Abstract
In the late 1960’s numerous investigators independently demonstrated that platelets are capable of synthesizing proteins. Studies continued at a steady pace over the next 30 years and into the 21st century. Collectively, these investigations confirmed that platelets synthesize proteins and that the pattern of protein synthesis changes in response to cellular activation. More recent studies have characterized the mechanisms by which platelets synthesize proteins and have shown that protein synthesis alters the phenotype and functions of platelets. Here, we chronologically review our increased understanding of protein synthetic responses in platelets and discuss how the field may evolve over the next decade.
“Seek simplicity and distrust it”
Alfred North Whitehead“Is there any thing whereof it may be said, See, this is new?”
Ecclesiastes 1:10
I. Early Studies of Protein Synthesis by Platelets
The blood platelet was discovered by Bizzozero in the late 19th century [1, 2] and shortly thereafter Wright determined that megakaryocytes are platelet precursors [3]. The elegant studies of Bizzozero and Wright were critical for assigning hemostatic roles to platelets and understanding the roots of platelet production [1-5]. These early studies also described the essential anatomy of the blood platelet, which is unique and deceptively simple. One of the key features of platelets is that they circulate without a nucleus. Because they lack nuclei, platelets were considered incapable of regulated gene expression and protein synthesis.
In 1966, however, Andrew Warshaw and his colleagues [6] demonstrated that platelets incorporate 14C-leucine as they oxidize glucose. They also found that puromycin, a protein synthesis inhibitor that blocks mRNA translation [7], reduced incorporation of 14C-leucine providing the first suggestion that anucleate platelets synthesize protein. This initial study was not only compelling in regards to the synthetic potential of platelets, but the experiments were rigorously performed and thoughtfully interpreted. The investigators determined that the average erythrocyte and leukocyte contamination was less than 1 cell per 3,000 platelets, contributing no more than 0.1% of the observed radiochemical yield in their experiments [6]. Warshaw and his group were also among the first to study the functions of platelets for extended periods of time. The premise of their study was that clot retraction occurs over hours and requires energy derived from glucose. Using an in vitro model of clot retraction they determined that thrombin induces glucose oxidation in platelets that lasts for at least 8 hours and is lessened, but not obliterated, when protein synthesis is inhibited [7]. Just a little over 40 years later, we found that protein synthesis regulates platelet-dependent clot retraction, validating their initial results [8].
A year after their initial discovery Warshaw’s group conducted a more focused study that, in their view, constituted the first definitive demonstration that mammalian platelets synthesize protein [9]. At roughly the same time, Francois Booyse and Max Rafelson Jr. published two articles demonstrating that platelets incorporate amino acids into contractile proteins [10, 11]. These investigators also concluded that platelets use stable messenger RNA (mRNA) transcripts to synthesize protein and speculated that the stability of mRNAs directing protein synthesis may determine the lifespan of the platelet [11]. Numerous studies ensued over the next five years (1968-1973) addressing whether platelets synthesize protein [12-23]. Two independent groups developed cell-free systems to demonstrate that platelets contain ribosomes and other constituents necessary for protein synthesis [14, 21]. There was also considerable effort to separate platelets into different populations and to identify a relationship between age and synthetic potential. Data from these studies suggested that large platelets, which were presumably younger cells, have the greatest synthetic potential [15, 19] and ultrastructural analyses identified rough endoplasmic reticulum and ribosomes in platelets after the induction of thrombocytopenia [24].
II. The Next 30 Years Provided More Evidence that Platelets Synthesize Proteins
Although several studies independently concluded that platelets synthesize protein, the physiological significance of this process was not clear. There were questions regarding the magnitude of protein synthesis, the contributions of leukocyte contaminants, and whether protein production was confined to mitochondria [16, 25]. In addition, it was hard to envision how protein synthesis controlled the function of platelets especially since aggregation, which was avidly studied at that time, was considered a terminal event [25]. Thus, after the initial burst of studies in the late 1960’s published observations became less frequent, but nonetheless remained steady over the next 30 years.
A recurrent theme in the 1970’s and 1980’s was that investigators were able to reproducibly demonstrate that platelets incorporate amino acids into protein [26-40]. The source and specificity of protein synthesis was confirmed with several types of classic translation inhibitors [26, 27, 32, 33, 35] and one study demonstrated that an extract from oriental hornet venom blocked protein synthesis by platelets [34]. Protein synthesis by platelets was shown to be influenced when platelets were exposed to extracellular factors [30], cigarette smoke [40], or during phagocytosis of foreign particles [28]. Agam and colleagues [41] also observed that platelets transcribe RNA, confirming an earlier report of DNA-dependent synthesis of RNA in platelets [20], which presumably occurred in mitochondria. In addition, Agam’s group observed DNA synthesis in platelets [41], a finding that was subsequently confirmed by Gerald Soslau in 1983 [42].
For the majority of studies described above, the index for protein synthesis was incorporation of radiolabelled amino acids into trichloroacetic-acid-precipitable material. This allowed for global assessment of protein synthesis but did not identify the types of proteins synthesized by platelets. In 1987, however, Kieffer et al. [38] separated proteins by electrophoresis and demonstrated that several of the proteins stained by Coomassie blue in newly-formed platelets isolated from splenectomized patients with idiopathic thrombocytopenic purpura (ITP) also incorporated radiolabelled amino acids. The pattern of protein synthesis was nearly identical, but reduced, in circulating platelets isolated from normal subjects. Using crossed-immunoelectrophoresis Kieffer’s group concluded that platelets synthesize GPIb, αIIbβ3, fibrinogen, thrombospondin, albumin, von Willebrand factor, various contractile proteins, HLA and coagulation factor XIIIa. Shortly thereafter, Francis Belloc and colleagues [36] provided definitive evidence that platelets synthesize and assemble the different subunits of thrombospondin and fibrinogen. Their results also indicated that normal and Glanzmann’s Thrombastenia platelets retain the capacity to synthesize fibrinogen, but in thrombastenia there is a defect in storage of the newly synthesized protein [36]. Athough these studies demonstrated that platelets synthesize additional amounts of constitutively expressed proteins, the biologic significance of this response in regards to platelet function was not resolved.
In 1988 Peter Newman’s group used polymerase chain reaction (PCR) to demonstrate that messenger RNA (mRNA) resides in platelets [43]. Although presumed by other investigators, this pivotal result was the first definitive evidence that platelets expressed mRNA templates necessary for protein synthesis [43]. Santoso and associates [44] subsequently used this technology to demonstrate that platelets express mRNA for HLA class I. They also found that platelets translate this mRNA into protein [44], confirming the earlier predictions of Nelly Kieffer’s group that platelets synthesize HLA [38]. Two subsequent studies demonstrated that environmental signals facilitate translational responses in platelets. Singh and Kaul [45] found that exogenous cholesterol induced the synthesis of Receptor-Ck protein, which was associated with the messenger ribonucleoprotein (mRNP) pool of platelets [45]. In the same year, Lemaitre and colleagues [46] found that n-3 polyunsaturated fatty acids increased the expression of glutathione-dependent peroxidase (GPx) protein and activity in platelets, responses that were abolished by the translational inhibitor cycloheximide.
III. The Last Decade: What Has It Revealed Regarding Protein Synthesis by Platelets?
Surprisingly, nearly 40 peer-reviewed manuscripts were published between 1966 and 1997 that together demonstrated anucleate platelets synthesize protein. These studies provided the framework for investigations during the last decade, which identified new proteins under synthetic control and characterized previously-unrecognized mechanisms used by activated platelets to synthesize proteins.
In 1998, we found that activated platelets translate B-cell lymphoma 3 (Bcl-3) mRNA into protein [47]. After its synthesis, Bcl-3 binds the SH3 domain of Fyn and regulates platelet-dependent clot retraction [8, 47]. Bcl-3 synthesis is dramatically enhanced by engagement of αIIbβ3 integrins, which regulate the intracellular distribution of mRNAs and the mRNA-binding protein eukaryotic initiation factor 4E (eIF4E) within platelets [48, 49]. Bcl-3 synthesis also requires signaling through the mammalian Target of Rapamycin (mTOR) [8, 47, 49]. Platelets express high levels of mTOR and each of its downstream targets, S6K1 and 4E-BP1 (eIF4E-binding protein-1) [8, 48]. The mTOR inhibitor, rapamycin, specifically blocks activation-dependent phosphorylation of S6K1 and 4E-BP1 and as a result controls the translation of a subset of mRNAs in platelets that include Bcl-3 [8, 48]. Studies from Evangelista and colleagues [50] also imply that mTOR controls de novo synthesis of cyclooxygenase-1 (COX-1) in platelets.
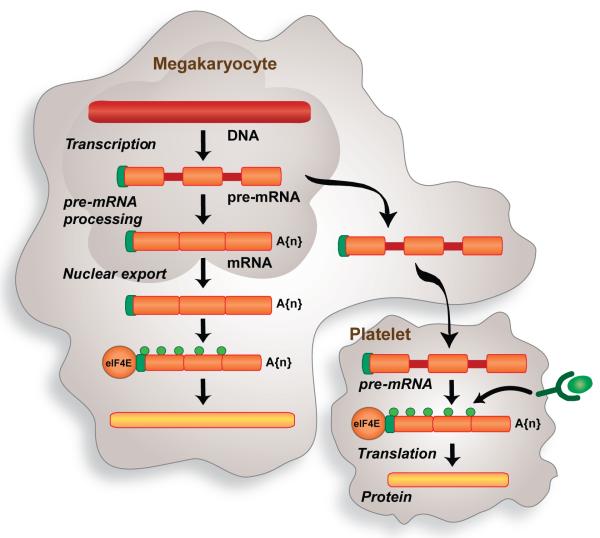
Earlier studies from independent laboratories demonstrated that activated, but not resting, platelets release and/or express interleukin-1β (IL-1β) [51-54]. This led us to the discovery that platelets synthesize IL-1β protein [55]. Anucleate platelets regulate IL-1β synthesis by splicing pre-mRNA [56], a novel mechanism of post-transcriptional signaling that has subsequently been observed in the cytoplasm of dendrites and proliferating fibroblasts [57, 58]. Spliceosome factors and IL-1β pre-mRNA are transferred from megakaryocytes to platelets (figure 1). Thrombin, lipopolysaccharide or clustering of FcαR1 induces IL-1β pre-mRNA splicing in platelets [56, 59, 60]. The mature transcript is subsequently translated into IL-1β protein [55, 56, 59, 60], which increases the adhesiveness of endothelial cells for polymorphonuclear leukocytes [55]. The majority of newly-synthesized IL-1β is retained in the platelet [55], a finding that was recently confirmed by Shashkin and colleagues [60] in platelet preparations that were filtered through a 5-μm mesh and then passed twice through columns to deplete CD14, CD15 and CD45 positive leukocytes. The tendency of platelets to accumulate intracellular IL-1β, rather than actively secrete it, may partially explain why a recent report did not detect mature IL-1β protein in platelet releasates [61].
Figure 1. Signal-dependent pre-mRNA splicing in platelets.
During the final stages of differentiation, splicing factors accumulate in the cytoplasm of megakaryocytes. The splicing components, along with specific pre-mRNAs, are transferred to anucleate platelets. Stimulated platelets activate their splicing machinery and generate mature mRNAs that are subsequently translated into protein. This novel pathway of control provides platelets with a mechanism to alter their transcriptome and proteome profile in response to cellular activation.
Activation-dependent splicing of IL-1β pre-mRNA sparked the search for other candidates, yielding tissue factor (TF) as a gene under a similar type of regulatory control [62]. TF is a critical procoagulant protein that is involved in the initiation and propagation stages of clot formation [63]. TF pre-mRNA splicing is controlled by Cdc2-like kinase 1 (Clk1), which induces phosphorylation of splicing factor 2 (SF2) in platelets [62]. Inhibition of Clk1 in platelets prevents TF pre-mRNA from being spliced and as a consequence, delays the onset of plasma clot formation [62]. A recent independent report demonstrates that ligation of the human IgA receptor FcαR1 on platelets induces TF pre-mRNA splicing and protein production [59]. Panes and colleagues [64] also observed varying expression of mature TF mRNA in platelets and confirmed that activated human platelets synthesize TF protein. The contribution of platelet-derived TF to in vivo clot formation and resolution, however, is not yet known [65].
Several independent investigations have also reported that platelets synthesize other proteins. Brogren and colleagues [66] recently demonstrated that platelets constitutively synthesize plasminogen activator inhibitor-1 (PAI-1), a response that is markedly increased in the presence of thrombin. A follow-up study from the same group suggests that PAI-1 synthesis by platelets is the primary source of plasma PAI because platelet-derived and plasma PAI-1 possess identical glycosylation patterns [67]. In addition, the authors demonstrated that the amount of PAI synthesized in vitro by platelets far exceeds what would be required to maintain normal plasma levels of PAI-1 [66, 67]. Platelets also compensate for fluctuations in intracellular ascorbate levels by modulating the expression of the Na+-dependent transporter SVCT2 at the translational level [68]. Depletion of ascorbic acid levels enhances SVCT2 synthesis by platelets and markedly reduces thrombus rigidity [68]. Evangelista and colleagues [50] also found that aspirin-treated platelets recover their capacity to generate thromboxane A2 through mechanisms that involve de novo synthesis of COX-1. COX-2 pre-mRNA splicing and expression of its protein has likewise been reported in platelets, but the significance of COX-2 protein in regards to arachidonate metabolism has not been established [60]. Circumstantial evidence also suggests that translational control mechanisms regulate tetrahydrobiopterin biosynthesis in platelets [69] and metabolic labeling experiments indicate that activated platelets synthesize many additional proteins, most of which are yet to be identified [47, 55, 70].
IV. Moving Forward on the Protein Synthetic Front
The findings to date demonstrate that platelets have developed extranuclear mechanisms to process and efficiently translate mRNAs into protein. Specialized post-transcriptional and translational mechanisms are now considered part of the platelet functional repertoire. It is also likely that additional new pathways and mechanisms that regulate protein synthesis will be discovered in the near future. Among these are cytoplasmic polyadenylation elements (CPEs), nucleotide sequences embedded in the 3′-untranslated region (3′-UTR) that facilitate translation by extending the length of the poly(A)-tail [71]. Comprehensive SAGE (serial analysis of gene expression) indicates that the 3′-UTRs of platelet mRNAs tend to be longer and more complex when compared to mRNAs of nucleated cells [72]. Platelet-derived mRNAs are also enriched for CPEs and Dittrich and colleagues [72] have identified several highly expressed transcripts with CPE elements whose corresponding proteins have not been previously identified or characterized in platelets. CPE-like elements are present in the 3′-UTR of inducible poly(A)-binding protein (iPABP), a protein that was previously detected in activated platelets by Houng and colleagues [73].
Other control elements, such as AU-rich sequences and Brd boxes, are also commonplace in platelet mRNAs [72]. Given the complexity, stability and abundance (~4000-6000 transcripts) of their transcriptome [55, 72, 74-78], it is attractive to predict that platelets use a variety of translational and post-translational mechanisms to fine-tune their protein profile once they enter the circulation. Our group has focused on signal-dependent translation [7, 79], but there is also recent evidence that constitutive protein synthesis occurs in platelets. Rosenwald and colleagues [80] found that platelets constitutively express eIF4E and also eIF-2α, a rate-limiting protein responsible for the transfer of initiator methionyl-tRNA to the 40S subunit, and that cooperatively these two translation initiation factors facilitate continual protein synthesis in stored platelets. A recent study by Thon and Devine [81] clearly demonstrates that platelets use their translational apparatus to synthesize integrin αIIbβ3 during storage. Full-length mRNA for αIIbβ3 was present throughout a 10-day storage period and translation of the message into protein was demonstrated by incorporation of [35S]-methionine into sequence-confirmed immunoprecipitated αIIbβ3 protein. These studies [80, 81], along with previous ones [36, 38], demonstrate that platelets have the capacity to continually synthesize proteins during their short lifespan. Whether or not constitutive protein synthesis is required to maintain threshold concentrations of essential platelet proteins, however, is not known.
Protein synthesis by platelets was described over 40 years ago, a mechanism of control that has been advanced by our group and others. Platelets synthesize numerous proteins, and several lines of evidence indicate that others will be discovered in the future [79]. There is also much to be learned regarding signaling intermediates that control pre-mRNA splicing, mRNA translation and the biologic significance of protein synthesis in this anucleate cell. Questions of this nature will occupy investigators for years to come and will undoubtedly reveal fascinating new discoveries about platelets, an anucleate cell that continues to defy conventional logic and preconceived notions.
Acknowledgements
The authors thank Diana Lim for preparing the figures and the trainees and collaborators who contributed to work cited in this review. We are also indebted to the funding agencies that have supported our work over the years, especially the American Heart Association, Deutsche Forschungsgemeinschaft, and the National Institutes of Health.
References
- 1.Bizzozero G. Su di un nuovo elemento morfologico del sangue dei mammiferi e della sua importanza nella trombosi e nella coagulazione. L’Osservatore. 1881;17:785–7. [Google Scholar]
- 2.Bizzozero G. Ueber einen neuen Formbestandtheil des Blutes und dessen Rolle bei der Thrombose und der Blutgerinnung. Virchows Arch Pathol Anat Physiol. 1882;90:261–332. [Google Scholar]
- 3.Wright JH. The origin and nature of blood plates. Boston Med Surg J. 1906;154:643–5. [Google Scholar]
- 4.Coller BS. A Brief History of Ideas about Platelets in Health and Disease. In: Michelson AD, editor. Platelets. 2nd edn Academic Press: An Imprint of Elsevier Science; San Diego: 2007. pp. xxiii–xlii. [Google Scholar]
- 5.Wright J. The histogenesis of the blood platelets. Journal of Morphology. 1910;21:263. [Google Scholar]
- 6.Warshaw AL, Laster L, Shulman NR. The stimulation by thrombin of glucose oxidation in human platelets. The Journal of clinical investigation. 1966;45:1923–34. doi: 10.1172/JCI105497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyrich AS, Lindemann S, Tolley ND, Kraiss LW, Dixon DA, Mahoney TM, Prescott SM, McIntyre TM, Zimmerman GA. Change in protein phenotype without a nucleus: translational control in platelets. Seminars in Thrombosis and Hemostasis. 2004;30:493–500. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- 8.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshaw AL, Laster L, Shulman NR. Protein synthesis by human platelets. The Journal of biological chemistry. 1967;242:2094–7. [PubMed] [Google Scholar]
- 10.Booyse F, Rafelson ME., Jr. In vitro incorporation of amino-acids into the contractile protein of human blood platelets. Nature. 1967;215:283–4. doi: 10.1038/215283a0. [DOI] [PubMed] [Google Scholar]
- 11.Booyse FM, Rafelson ME., Jr. Stable messenger RNA in the synthesis of contractile protein in human platelets. Biochim Biophys Acta. 1967;145:188–90. doi: 10.1016/0005-2787(67)90673-9. [DOI] [PubMed] [Google Scholar]
- 12.FaRJ M.E. Booyse. Protein synthesis and platelet function. In: SAaG M. Johnson., editor. Dynamics of thrombus formation and dissolution. J.B. Lippencott and Co.; Philadelphia: 1969. p. 149. [Google Scholar]
- 13.Booyse FM, Hoveke TP, Rafelson ME. Studies on human platelets. II. Protein synthetic activity of various platelet populations. Biochim Biophys Acta. 1968;157:660–3. [PubMed] [Google Scholar]
- 14.Booyse FM, Rafelson ME., Jr. Studies on human platelets. I. synthesis of platelet protein in a cell-free system. Biochim Biophys Acta. 1968;166:689–97. doi: 10.1016/0005-2787(68)90376-6. [DOI] [PubMed] [Google Scholar]
- 15.Booyse FM, Zschocke D, Hoveke TP, Rafelson ME. Studies on human platelets. IV. Protein synthesis in maturing human platelets. Thromb Diath Haemorrh. 1971;26:167–76. [PubMed] [Google Scholar]
- 16.Boullin DJ, Votavova M, Green AR. Protein synthesis by human blood platelets after accumulation of leucine and arginine. Thromb Diath Haemorrh. 1972;28:54–64. [PubMed] [Google Scholar]
- 17.Freedman ML, Karpatkin S. Requirement of iron for platelet protein synthesis. Biochemical and biophysical research communications. 1973;54:475–81. doi: 10.1016/0006-291x(73)91445-9. 0006-291X(73)91445-9 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Hirsh J. Platelet age: its relationship to platelet size, function and metabolism. British journal of haematology. 1972;23(Suppl):209–14. doi: 10.1111/j.1365-2141.1972.tb03520.x. [DOI] [PubMed] [Google Scholar]
- 19.Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. The Journal of clinical investigation. 1969;48:1073–82. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider W, Dries R, Kulenkampff G. Studies on the protein and nucleic acid synthesis of normal human blood platelets. Acta Univ Carol Med Monogr. 1972;53:113–7. [PubMed] [Google Scholar]
- 21.Steiner M. Platelet protein synthesis studied in a cell-free system. Experientia. 1970;26:786–9. doi: 10.1007/BF02232552. [DOI] [PubMed] [Google Scholar]
- 22.Steiner M, Baldini M. Protein synthesis in aging blood platelets. Blood. 1969;33:628–33. [PubMed] [Google Scholar]
- 23.Schneider W, Lampe K, Scheurlen PG. Studies on protein synthesis of human blood platelets. Verh Dtsch Ges Inn Med. 1971;77:400–3. [PubMed] [Google Scholar]
- 24.Ts’ao CH. Rough endoplasmic reticulum and ribosomes in blood platelets. Scand J Haematol. 1971;8:134–40. doi: 10.1111/j.1600-0609.1971.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 25.Michal F, Firkin BG. Physiological and pharmacological aspects of the platelet. Annu Rev Pharmacol. 1969;9:95–118. doi: 10.1146/annurev.pa.09.040169.000523. 10.1146/annurev.pa.09.040169.000523. [DOI] [PubMed] [Google Scholar]
- 26.Agam G, Gasner S, Bessler H, Fishman P, Djaldetti M. Chloramphenicol induced inhibition of platelet protein synthesis: in vitro and in vivo studies. British journal of haematology. 1976;33:53–9. doi: 10.1111/j.1365-2141.1976.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 27.Agam G, Djaldetti M. Inhibition of human platelet mitochondrial protein synthesis by chloramphenicol. Biomedicine. 1977;27:66–9. [PubMed] [Google Scholar]
- 28.Bessler H, Agam G, Djaldetti M. Increased protein synthesis by human platelets during phagocytosis of latex particles in vitro. Thrombosis and haemostasis. 1976;35:350–7. [PubMed] [Google Scholar]
- 29.Malik Z, Agam G, Djaldetti M. Effect of hemin and Protoporphyrin IX on the protein-synthesizing activity of human granulocytes, lymphocytes and platelets. Acta Haematol. 1979;61:138–43. doi: 10.1159/000207646. [DOI] [PubMed] [Google Scholar]
- 30.Plow EF. Extracellular factors influencing the in vitro protein synthesis of platelets. Thrombosis and haemostasis. 1979;42:666–78. [PubMed] [Google Scholar]
- 31.Fishman P, Levi J, Notti I, Djaldetti M. Synthesizing activity and ultrastructural findings of human blood cells after incubation with cis-platinum “in vitro”. Biomedicine. 1981;34:78–82. [PubMed] [Google Scholar]
- 32.Soslau G, Rybicki A. In vitro incorporation of fucose and methionine into human platelet proteins. Biochemical and biophysical research communications. 1982;109:1256–63. doi: 10.1016/0006-291x(82)91912-x. 0006-291X(82)91912-X [pii] [DOI] [PubMed] [Google Scholar]
- 33.Shaw T, Chesterman CN, Morgan FJ. In vitro synthesis of low molecular weight proteins in human platelets: absence of labelled release products. Thrombosis research. 1984;36:619–31. doi: 10.1016/0049-3848(84)90201-9. [DOI] [PubMed] [Google Scholar]
- 34.Friedman J, Ishay JS. Inhibition of protein synthesis by an extract of the venom sac of the oriental hornet (Vespa orientalis) Toxicon. 1987;25:673–6. doi: 10.1016/0041-0101(87)90114-0. 0041-0101(87)90114-0 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Bruce IJ, Kerry R. The effect of chloramphenicol and cycloheximide on platelet aggregation and protein synthesis. Biochem Pharmacol. 1987;36:1769–73. doi: 10.1016/0006-2952(87)90236-x. 0006-2952(87)90236-X [pii] [DOI] [PubMed] [Google Scholar]
- 36.Belloc F, Heilmann E, Combrie R, Boisseau MR, Nurden AT. Protein synthesis and storage in human platelets: a defective storage of fibrinogen in platelets in Glanzmann’s thrombasthenia. Biochim Biophys Acta. 1987;925:218–25. doi: 10.1016/0304-4165(87)90112-7. [DOI] [PubMed] [Google Scholar]
- 37.Belloc F, Hourdille P, Boisseau MR, Bernard P. Protein synthesis in human platelets correlation with platelet size. Nouv Rev Fr Hematol. 1982;24:369–73. [PubMed] [Google Scholar]
- 38.Kieffer N, Guichard J, Farcet JP, Vainchenker W, Breton-Gorius J. Biosynthesis of major platelet proteins in human blood platelets. Eur J Biochem. 1987;164:189–95. doi: 10.1111/j.1432-1033.1987.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 39.Ludany A, Kellermayer M. Protein synthesis in human platelets. Clin Biochem. 1988;21:107–10. doi: 10.1016/s0009-9120(88)80097-3. [DOI] [PubMed] [Google Scholar]
- 40.Djaldetti M, Agam G, Creter D. Effect of cigarette smoking on platelet function. Haematologica. 1977;62:575–80. [PubMed] [Google Scholar]
- 41.Agam G, Bessler H, Djaldetti M. In vitro DNA and RNA synthesis by human platelets. Biochim Biophys Acta. 1976;425:41–8. doi: 10.1016/0005-2787(76)90214-8. [DOI] [PubMed] [Google Scholar]
- 42.Soslau G. De novo synthesis of DNA in human platelets. Arch Biochem Biophys. 1983;226:252–6. doi: 10.1016/0003-9861(83)90291-6. [DOI] [PubMed] [Google Scholar]
- 43.Newman PJ, Gorski J, White GC, 2nd, Gidwitz S, Cretney CJ, Aster RH. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. The Journal of clinical investigation. 1988;82:739–43. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoso S, Kalb R, Kiefel V, Mueller-Eckhardt C. The presence of messenger RNA for HLA class I in human platelets and its capability for protein biosynthesis. British journal of haematology. 1993;84:451–6. doi: 10.1111/j.1365-2141.1993.tb03100.x. [DOI] [PubMed] [Google Scholar]
- 45.Singh J, Kaul D. RNA-mediated regulation of Receptor-Ck gene in human platelets. Mol Cell Biochem. 1997;173:189–92. doi: 10.1023/a:1006872904778. [DOI] [PubMed] [Google Scholar]
- 46.Lemaitre D, Vericel E, Polette A, Lagarde M. Effects of fatty acids on human platelet glutathione peroxidase: possible role of oxidative stress. Biochem Pharmacol. 1997;53:479–86. doi: 10.1016/s0006-2952(96)00734-4. [DOI] [PubMed] [Google Scholar]
- 47.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5556–61. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindemann S, Tolley ND, Eyre JR, Kraiss LW, Mahoney TM, Weyrich AS. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. The Journal of biological chemistry. 2001;276:33947–51. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- 49.Pabla R, Weyrich AS, Dixon DA, Bray PF, McIntyre TM, Prescott SM, Zimmerman GA. Integrin-dependent control of translation: engagement of integrin alphaIIbbeta3 regulates synthesis of proteins in activated human platelets. The Journal of cell biology. 1999;144:175–84. doi: 10.1083/jcb.144.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evangelista V, Manarini S, Di Santo A, Capone ML, Ricciotti E, Di Francesco L, Tacconelli S, Sacchetti A, D’Angelo S, Scilimati A, Sciulli MG, Patrignani P. De novo synthesis of cyclooxygenase-1 counteracts the suppression of platelet thromboxane biosynthesis by aspirin. Circulation research. 2006;98:593–5. doi: 10.1161/01.RES.0000214553.37930.3e. [DOI] [PubMed] [Google Scholar]
- 51.Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 1991;174:785–90. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawrylowicz CM, Santoro SA, Platt FM, Unanue ER. Activated platelets express IL-1 activity. J Immunol. 1989;143:4015–8. [PubMed] [Google Scholar]
- 53.Kaplanski G, Porat R, Aiura K, Erban JK, Gelfand JA, Dinarello CA. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993;81:2492–5. [PubMed] [Google Scholar]
- 54.Loppnow H, Bil R, Hirt S, Schonbeck U, Herzberg M, Werdan K, Rietschel ET, Brandt E, Flad HD. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood. 1998;91:134–41. [PubMed] [Google Scholar]
- 55.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. The Journal of cell biology. 2001;154:485–90. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glanzer J, Miyashiro KY, Sul JY, Barrett L, Belt B, Haydon P, Eberwine J. RNA splicing capability of live neuronal dendrites. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16859–64. doi: 10.1073/pnas.0503783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konig H, Matter N, Bader R, Thiele W, Muller F. Splicing segregation: the minor spliceosome acts outside the nucleus and controls cell proliferation. Cell. 2007;131:718–29. doi: 10.1016/j.cell.2007.09.043. S0092-8674(07)01279-2 [pii] 10.1016/j.cell.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 59.Qian K, Xie F, Gibson AW, Edberg JC, Kimberly RP, Wu J. Functional expression of IgA receptor Fc{alpha}RI on human platelets. Journal of leukocyte biology. 2008 doi: 10.1189/jlb.0508327. jlb.0508327 [pii] 10.1189/jlb.0508327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shashkin PN, Brown GT, Ghosh A, Marathe GK, McIntyre TM. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–502. doi: 10.4049/jimmunol.181.5.3495. 181/5/3495 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pillitteri D, Bassus S, Boller K, Mahnel R, Scholz T, Westrup D, Wegert W, Kirchmaier CM. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18:119–27. doi: 10.1080/09537100600800792. 772843567 [pii] 10.1080/09537100600800792. [DOI] [PubMed] [Google Scholar]
- 62.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenecity of human platelets. J Exp Med. 2006;203:2433–40. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. blood-2006-06-030619 [pii] 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 65.Weyrich A, Schwertz H, Mackman N. Platelet Tissue Factor Comes of Age. Blood. 2007;109:5069–70. [Google Scholar]
- 66.Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–8. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
- 67.Brogren H, Sihlbom C, Wallmark K, Lonn M, Deinum J, Karlsson L, Jern S. Heterogeneous glycosylation patterns of human PAI-1 may reveal its cellular origin. Thrombosis research. 2008;122:271–81. doi: 10.1016/j.thromres.2008.04.008. S0049-3848(08)00132-1 [pii] 10.1016/j.thromres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Savini I, Catani MV, Arnone R, Rossi A, Frega G, Del Principe D, Avigliano L. Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic Biol Med. 2007;42:608–16. doi: 10.1016/j.freeradbiomed.2006.11.028. S0891-5849(06)00766-0 [pii] 10.1016/j.freeradbiomed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 69.Franscini N, Bachli EB, Blau N, Fischler M, Walter RB, Schaffner A, Schoedon G. Functional tetrahydrobiopterin synthesis in human platelets. Circulation. 2004;110:186–92. doi: 10.1161/01.CIR.0000134281.82972.57. [DOI] [PubMed] [Google Scholar]
- 70.Lindemann S, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Platelet Signal-Dependent Protein Synthesis. In: DJaQ M. Fitzgerald., editor. Platelet Function: Assessment, Diagnosis, and Treatment. The Humana Press Inc.; Totowa: 2005. pp. 151–76. [Google Scholar]
- 71.Richter JD. Cytoplasmic polyadenylation in development and beyond. Microbiol Mol Biol Rev. 1999;63:446–56. doi: 10.1128/mmbr.63.2.446-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dittrich M, Birschmann I, Pfrang J, Herterich S, Smolenski A, Walter U, Dandekar T. Analysis of SAGE data in human platelets: features of the transcriptome in an anucleate cell. Thrombosis and haemostasis. 2006;95:643–51. [PubMed] [Google Scholar]
- 73.Houng AK, Maggini L, Clement CY, Reed GL. Identification and structure of activated-platelet protein-1, a protein with RNA-binding domain motifs that is expressed by activated platelets. Eur J Biochem. 1997;243:209–18. doi: 10.1111/j.1432-1033.1997.0209a.x. [DOI] [PubMed] [Google Scholar]
- 74.Bugert P, Dugrillon A, Gunaydin A, Eichler H, Kluter H. Messenger RNA profiling of human platelets by microarray hybridization. Thrombosis and haemostasis. 2003;90:738–48. [PubMed] [Google Scholar]
- 75.Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–93. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 76.Macaulay IC, Carr P, Farrugia R, Watkins NA. Analysing the platelet transcriptome. Vox Sang. 2004;87(Suppl 2):42–6. doi: 10.1111/j.1741-6892.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 77.Macaulay IC, Carr P, Gusnanto A, Ouwehand WH, Fitzgerald D, Watkins NA. Platelet genomics and proteomics in human health and disease. The Journal of clinical investigation. 2005;115:3370–7. doi: 10.1172/JCI26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McRedmond JP, Park SD, Reilly DF, Coppinger JA, Maguire PB, Shields DC, Fitzgerald DJ. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol Cell Proteomics. 2004;3:133–44. doi: 10.1074/mcp.M300063-MCP200. [DOI] [PubMed] [Google Scholar]
- 79.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:s17–24. doi: 10.1161/ATVBAHA.107.160218. 28/3/s17 [pii] 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenwald IB, Pechet L, Han A, Lu L, Pihan G, Woda B, Chen JJ, Szymanski I. Expression of translation initiation factors elF-4E and elF-2alpha and a potential physiologic role of continuous protein synthesis in human platelets. Thrombosis and haemostasis. 2001;85:142–51. [PubMed] [Google Scholar]
- 81.Thon JN, Devine DV. Translation of glycoprotein IIIa in stored blood platelets. Transfusion. 2007;47:2260–70. doi: 10.1111/j.1537-2995.2007.01455.x. TRF01455 [pii] 10.1111/j.1537-2995.2007.01455.x. [DOI] [PubMed] [Google Scholar]