLC2 OX both suppress PIP5K6 OX induced growth inhibition in tobacco pollen tubes (n = 30, error bars indicate sd, Student’s t test value P < 0.05). Length of tobacco pollen tubes was measured 4 to 5 h after bombardment.
(E) PI4Kβ1 OX or PLC2 OX both suppress PIP5K6 OX induced width increase in tobacco pollen tubes (n = 30, error bars indicate sd, Student’s t test value P < 0.05). Width of tobacco pollen tubes was measured 4 to 5 h after bombardment.
Since suppression of PI(4,5)P2 overaccumulation did not completely restore normal pollen tube tips, we hypothesized that PIP5K6 OX–induced depletion of PI4P in the apical PM of pollen tubes also contributed to the PIP5K6 OX phenotype. To test this hypothesis, we examined the effect of overexpressing Arabidopsis PI4Kβ1, which rescued the reduction of PI4P marker localization caused by PIP5K6 overexpression (see Supplemental Figure 9 online). Co-overexpression of PI4Kβ1 with PIP5K6-GFP not only partially suppressed PIP5K6 OX growth phenotype (Figures 6D and 6E; n = 30, Student’s t test value P < 0.05) but also partially suppressed the PM invagination phenotype induced by PIP5K6-GFP overexpression (Figure 6C). At 4 to 5 h after bombardment, the percentage of PIP5K6-GFP and PI4Kβ1 co-overexpressing tobacco pollen tubes that displayed little, moderate, and severe PM deformation was 30, 45, and 25%, respectively (n = 100, Fisher’s exact probability test value P < 0.05). Furthermore, treatment of wild-type pollen tubes with PLC inhibitor U73122, which increased PI(4,5)P2 level without decreasing PI4P level on the PM (see Supplemental Figure 10 online) (Helling et al., 2006), did not cause PM invagination (see Supplemental Figure 10 online; n = 30). These results further support the notion that overaccumulation of PI(4,5)P2 at the apical PM alone was insufficient to cause the PM invagination phenotype induced by PIP5K6 OX. Taken together, our data suggest that the PM invagination phenotype in PIP5K6 OX pollen tubes was the result of simultaneous overaccumulation of PI(4,5)P2 and reduction of its precursor PI4P.
DISCUSSION
Several recent reports support a role for both PI4P and PI(4,5)P2 in the regulation of tip growth in root hairs and pollen tubes (Monteiro et al., 2005a, 2005b; Dowd et al., 2006; Helling et al., 2006; Preuss et al., 2006; Ischebeck et al., 2008; Kusano et al., 2008; Sousa et al., 2008; Stenzel et al., 2008; Thole et al., 2008; Szumlanski and Nielsen, 2009), but the mechanism by which these membrane-localized signaling molecules affect polarized cell growth remained unclear. Our results support the hypothesis that both PI4P and PI(4,5)P2 play a critical role in the regulation of clathrin-dependent endocytosis at the tip of pollen tubes and that a proper balance between PI4P and PI(4,5)P2 accumulation in the apical PM is important for clathrin-dependent endocytosis. We propose that PI(4,5)P2 promotes the formation and invagination of clathrin-coated pits as shown in yeast and animal cells, while PI4P participates in the final stage of clathrin-dependent endocytosis at the tip of pollen tubes.
PIP5K-Dependent Conversion of PI4P to PI(4,5)P2 Promotes the Early Stages of Clathrin-Dependent Endocytosis at the Tip of Pollen Tubes
Our results strongly support the hypothesis that PIP5K-mediated conversion of PI4P to PI(4,5)P2, which preferentially occurs at the subapical PM of pollen tubes, promotes the early steps of clathrin-dependent endocytosis. This conclusion is based on two key findings: (1) RNAi-mediated suppression of PIP5K6 inhibits endocytosis and clathrin assembly onto the apical PM, and (2) PIP5K6 overexpression induces clathrin-dependent excessive PM invagination. Our results suggest that this excessive PM invagination was not caused by overactivation of exocytosis. On the contrary, we showed PIP5K6 overexpression appeared to inhibit exocytosis, a phenomenon consistent with a role for PI4P (the PIP5K substrate) in the positive regulation of exocytosis (Preuss et al., 2006). It was recently proposed that PIP5K OX–induced PI(4,5)P2 overaccumulation leads to overactivated exocytosis, causing the PM to fold backward from the cell wall (Ischebeck et al., 2008; Sousa et al., 2008). This proposition was based on the observation that pectin overaccumulated outside of the invaginated region of the PM in pollen tubes overexpressing PIP5K. An alternative explanation for this observation could be that the endocytosis of pectin components is abortive in these pollen tubes. Pectin is found to accumulate in BFA-induced compartments in plant cells, which are thought to be enlarged endocytic compartments (Baluska et al., 2002). Thus overinitiated abortive endocytosis could lead to pectin overaccumulation in the tip of PIP5K-overexpressing pollen tubes.
Is PIP5K-Dependent Endocytosis Mediated by Its Immediate Product PI(4,5)P2 or Secondary Products?
PI(4,5)P2 could be converted into PI(3,4,5)P3 by PI(4,5)P2-3-kinase. Homologs of this enzyme are absent in plants, and PI(3,4,5)P3 has not been detected in plants to date. Thus, PI(3,4,5)P3 is unlikely to participate in clathrin-dependent endocytosis in plant cells. PI(4,5)P2 could also be converted into inositol-3-phosphate and diacylglycerol by PLC. However, overexpression of PLC in tobacco pollen tubes did not induce excessive PM invagination seen in PIP5K-overexpressing tubes (Helling et al., 2006), arguing against a role for PLC products in the promotion of early clathrin-dependent endocytosis. Importantly, we found that the clathrin-dependent PM invagination in PIP5K6-overexpressing tubes was partially suppressed when PM-localized PLC2 was overproduced. PI(4,5)P2 has been found in clathrin-enriched vesicles upon salt stress, supporting a link between calthrin-dependent endocytosis and PI(4,5)P2 (König et al., 2008). Therefore, we propose that PI(4,5)P2, not its products, promotes early steps of clathrin-dependent endocytosis.
A role for PI(4,5)P2 in the regulation of clathrin-dependent endocytosis in pollen tubes is consistent with the findings in animal and yeast cells (Sun et al., 2005, 2007; Zoncu et al., 2007). Through the study of PI(4,5)P2 dynamics and clathrin-dependent endocytic compartment, it was shown in yeast cells that PI(4,5)P2-enriched region is important for the initiation of clathrin-dependent endocytosis (Sun et al., 2005, 2007). The most convincing study that supports a direct involvement of PI(4,5)P2 in clathrin-dependent endocytosis was the demonstration that acute depletion of PI(4,5)P2 by inducible activation of PI(4,5)P2 phosphatase causes loss of coated pits in mammalian COS-7 cells (Zoncu et al., 2007). Moreover, several PI(4,5)P2 binding proteins, including AP2 and AP180, are involved in the assembly of clathrin coat and/or invagination of clathrin-coated pits. Importantly, homologs for AP180 are present in plants and are expressed in pollen (Holstein and Oliviusson, 2005). Therefore, PI(4,5)P2 regulation of the early stage of clathrin-dependent endocytosis appears to be conserved in eukaryotic kingdoms.
PI(4,5)P2 also regulates the organization and dynamics of the actin cytoskeleton, which impacts clathrin-mediated endocytosis in yeast (Yin and Janmey, 2003; Kaksonen et al., 2006; Sun et al., 2007). Could PI(4,5)P2 regulation of actin be part of the mechanism by which PI(4,5)P2 promotes the early stages of clathrin-dependent endocytosis? When overexpressed, a dominant-negative mutant of PLC was found to increase F-actin in pollen tubes, implicating the involvement of PI(4,5)P2 in the regulation of the actin cytoskeleton (Dowd et al., 2006). A previous report disagreed with the idea that the membrane invagination induced by PIP5K4 and PIP5K5 overexpression could be attributed to changes in the actin cytoskeleton (Ischebeck et al., 2008). Nonetheless, actin as a potential bridge between PIP5Ks and endocytosis is worthy of further investigation, given that PI(4,5)P2 regulation of endocytosis is conserved in different eukaryotic systems.
PI4P May Participate in a Late Stage of Clathrin-Dependent Endocytosis
Our results also support a requirement for the PI(4,5)P2 precursor, PI4P, in the regulation of clathrin-dependent endocytosis. Using a PI4P marker, GFP-FAPP1 PH, we found that PI4P is enriched in the apical PM and is localized in putative Golgi-derived vesicles. Similar PI4P distribution was also found in root hairs (Vermeer et al., 2009). In plant cells, PI4P is the most abundant form of phosphoinositide (Im et al., 2007b). Genetic studies of enzymes involved in PI4P synthesis and degradation in root hairs have implicated PI4P in the regulation of polar exocytosis (Preuss et al., 2006; Thole et al., 2008). Because PI4P is the immediate precursor of PI(4,5)P2, however, it has been difficult to determine whether PI4P merely provides a precursor for PI(4,5)P2 or has a direct role in signaling.
The excessive PM invagination phenotype in PIP5K6-overexpressing pollen tubes provided us with a unique opportunity to investigate the role of PI4P. As discussed above, our findings suggest that overaccumulation of PI(4,5)P2 contributes to this phenotype. However, this phenotype cannot be explained solely by overproduction of PI(4,5)P2 because inhibition of PLC, which also causes overaccumulation of PI(4,5)P2, did not induce the PM invagination phenotype. Furthermore, co-overexpression with PLC2 was able to only partially suppress the PIP5K6 overexpression phenotype. Importantly, co-overexpression with PI4K, which synthesizes PI4P, also partially suppressed the PIP5K6 overexpression phenotype. These observations suggest that PI4P not only provides a precursor for PI(4,5)P2 but also plays a more direct role in the regulation of clathrin-dependent endocytosis.
In yeast, knockout mutants for SJL genes [which encode PI(4,5)P2 5-phosphatases] show abnormal PM invagination (Sun et al., 2005, 2007), which highly resembles the PIP5K6 overexpression PM phenotype. Using markers for different stages of endocytosis, it was shown that the abnormal PM invagination in the sjl mutants was caused by a defect in the late stage of clathrin-dependent endocytosis. The PM invagination phenotype in sjl knockout yeast cells was due to overaccumulation of abortive endocytic compartment. Based on these observations, it was proposed that the process of hydrolyzing PI(4,5)P2 into PI4P is required for the later steps of clathrin-dependent endocytosis. Our finding that increasing the pool of PI4P in PIP5K6-overexpressing tubes by overexpressing PI4K can rescue the PM invagination defect clearly supports a direct role for PI4P in the regulation of the late stage of endocytosis. Thus, we propose that a PI4P-dependent process may provide a conserved mechanism underlying the completion of clathrin-dependent endocytosis and that the right balance of (or interconversion between) PI4P and PI(4,5)P2 is critical for the initiation, progression, and maturation of clathrin-dependent endocytosis. Identification of PI4P binding proteins involved in this process will be important for testing this hypothesis.
METHODS
Plant Growth and Pollen Germination Conditions
Arabidopsis thaliana plants (Col-0) were grown at 22°C in growth rooms under a light regime of 16 h of light and 8 h of dark. Arabidopsis pollen grains were germinated on a solid germination medium (Li et al., 1999) at room temperature. Nicotiana tabacum plants were grown in a growth chamber at 25°C under a light regime of 12 h of light and 12 h of dark. Tobacco pollen grains were germinated in a liquid germination medium (Fu et al., 2001) at room temperature.
Cloning of Arabidopsis PIP5K6, PLC2, PI4Kβ1, HUB, and AP180 Coding Sequences
The PIP5K6 coding cDNA without the stop codon was amplified from cDNA obtained from Col-0 flowers using forward (5′-GCTCTAGAATGTCGGTAGCACACGC-3′) and reverse (5′-GCTCTAGAAGCGTCTTCAACGAAGAC-3′) primers carrying the XbaI site (underlined). The PLC2 coding cDNA was amplified from cDNA obtained from Col-0 flowers using forward (5′- GCTCTAGAATGTCGAAGCAAACGTAC-3′) and reverse (5′-CGGGATCCCCACAAACTCCACCTTCACG-3′) primers carrying XbaI and BamHI sites (underlined), respectively. The PI4Kβ1 coding cDNA was amplified from cDNA obtained from Col-0 flowers using forward (5′-GCTCTAGAATGCCGATGGGACGC-3′) and reverse (5′-CGGGATCCCCAATATTCCATTTTAAGACCC-3′) primers carrying XbaI and BamHI sites (underlined), respectively. HUB (1860 bp of C-terminal CHC At3g11130) coding cDNA was amplified from cDNA obtained from Col-0 flowers using forward (5′-CCATGGAGAAGAAGTTTAACTTAAATGTTCAGGC-3′) and reverse (5′-GGTACCTTAGTAGCCGCCCATCGGTGG-3′) primers carrying NcoI and KpnI sites (underlined), respectively. Coding cDNAs for PIP5K6, PLC2, and PI4Kβ1 were cloned into the vector pGEM-Teasy vector (Promega) and sequenced. Coding cDNA for HUB was cloned into vector pCR2.1 (Invitrogen) and sequenced. AP180 coding cDNA was amplified from cDNA obtained from Col-0 flowers using forward (5′- GGGGACAAGTTTGTACAAAAAAGCAGGCTCCATGCCGAGCAAGCTTAAAAAAG-3′) and reverse (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTAACTCAAGTGCTTGGCTATGATC-3′) primers and then cloned into Gateway DONR vector and sequenced.
Mutagenesis of the PIP5K6 Coding Sequence
To obtain the point-mutated coding sequence of PIP5K6 K443S, two cDNA fragments were amplified from pGEM-T easy PIP5K6 using the primer combinations (5′-GCTCTAGAATGTCGGTAGCACACGC-3′/5′-TTCTTCATAGTCGATATCATG-3′) and (5′-CTACATGATATCGACTATGAA-3′/5′-GCTCTAGAAGCGTCTTCAACGAAGAC-3′) and then cloned into pGEM-Teasy, respectively. Sequencing confirmed the presence of the K443S mutation. These two fragments were then ligated using the EcoRV site, generating the PIP5K6 K443S cDNA.
Constructs for Transient Expression in Tobacco Pollen Tubes
To generate the pLAT52:PIP5K6-GFP construct, PIP5K6 coding cDNA was subcloned from pGEM-T easy-PIP5K6 into pUC pLAT52:GFP vector using XbaI site (in frame with the 5′ end of GFP sequence) (Fu et al., 2001). To generate the pLAT52:PIP5K6 K443S-GFP construct, PIP5K6 K443S coding cDNA was subcloned from pGEM-T easy-PIP5K6 K443S into the pUC pLAT52:GFP vector using the XbaI site (in frame with 5′ end of GFP sequence). To generate the pLAT52:PIP5K6 construct, PIP5K6 coding cDNA was subcloned from pGEM-T easy-PIP5K6 into the pUC pLAT52:GFP vector using the XbaI site. To generate the pLAT52:PIP5K6 K443S construct, PIP5K6 K443S coding cDNA was subcloned from pGEM-T easy-PIP5K6 into the pLAT52:GFP vector using the XbaI site. The pLAT52:GFP-RLK PM marker construct has been described previously (Lee et al., 2008). DNA encoding the human PLCδ1 PH domain was cut using BglII and BamHI from YFP-PLCδ1 construct (from M. Furutani; Furutani et al., 2006) and then insert into the pUC pLAT52:GFP vector using the BglII site (downstream of the 3′ end of GFP) to obtain pLAT52:GFP-PLCδ1 PH. Human FAPP1 PH domain was cut using BglII and BamHI from pDriveFAPP1 construct (Furutani et al., 2006) and then inserted into pUC pLAT52:GFP using the BglII site (downstream of the 3′ end of GFP) to produce the pLAT52:GFP-FAPP1 construct. HUB coding cDNA was subcloned from pCR2.1-HUB construct into the pUC pLAT52 vector using NcoI and KpnI sites to produce the pUC pLAT52:HUB construct. PLC2 coding cDNA was subcloned from pGEM-T easy PLC2 construct into the pUC pLAT52 vector using XbaI and BamHI sites to generate the pUC pLAT52:PLC2 construct. PI4Kβ1 coding cDNA was subcloned from pGEM-T easy PI4Kβ1 construct into the pUC pLAT52 vector using XbaI and BamHI sites to obtain the pUC pLAT52:PI4Kβ1 construct. AP180 coding cDNA was subcloned from Gateway DONR vector to destination vector to obtain the pGWLAT52:GFP-AP180 construct.
PIP5K6 RNAi Construct and Transgenic Lines
To generate the PIP5K6 RNAi construct, a 340-bp antisense cDNA fragment of the specific sequence of the PIP5K6 was amplified using primer combination (5′-AAATCGATTTACCCTTCGACTTCTTCC-3′/5′-AACCATGGCCTTTAGAGATAGTTTGTCCT-3′), cloned into the ClaI-SacI sites in the pGEM-7Zf vector, yielding the plasmid pGEM-7Z-PIP5K6A. The antisense fragment was cloned as an NcoI-SmaI fragment into pUC pLAT52:GFP vector; the LAT52:PIP5K6A fragment (HindIII/SacI) was subcloned into the pBI121 vector by replacing the cauliflower mosaic virus 35S promoter and GUS cassette, generating the plasmid pBI121 LAT52:PIP5K6A. The sense fragment of PIP5K6 amplified using the primer set (5′- AAGGATCCTTACCCTTCGACTTCTTCC-3′/5′-AATCTAGACCTTTAGAGATAGTTTGTCCT-3′) was inserted into BamHI-XbaI sites in the vector pFGC5941 LAT52 to generate the sense construct pFGC594-LAT52-PIP5K6S (Gu et al., 2005). The intron-PIP5K6S fragment (ClaI/XbaI) was inserted into the pGEM-3Zf vector, and then the fragment of intron-PIP5K6S (KpnI/XbaI) was ligated into the pBI121 LAT52:PIP5K6A, generating the plasmid pBI-LAT52-PIP5K6 RNAi construct.
The PIP5K6 RNAi construct was introduced into Agrobacterium tumefaciens GV3101 and transformed into Arabidopsis (Col-0) using the floral dipping method (Clough and Bent, 1998). Independent T3 homozygous plants were isolated using kanamycin selection for further analysis. Pollen mRNA was extracted from wild-type and PIP5K6 RNAi plants and then used for synthesis of cDNA using the Qiagen RNAeasy kit and the Invitrogen Superscript III reverse transcriptase kit. RT-PCR experiments (30 cycles) with primer sets (PIP5K6-F, 5′-ATGTCGGTAG CACACGCAGA-3′/PIP5K6-R, 5′-TAAGCATGAGTTCATAATTCTTATGACC-3′; UBQ10-F, 5′-GATCTTTGCCGGAAAACAATTGGAGGATGGT-3′/UBQ10-R, 5′-CGACTTGTCATTAGAAAGAAAGAGATAACAGG-3′) were performed to determine the mRNA level of PIP5K6 in both wild-type Col-0 and PIP5K6 RNAi pollen.
Transient Gene Expression in Tobacco Pollen Tubes
Mature pollen grains collected from tobacco flowers were used for transient expression using a particle bombardment procedure as described previously (Fu et al., 2001). For all plasmid constructs used in our experiments, 0.2 to 1.0 μg plasmid DNA was used for each bombardment. Bombarded pollen grains were germinated in a liquid germination medium for various times before observation under a microscope (Fu et al., 2001).
Analysis of Arabidopsis Pollen Tube Growth
Flowers from Arabidopsis plants 2 weeks after bolting were used as the source of pollen. Pollen grains were germinated on a solid germination medium (Li et al., 1999). Unless indicated otherwise, ~3 to 5 h after germination, images of pollen tubes were recorded through a cooled CCD camera (model C4742-95; Hamamatsu) attached to an Eclipse inverted microscope (model TE300; Nikon). The images were analyzed using the MetaMorph v4.5 (Molecular Devices) measurement function.
PLC Inhibitor Treatment
U-73122 (Sigma-Aldrich) PLC inhibitor (in 0.1% DMSO) was added into tobacco pollen to a final concentration of 10 μM immediately following bombardment with a particular construct. A mock treatment (0.1% DMSO) was used as control. Five hours after pollen germination, pollen tubes were examined under a confocal microscope (Leica SP2).
FM 4-64 Dye Staining in Pollen Tubes
FM 4-64 dye staining was performed at 4 to 5 h after pollen germination. For tobacco pollen tubes, FM 4-64 dye (Sigma-Aldrich) was added to a final concentration of 2.5 μM into liquid pollen germination medium. For FM 4-64 dye staining with BFA treatment, FM dye (Invitrogen) and BFA (Invitrogen) was added to final concentrations of 2.5 and 10 μM into liquid pollen germination medium. For Arabidopsis pollen tubes, a droplet (10 μL) of liquid Arabidopsis pollen germination medium containing 10 μM FM 4-64 dye was applied onto thin layers of solid Arabidopsis pollen germination medium. Pollen tubes were incubated with FM 4-64 dye for 10 to 15 min before examination under a confocal microscope (Leica SP2 or Zeiss LSM510).
Immunostaining of CHC in Pollen Tubes
Pollen grains of Arabidopsis flowers were germinated in solid germination medium for 4 h at room temperature and stained as described previously (Hwang et al., 2008). Pollen tubes were treated with fixative (4% paraformaldehyde, 3 mM MgSO4, 2 mM CaCl2, 18% sucrose, and 50 mM PIPES buffer, pH 6.9) for 1 h. After washing gently with PBST (0.05% Triton X-100) buffer (Hwang et al., 2008), three times for 5 min each, pollen tubes were treated with digestion buffer (2% cellulase R-10, 400 mM mannitol, 5 mM CaCl2, and 15 mM MES buffer, pH 5.5) at room temperature for 3 to 5 min. Digested pollen tubes were washed gently with PBST buffer three times for 5 min each and then were blocked with 1% nonfat milk (Nestle) in PBS buffer (Hwang et al., 2008) at room temperature for 1 h. Pollen tubes were incubated with the purified primary anti-CHC (Kim et al., 2001) polyclonal antibody (1:200 dilution with 1% nonfat milk in PBS buffer) at room temperature for 1 h. After washing three times for 10 min each in PBST buffer, pollen tubes were incubated with secondary antibody and fluorescein isothiocyanate (FITC)–conjugated goat anti-rabbit IgG (Sigma-Aldrich) (1:300 dilution with 1% nonfat milk in PBS buffer) at room temperature for 2 h. After three washes with PBST buffer, slides were mounted with mount solution (0.1% p-phenylenediamine, 50% glycerol, and PBS buffer). Mounted pollen tubes were observed under a confocal microscope (Leica SP2).
Confocal Microscopy and Imaging Analysis for GFP Localization
Localization patterns for GFP-tagged proteins in pollen tubes were observed under a confocal microscope (Leica SP2 or Zeiss LSM510). The signal intensities of GFP on the PM and in the cytoplasm were measured using the MetaMorph v4.5 (Molecular Devices) measurement function. The PM region for GFP intensity measurement was measured by defining the peripheral region of the pollen tube as the PM. Apical PM region for intensity measurement in tobacco pollen tubes was defined as the first 6 μm along PM from the apical point. A circular region (4 μm in diameter) 4 μm away from tip was chosen for the measurement of cytosolic GFP intensity. The relative localization of GFP-labeled protein on the PM was calculated as the ratio of PM intensity versus cytosolic intensity.
FM 4-64 Dye Localization
FM 4-64 dye–labeled pollen tubes were observed under a Leica SP2 confocal microscope or a Zeiss LSM510 confocal microscope. The signal intensities of FM dye on the PM and in the cytoplasm were measured using the MetaMorph v4.5 measurement function. Apical PM region for intensity measurement in tobacco and Arabidopsis pollen tubes were defined as the first 6 and 4 μm along PM from the tip point, respectively. A circular region (4 μm in diameter) 4 μm away from tip was chosen for measurement of cytosol intensity. Relative localization of FM dye on the PM was calculated to show the degree of internalization of FM dye.
CHC Localization
Arabidopsis pollen tubes stained with anti-CHC primary antibody and FITC-conjugated secondary antibody were observed under a Leica SP2 confocal microscope. Median planes of pollen tubes were scanned to show the subcellular localization pattern of CHC.
The signal intensities of FITC antibody on the PM and in the cytoplasm were measured using the MetaMorph v4.5 measurement function. Apical PM region for intensity measurement in Arabidopsis pollen tubes was defined as the first 4 μm along PM from the tip point. A circular region (4 μm in diameter) 4 μm away from tip was chosen for measurement of cytosol intensity. Relative localization of FITC antibody on the PM was calculated as the ratio of PM intensity versus cytosolic intensity.
FRAP Analysis of Exocytosis at the Pollen Tube Tip
Tobacco pollen tubes expressing RLK-GFP PM marker alone and coexpressing RLK-GFP PM marker and PIP5K6 were used for FRAP analysis (Lee et al., 2008). The apical region of pollen tubes was photobleached using 100% power with a 488-nm laser (Leica SP2), and the recovery of fluorescence in pollen tubes was tracked in the following 3 min using a confocal microscope (Leica SP2). Time interval between adjacent frames was 10 s. Relative intensity of membrane-localized RLK-GFP compared with fluorescence before photobleaching was used to quantify the speed of fluorescence recovery.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: PIP5K6 (At3g07960), PLC2 (At3g08510), PI4Kβ1 (At5g64070), RLK PM marker (At5g35390), CHC HUB (At3g11130), and AP180 (At1g05020). Sequence data for human genes used in this article can be found in GenBank under accession numbers AF286162 (FAPP1) and NM1130964 (PLCδ1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. PIP5K6 OX Increases PI(4,5)P2 on Apical PM of Tobacco Pollen Tubes.
Supplemental Figure 2. PIP5K6 OX Suppresses PI4P on Apical PM of Tobacco Pollen Tubes.
Supplemental Figure 3. PIP5K6 OX Induces PM Deformation in Tobacco Pollen Tubes.
Supplemental Figure 4. PIP5K6 OX Does Not Change RabA4d Localization Pattern in Tobacco Pollen Tube Tips.
Supplemental Figure 5. DN-CHC HUB OX Slightly Inhibits Pollen Tube Growth.
Supplemental Figure 6. PIP5K6 OX Causes Mislocalization of GFP-AP180 in Tobacco Pollen Tubes.
Supplemental Figure 7. GFP-PLC2 Is Preferentially Localized to the Subapical PM of Pollen Tubes.
Supplemental Figure 8. PLC2 OX Suppresses Increase of PI(4,5)P2 Induced by PIP5K6 OX.
Supplemental Figure 9. PI4Kβ1 OX Suppresses PI4P Decrease Induced by PIP5K6 OX.
Supplemental Figure 10. PLC Inhibitor Treatment Increases PI4P Localization and Does Not Cause PM Invagination in Tobacco Pollen Tube.
Supplemental Movie 1. Recovery of GFP-RLK in the Apical PM after Photobleaching in a Tobacco Pollen Tube Expressing GFP-RLK Alone.
Supplemental Movie 2. Recovery of GFP-RLK on Apical PM after Photobleaching in a Tobacco Pollen Tube Expressing GFP-RLK and Low PIP5K6.
Supplemental Movie 3. Dynamics of GFP-AP180 in a Growing Tobacco Pollen Tube.
Supplementary Material
Acknowledgments
This work was supported by the 973 Basic Science Project (2007cb108700), the Department of Energy to Z.Y. (DE-FG02-04ER15555), the National Institute of General Medical Sciences to Z.Y. (GM081451), and the Natural Science Foundation of China to Y.Z. J.A.F.’s lab is funded by Centro de Biologia do Desenvolvimento (FCT U664) and Fundacao para a Ciencia e Tecnolgia grant PTDC/BIA-BCM/108044/2008.
References
- Baluska F., Hlavacka A., Samaj J., Palme K., Robinson D.G., Matoh T., McCurdy D.W., Menzel D., Volkmann D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130: 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth M., Holstein S.E. (2004). Identification and functional characterization of Arabidopsis AP180, a binding partner of plant alphaC-adaptin. J. Cell Sci. 117: 2051–2062 [DOI] [PubMed] [Google Scholar]
- Blackbourn H.D., Jackson A.P. (1996). Plant clathrin heavy chain: Sequence analysis and restricted localisation in growing pollen tubes. J. Cell Sci. 109: 777–786 [DOI] [PubMed] [Google Scholar]
- Cheung A.Y., Wu H.M. (2008). Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conner S.D., Schmid S.L. (2003). Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- de Graaf B.H., Cheung A.Y., Andreyeva T., Levasseur K., Kieliszewski M., Wu H.M. (2005). Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell 17: 2564–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J., Rutten T., Lichtscheidl I.K., Win A.H.N.d., Pierson E.S., Rongen G. (1995). Quantitative analysis of the distribution of organelles in tobacco pollen tubes: Implications for exocytosis and endocytosis. Protoplasma 188: 267–276 [Google Scholar]
- Dhonukshe P., Aniento F., Hwang I., Robinson D.G., Mravec J., Stierhof Y.D., Friml J. (2007). Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., et al. (2008). Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Di Paolo G., De Camilli P. (2006). Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Dowd P.E., Coursol S., Skirpan A.L., Kao T.H., Gilroy S. (2006). Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell 18: 1438–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Wu G., Yang Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M., Tsujita K., Itoh T., Ijuin T., Takenawa T. (2006). Application of phosphoinositide-binding domains for the detection and quantification of specific phosphoinositides. Anal. Biochem. 355: 8–18 [DOI] [PubMed] [Google Scholar]
- Geldner N., Hyman D.L., Wang X., Schumacher K., Chory J. (2007). Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Fu Y., Dowd P., Li S., Vernoud V., Gilroy S., Yang Z. (2005). A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling D., Possart A., Cottier S., Klahre U., Kost B. (2006). Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 18: 3519–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., van Der Kaay J., Downes C.P., Smythe E. (2001). The role of dynamin and its binding partners in coated pit invagination and scission. J. Cell Biol. 152: 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Feijo J.A., Hackett G.R., Kunkel J.G., Hepler P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein S.E., Oliviusson P. (2005). Sequence analysis of Arabidopsis thaliana E/ANTH-domain-containing proteins: Membrane tethers of the clathrin-dependent vesicle budding machinery. Protoplasma 226: 13–21 [DOI] [PubMed] [Google Scholar]
- Hwang J.U., Vernoud V., Szumlanski A., Nielsen E., Yang Z. (2008). A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 18: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im Y.J., Davis A.J., Perera I.Y., Johannes E., Allen N.S., Boss W.F. (2007a). The N-terminal membrane occupation and recognition nexus domain of Arabidopsis phosphatidylinositol phosphate kinase 1 regulates enzyme activity. J. Biol. Chem. 282: 5443–5452 [DOI] [PubMed] [Google Scholar]
- Im Y.J., Perera I.Y., Brglez I., Davis A.J., Stevenson-Paulik J., Phillippy B.Q., Johannes E., Allen N.S., Boss W.F. (2007b). Increasing plasma membrane phosphatidylinositol(4,5)bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell 19: 1603–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T., Stenzel I., Heilmann I. (2008). Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 20: 3312–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Toret C.P., Drubin D.G. (2006). Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 7: 404–414 [DOI] [PubMed] [Google Scholar]
- Kang B.H., Busse J.S., Bednarek S.Y. (2003). Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15: 899–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.W., Park D.S., Park S.C., Kim S.H., Cheong G.W., Hwang I. (2001). Arabidopsis dynamin-like 2 that binds specifically to phosphatidylinositol 4-phosphate assembles into a high-molecular weight complex in vivo and in vitro. Plant Physiol. 127: 1243–1255 [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Friml J. (2008). Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 24: 447–473 [DOI] [PubMed] [Google Scholar]
- König S., Ischebeck T., Lerche J., Stenzel I., Heilmann I. (2008). Salt-stress-induced association of phosphatidylinositol 4,5-bisphosphate with clathrin-coated vesicles in plants. Biochem. J. 415: 387–399 [DOI] [PubMed] [Google Scholar]
- Kost B., Lemichez E., Spielhofer P., Hong Y., Tolias K., Carpenter C., Chua N.H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H., Testerink C., Vermeer J.E., Tsuge T., Shimada H., Oka A., Munnik T., Aoyama T. (2008). The Arabidopsis Phosphatidylinositol Phosphate 5-Kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Szumlanski A., Nielsen E., Yang Z. (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 181: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Yang Z. (2008). Tip growth: Signaling in the apical dome. Curr. Opin. Plant Biol. 11: 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lin Y., Heath R.M., Zhu M.X., Yang Z. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11: 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wang Y., Zhu J.K., Yang Z. (1996). Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8: 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Wong M.L., Craik C.S., Brodsky F.M. (1995). Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell 83: 257–267 [DOI] [PubMed] [Google Scholar]
- Monteiro D., Castanho Coelho P., Rodrigues C., Camacho L., Quader H., Malhó R. (2005a). Modulation of endocytosis in pollen tube growth by phosphoinositides and phospholipids. Protoplasma 226: 31–38 [DOI] [PubMed] [Google Scholar]
- Monteiro D., Liu Q., Lisboa S., Scherer G.E., Quader H., Malhó R. (2005b). Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J. Exp. Bot. 56: 1665–1674 [DOI] [PubMed] [Google Scholar]
- Moscatelli A., Ciampolini F., Rodighiero S., Onelli E., Cresti M., Santo N., Idilli A. (2007). Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 120: 3804–3819 [DOI] [PubMed] [Google Scholar]
- Mousavi S.A., Malerød L., Berg T., Kjeken R. (2004). Clathrin-dependent endocytosis. Biochem. J. 377: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Roeber B., Pical C. (2002). Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 130: 22–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C., Romanowsky S.M., Barron Y.D., Garg S., Azuse C.L., Curran A., Davis R.M., Hatton J., Harmon A.C., Harper J.F. (2009). Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 59: 528–539 [DOI] [PubMed] [Google Scholar]
- Perera R.M., Zoncu R., Lucast L., De Camilli P., Toomre D. (2006). Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc. Natl. Acad. Sci. USA 103: 19332–19337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss M.L., Schmitz A.J., Thole J.M., Bonner H.K., Otegui M.S., Nielsen E. (2006). A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 172: 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa E., Kost B., Malhó R. (2008). Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell 20: 3050–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I., Ischebeck T., König S., Hołubowska A., Sporysz M., Hause B., Heilmann I. (2008). The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 20: 124–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Carroll S., Kaksonen M., Toshima J.Y., Drubin D.G. (2007). PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J. Cell Biol. 177: 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Kaksonen M., Madden D.T., Schekman R., Drubin D.G. (2005). Interaction of Sla2p’s ANTH domain with PtdIns(4,5)P2 is important for actin-dependent endocytic internalization. Mol. Biol. Cell 16: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlanski A.L., Nielsen E. (2009). The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell 21: 526–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J.M., Vermeer J.E., Zhang Y., Gadella T.W., Jr, Nielsen E. (2008). Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell 20: 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer J.E., Thole J.M., Goedhart J., Nielsen E., Munnik T., Gadella T.W., Jr (2009). Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J. 57: 356–372 [DOI] [PubMed] [Google Scholar]
- Vidali L., McKenna S.T., Hepler P.K. (2001). Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 12: 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S., Bloch D., Sorek N., Kost B. (2008). Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 147: 1527–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2008). Cell polarity signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol. 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.L., Janmey P.A. (2003). Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65: 761–789 [DOI] [PubMed] [Google Scholar]
- Yoon G.M., Dowd P.E., Gilroy S., McCubbin A.G. (2006). Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18: 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R., Perera R.M., Sebastian R., Nakatsu F., Chen H., Balla T., Ayala G., Toomre D., De Camilli P.V. (2007). Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 104: 3793–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2008). Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J. Exp. Bot. 59: 861–873 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.