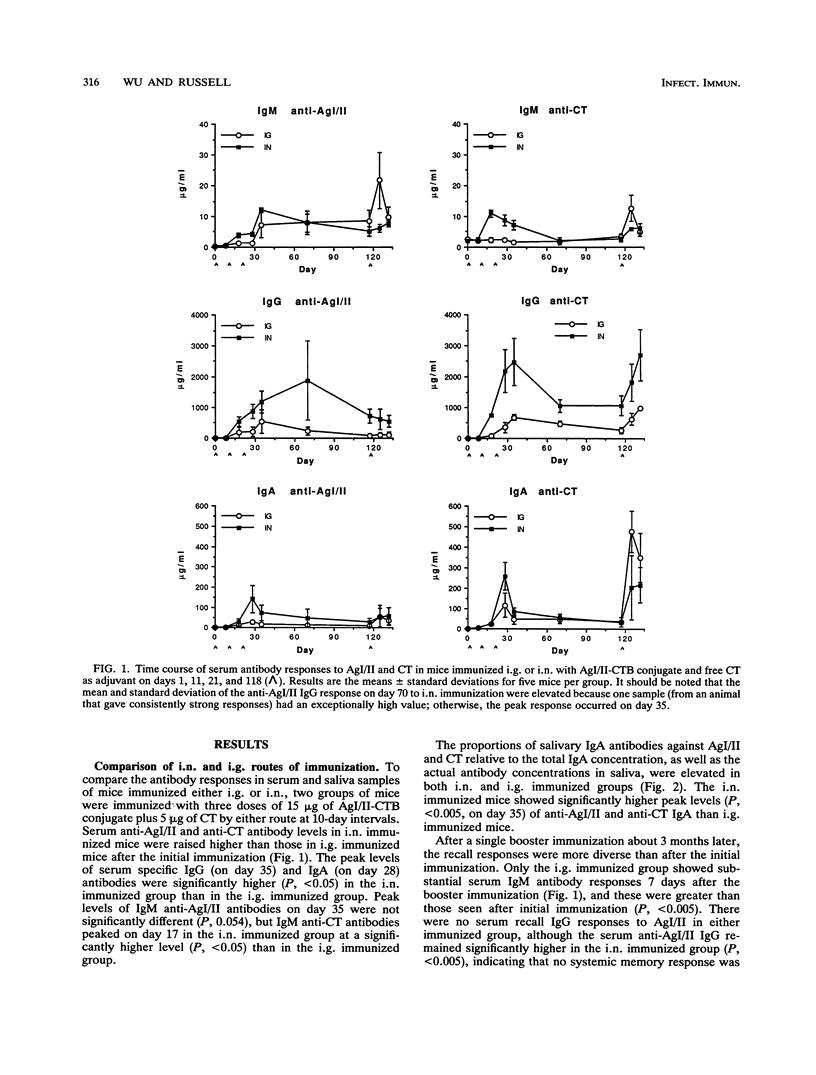
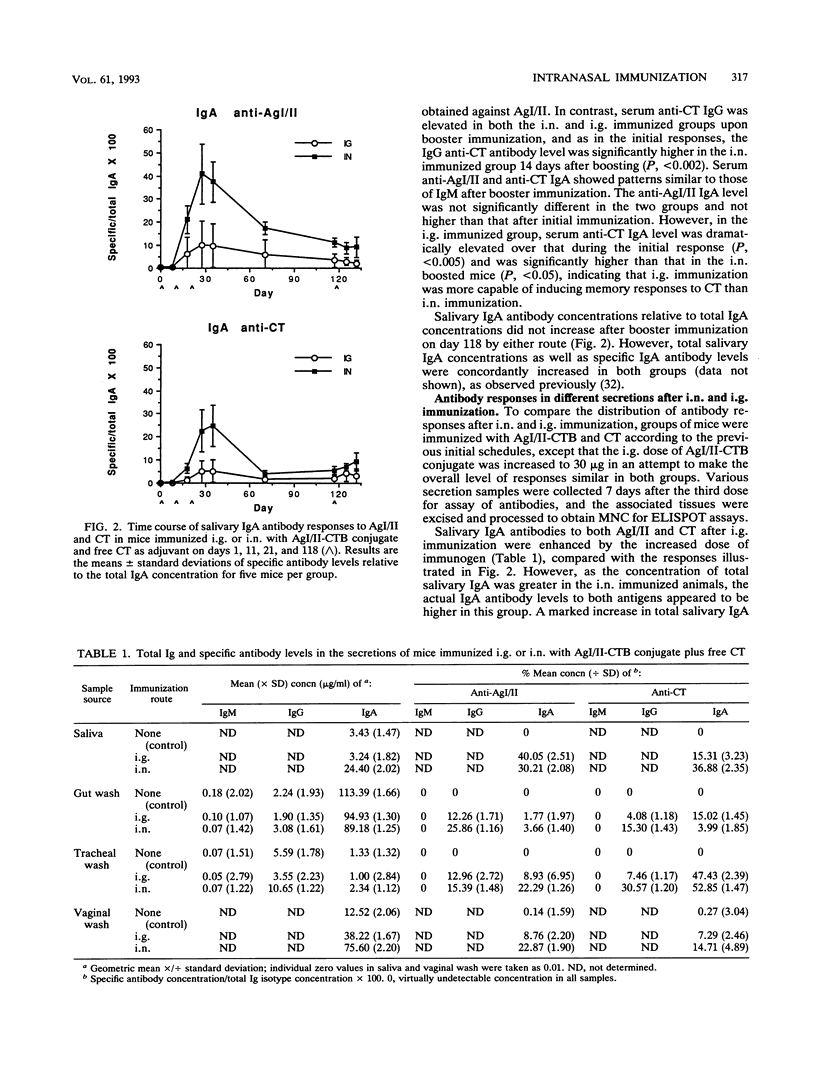
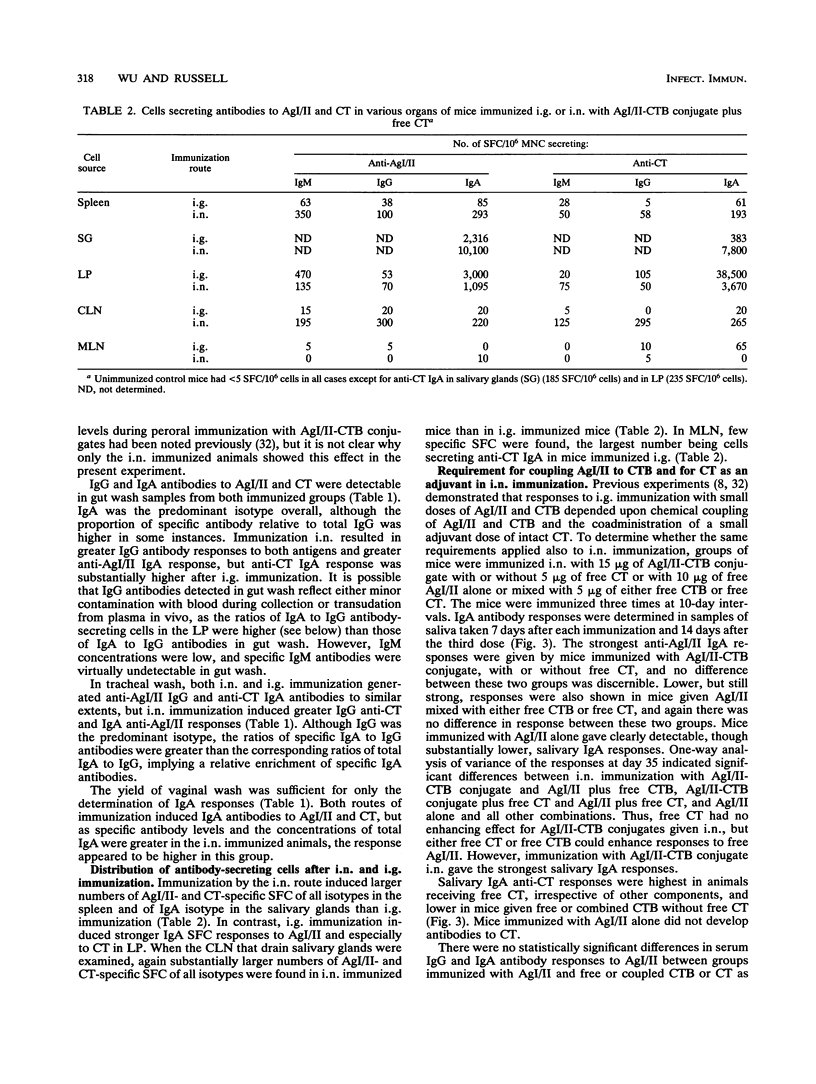
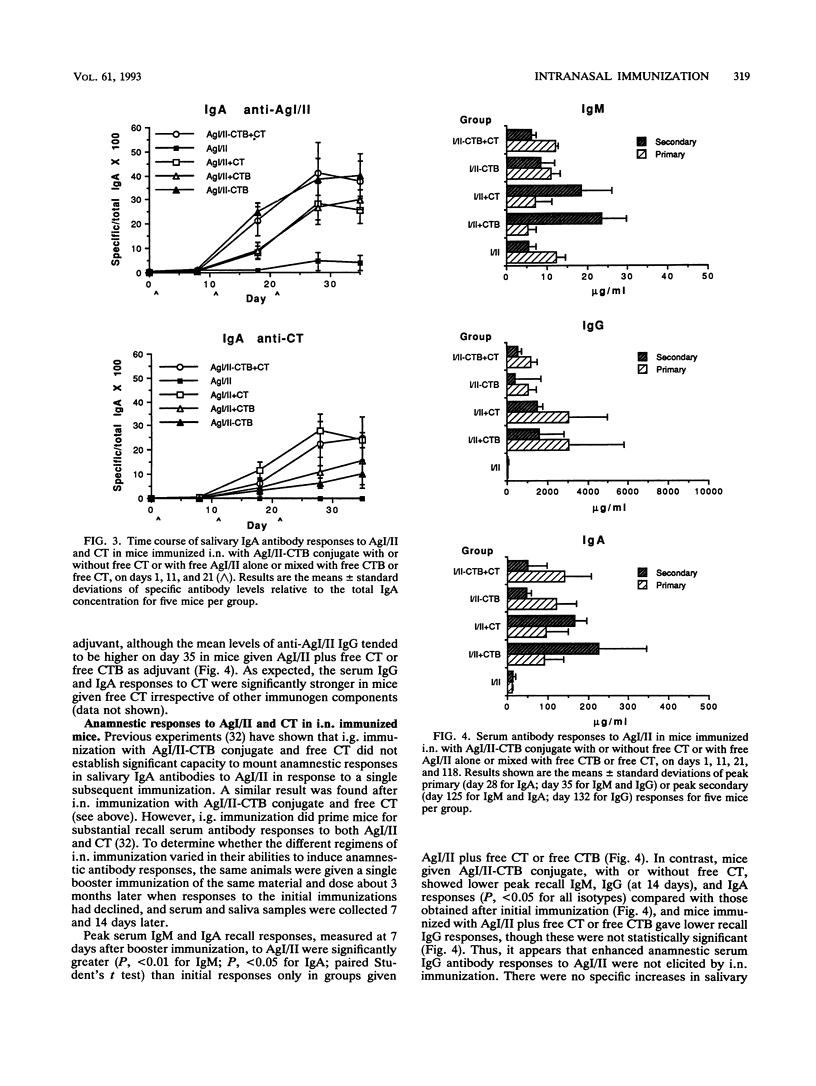
Abstract
The level and distribution of isotype-specific antibodies in various secretions and of antibody-secreting cells in corresponding lymphoid organs and tissues were compared in mice immunized with Streptococcus mutans surface protein antigen I/II (AgI/II) conjugated to the cholera toxin B subunit (CTB), given intranasally (i.n.) or intragastrically (i.g.), with or without free cholera toxin (CT) as an adjuvant. Immunization i.n. induced stronger initial antibody responses to AgI/II in both serum and saliva than immunization i.g., but salivary immunoglobulin A (IgA)-specific antibody responses to immunization about 3 months later were not increased relative to total salivary IgA concentrations. Specific antibodies induced by i.n. immunization were as widely distributed in serum, saliva, tracheal wash, gut wash, and vaginal wash as those induced by i.g. immunization. Likewise, specific antibody-secreting cells were generated in the spleen, salivary glands, intestinal lamina propria, and mesenteric and cervical lymph nodes by either route of immunization. The strongest salivary IgA antibody response was induced by AgI/II-CTB conjugate given i.n., but the addition of CT did not further enhance it. However, free CTB could effectively replace CT as an adjuvant in i.n. immunization with unconjugated AgI/II. Booster i.n. immunization with AgI/II plus either free CT or CTB induced stronger recall serum antibody responses than conjugated AgI/II-CTB with or without CT as an adjuvant. Therefore, i.n. immunization with a protein antigen and free or coupled CTB is an effective means of generating IgA antibody responses expressed at several mucosal sites where protective immunity may be beneficial.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A. T., Zwart R. J., Van der Heijden P. J. Induction of an enteric Ig-response against ovalbumin and stimulation of the response by cholera toxin and its B-subunit in mice. Reg Immunol. 1990;3(3):131–138. [PubMed] [Google Scholar]
- Bolduc C., Waterfield J. D., Deslauriers N. Tissue distribution and cytofluorometric analysis of oral mucosal T cells in the BALB/c mouse. Res Immunol. 1990 Jul-Aug;141(6):461–475. doi: 10.1016/0923-2494(90)90016-r. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Sollid L. M., Thrane P. S., Kvale D., Bjerke K., Scott H., Kett K., Rognum T. O. Lymphoepithelial interactions in the mucosal immune system. Gut. 1988 Aug;29(8):1116–1130. doi: 10.1136/gut.29.8.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromander A., Holmgren J., Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991 May 1;146(9):2908–2914. [PubMed] [Google Scholar]
- Chen K. S., Strober W. Cholera holotoxin and its B subunit enhance Peyer's patch B cell responses induced by orally administered influenza virus: disproportionate cholera toxin enhancement of the IgA B cell response. Eur J Immunol. 1990 Feb;20(2):433–436. doi: 10.1002/eji.1830200230. [DOI] [PubMed] [Google Scholar]
- Clarke C. J., Wilson A. D., Williams N. A., Stokes C. R. Mucosal priming of T-lymphocyte responses to fed protein antigens using cholera toxin as an adjuvant. Immunology. 1991 Mar;72(3):323–328. [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh M. T., Peterson D. L., Macrina F. L. Cholera toxin B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect Immun. 1990 Jan;58(1):70–79. doi: 10.1128/iai.58.1.70-79.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers N., Néron S., Mourad W. Immunobiology of the oral mucosa in the mouse. Immunology. 1985 Jul;55(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O. Cholera toxin and its subunits as potential oral adjuvants. Curr Top Microbiol Immunol. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- Elson C. O. Cholera toxin as a mucosal adjuvant: effects of H-2 major histocompatibility complex and lps genes. Infect Immun. 1992 Jul;60(7):2874–2879. doi: 10.1128/iai.60.7.2874-2879.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Francis M. L., Ryan J., Jobling M. G., Holmes R. K., Moss J., Mond J. J. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J Immunol. 1992 Apr 1;148(7):1999–2005. [PubMed] [Google Scholar]
- Gizurarson S., Tamura S., Aizawa C., Kurata T. Stimulation of the transepithelial flux of influenza HA vaccine by cholera toxin B subunit. Vaccine. 1992;10(2):101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Kurata H., Funato H., Nagamine T., Aizawa C., Tamura S., Shimada K., Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990 Jun;8(3):243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Tamura S. I., Shimada K., Kurata T. Involvement of antigen-presenting cells in the enhancement of the in vitro antibody responses by cholera toxin B subunit. Immunology. 1992 Mar;75(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Koornstra P. J., de Jong F. I., Vlek L. F., Marres E. H., van Breda Vriesman P. J. The Waldeyer ring equivalent in the rat. A model for analysis of oronasopharyngeal immune responses. Acta Otolaryngol. 1991;111(3):591–599. doi: 10.3109/00016489109138388. [DOI] [PubMed] [Google Scholar]
- Kuper C. F., Koornstra P. J., Hameleers D. M., Biewenga J., Spit B. J., Duijvestijn A. M., van Breda Vriesman P. J., Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992 Jun;13(6):219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Lycke N. A sensitive method for the detection of specific antibody production in different isotypes from single lamina propria plasma cells. Scand J Immunol. 1986 Oct;24(4):393–403. doi: 10.1111/j.1365-3083.1986.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Bromander A. K., Ekman L., Karlsson U., Holmgren J. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. J Immunol. 1989 Jan 1;142(1):20–27. [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986 May;23(5):611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Karlsson U., Sjölander A., Magnusson K. E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991 Jun;33(6):691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989 Jun 1;142(11):3781–3787. [PubMed] [Google Scholar]
- Maghazachi A. A. Cholera toxin inhibits interleukin-2-induced, but enhances pertussis toxin-induced T-cell proliferation: regulation by cyclic nucleotides. Immunology. 1992 Jan;75(1):103–107. [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Russell M. W. Immunization against dental caries. Curr Opin Dent. 1992 Sep;2:72–80. [PubMed] [Google Scholar]
- Russell M. W., Wu H. Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991 Nov;59(11):4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Kanamoto T., Asakawa H., Koga T. Intranasal immunization of mice with recombinant protein antigen of serotype c Streptococcus mutans and cholera toxin B subunit. Arch Oral Biol. 1990;35(6):475–477. doi: 10.1016/0003-9969(90)90211-r. [DOI] [PubMed] [Google Scholar]
- Tamura S. I., Samegai Y., Kurata H., Kikuta K., Nagamine T., Aizawa C., Kurata T. Enhancement of protective antibody responses by cholera toxin B subunit inoculated intranasally with influenza vaccine. Vaccine. 1989 Jun;7(3):257–262. doi: 10.1016/0264-410x(89)90240-5. [DOI] [PubMed] [Google Scholar]
- Tilney N. L. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971 Sep;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Trier J. S. Structure and function of intestinal M cells. Gastroenterol Clin North Am. 1991 Sep;20(3):531–547. [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Bailey M., Williams N. A., Stokes C. R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991 Oct;21(10):2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- Wilson A. D., Clarke C. J., Stokes C. R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990 Apr;31(4):443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Antibody-secreting cell responses in the mouse liver. Immunology. 1992 Nov;77(3):443–448. [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Immunological cross-reactivity between Streptococcus mutans and human heart tissue examined by cross-immunization experiments. Infect Immun. 1990 Nov;58(11):3545–3552. doi: 10.1128/iai.58.11.3545-3552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


