Abstract
Objective
To study the role of vastus lateralis/vastus medialis cross-sectional area ratio (VL/VM CSA ratio) in preclinical knee osteoarthritis (OA) using MRI-based cartilage T2 mapping technique and morphological analysis at 3.0T in non-symptomatic, middle-aged subjects.
Material and Methods
174 non-symptomatic individuals aged 45–55 years with OA risk factors were selected from the Osteoarthritis Initiative incidence cohort. OA-related knee abnormalities were analyzed using the whole-organ MR imaging score (WORMS). Knee cartilage T2 maps were generated using sagittal 2D multiecho spin echo images of the right knee. Cross-sectional area (CSA) of thigh muscles was measured using axial T1W images of the right mid thigh. Spline-based segmentation of cartilage and muscles was performed on a SUN/SPARC workstation. Muscle measurements were normalized to body size using body surface area. Statistical significance was determined using Student’s t-test, Pearson correlation test, and multiple regression models. To correct for multiple testing, Bonferroni adjustments were applied across all tests within each of the primary results tables (Tables 3 – 7).
Results
Higher T2 values were associated with increased prevalence and severity of cartilage degeneration. In our study, male and female subjects with higher VL/VM CSA ratio demonstrated significantly lower mean cartilage T2 values (all compartments combined) (mean 44.10 versus 45.17, p = 0.0017), and significantly lower WORMS scores (mean 14.12 versus 18.68, p = 0.0316). Regression analyses of combined mean cartilage T2 using VL/VM CSA ratio as a continuous predictor showed a significant curvilinear relationship between these two variables (p = 0. 0.0082).
Conclusion
Our results suggested that higher VL/VM CSA ratio is associated with lower T2 values and decreased presence and severity of OA-related morphological changes. Additional studies will be needed to determine causality.
Keywords: osteoarthritis, OAI, cartilage T2, WORMS, vastus lateralis, vastus medialis
Introduction
A number of risk factors associated with the development of osteoarthritis (OA) have been identified, including biomechanical and neuromuscular factors, such as knee adduction moment and muscle weakness [1, 2]. Lower extremity muscle strength has been shown to influence knee joint loading and dynamic stability [1, 2]. Individuals with less strength of the quadriceps and hamstrings were found to have significantly higher knee joint loads [3]. Quadriceps weakness was shown to precede the onset of knee OA, particularly in women [4, 5]. On the other hand, higher quadriceps strength is associated with significantly reduced risk of developing knee OA in women [6, 7]. Despite extensive studies of the role of quadriceps and quadriceps/hamstring balance in OA, the role of vastus lateralis and medialis in OA remains unclear. Vastus lateralis/medialis imbalance, particularly vastus medialis weakness has been implicated in patellofemoral pain syndrome (PFPS) [8]. Physiotherapy directed at restoring vastus muscle balance has been shown to be efficacious in PFPS patients [8, 9]. However, the role of vastus lateralis and medialis balance in the development of OA is not well understood. It is also noteworthy that most studies of muscle function in knee OA have focused on muscle strength, the measurement of which is not straightforward and has not always been well conducted in OA studies [1]. There is a paucity of data assessing the role of muscle cross-sectional area (CSA), which has been shown to be proportional to muscle strength [10, 11] and can be reliably quantified using modern magnetic resonance imaging (MRI) techniques.
Morphologically, OA is characterized by the progressive loss of hyaline articular cartilage. However, cartilage loss and OA symptoms are preceded by significant damage to the collagen-proteoglycan (PG) matrix and elevation of cartilage water content [12]. MRI enables characterization of changes in biochemical composition of cartilage in early OA [13]. Emerging techniques such as T2 mapping [14] may potentially be used as biomarkers to non-invasively assess early changes in cartilage matrix and help in prevention of OA progression by identifying individuals at risk for OA. These individuals may benefit from non-pharmacological and/or pharmacological treatments, or potentially in the future treatment with chondroprotective, disease-modifying osteoarthritis drugs (DMOAD) [15] before irreversible cartilage loss occurs.
Here we study the role of vastus lateralis and vastus medialis CSA and their ratio in preclinical knee OA using MRI based cartilage T2 mapping techniques and morphological analyses at 3.0T in non-symptomatic, middle-aged subjects selected from the OAI incidence cohort who have risk factors of OA.
Material and Methods
Subjects
One hundred and seventy four subjects, a subset of the 4796 participants in the Osteoarthritis Initiative (OAI), were included in this study. The study protocol, amendments, and informed consent documentation including analysis plans were reviewed and approved by the local institutional review boards. Specific datasets from the OAI database used for this study are baseline clinical dataset 0.2.2 and baseline image dataset 0.E.1, which are available for public access at http://www.oai.ucsf.edu/. The subjects analyzed in this study were part of the incidence cohort of the OAI. These individuals are characterized by absence of symptomatic knee OA but having risk factors for OA. These risk factors include history of knee surgery or injury, family history of total knee replacement, Heberden’s nodes and repetitive knee bending activities. Exclusion criteria were rheumatoid arthritis, severe bilateral joint space narrowing and contraindications or inability for MRI. Specific inclusion criteria for this project were: (1) baseline Western Ontario and McMaster University (WOMAC) pain score of zero for both knees, (2) age range of 45–55, and (3) BMI of 19–27. These criteria were used to exclude obesity as a risk factor and to focus on younger subjects with less severe abnormalities. The WOMAC osteoarthritis index is a multidimensional health status questionnaire that measures pain, stiffness and physical function in patients with osteoarthritis of the knee and hip [16, 17]. In this study we included only subjects with WOMAC pain score of zero of both knees for the past 7 days at the baseline visit. Based on these criteria 98 women and 76 men were included in this project.
Questionnaires and clinical examinations
KOOS
The Knee Injury and Osteoarthritis Outcome Score (KOOS) is an extension of the WOMAC Osteoarthritis Index for evaluating short- and long-term symptoms and function in subjects with knee injury and OA. The KOOS adds three subscales to the WOMAC Osteoarthritis Index: other knee symptoms, physical function in sport and recreation, and knee-related quality of life [18].
PASE
Levels of physical activity were obtained using the Physical Activity Scale for the Elderly (PASE), which is a reliable and valid instrument for the assessment of physical activity in epidemiologic studies [19]. The scale range is 0–400. Based on their physical activity level, subjects with PASE values of 0–199 were defined as low activity group, and those with PASE values of 200–400 were defined as the high activity group. The PASE score of 200 was used as threshold because it is the median of the PASE scale and is very close to the mean of the scale (197.72).
Clinical examinations
Subjects completed a 400-meter walk, repeated chair stands, and isometric muscle strength tests. The time for a 400-meter walk of each subject was measured in seconds [20]. The pace of repeated chair stand was measured as stands per second [21]. The maximum isometric flexion and extension strength of the right knee was obtained in Newtons using the “Good Strength” chair (Metitur, Jyvaskyla, Finland) [22].
MR imaging
MRI examinations were obtained with identical 3T MRI systems (Trio, Siemens, Erlangen, Germany) for the OAI. Both knees were examined with standard morphological sequences, and T2 mapping sequences were obtained of the right knee only. Identical knee coils were used for all studies at all scanners. Three sequences were used for morphological analysis of the knee: coronal intermediate-weighted (IW) 2D fast spin-echo (FSE) sequence (TE 29 ms, TR 3700 ms), sagittal 2D IW FSE sequence with fat suppression (FS) (TE 30 ms, TR 3200 ms) and sagittal 3D dual-echo in steady state (DESS) sequence with selective water excitation (WE) with coronal and axial reformations (TE 4.7 ms, TR 16.3 ms, flip angle 25°). For quantitative T2 relaxation time assessment a sagittal 2D multi-echo (ME) spin-echo (SE) sequence (TE 10, 20, 30, 40, 50, 60, and 70 ms, and TR 2700) was used [23]. For MR imaging of the right thigh, participants were positioned supine on the table with their legs in a neutral position. The patellar apex was palpated and the mid thigh region was defined as 15 cm above the patellar apex according to OAI protocol. A bi-planar (axial and coronal) localizer was used to visualize the right femoral epiphysis. Axial T1-weighted scans (T1W) (TE 13 ms, TE 600 ms) were positioned such that the bottom slice is at the medial femoral growth plate. A set of 15 contiguous axial images were generated. Further details regarding MRI techniques and protocols are available online at www.oai.ucsf.edu.
Image analysis
Semiquantitative morphological analyses
MR images of the right knee were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ) by two radiologists with 20 and 4 years of experience respectively. When scores were not identical, consensus readings by both radiologists were performed. A modified whole-organ magnetic resonance imaging score (WORMS) system was used to evaluate OA-related abnormalities of the knee [24, 25]. Findings in three subregions of the knee (patellofemoral, medial, and lateral compartments) were recorded, instead of the originally described 15 subregions [26]. Using the semi-quantitative scoring system the following structures were separately evaluated: cartilage, ligaments, menisci, bone marrow lesions, osteophytes, synovitis/effusion, subchondral cysts, loose bodies, and popliteal cysts. Details of scoring have been described previously [25]. The potential maximal WORMS summation score is 31. Cartilage lesions were also analyzed using the four-grade MRI classification described by Recht et al. [27, 28] based on the arthroscopic Noyes and Stabler [29] scoring system.
T2 measurements
Higher T2 values were associated with increased prevalence and severity of cartilage degeneration [13]. T2 maps were created using the sagittal 2D ME SE images of the right knee and provided exponential curve fits and T2 for each pixel [30]. The relaxation time, T2, was estimated by fitting an exponential function to the signal intensity at different echo times as follows: SI(TE) ~ exp(−TE/T2), where SI(TE) is signal intensity as a function of echo time, TE is the echo time, and T2 is the transverse relaxation time. We used a simplified mono-exponential decay model that has been previously described [14]. The seven echoes (10, 20, 30, 40, 50, 60, and 70 milliseconds) that are available from the OAI image database provided stable T2 data. Images were transferred to a remote workstation and analyzed by using software developed at our institution with an interactive display language (IDL) (Research Systems, Boulder, CO) environment. An IDL routine was used for segmentation of the patella, trochlea, medial and lateral femoral, and medial and lateral tibial cartilage to simplify the manual drawing of splines delineating cartilage areas. Only areas of artifact-free cartilage were segmented. To exclude both fluid and water-fat shift artifacts from the regions of interest, adjustment of the splines was performed simultaneously by opening both image panels and using a synchronized cursor, section number, and zoom. An IDL routine was used to calculate the mean T2 values from the regions of interest created in the T2 maps.
Thigh muscle cross-sectional area measurements
The three central sections (image #7–9) of 15 standardized axial T1W images through the right mid thigh region were used for segmentation of the thigh muscle groups. Spline-based segmentation was performed on a SUN/SPARC workstation using the Qbrain software. The CSA of the quadriceps, hamstring, vastus lateralis, and vastus medialis were calculated. Sartorius and gracilis muscles were not included in the analysis. While subcutaneous and peripheral fat was excluded during segmentation, fatty infiltration within each muscle group was not evaluated separately. To exclude variation in body size as a confounding factor, we performed allometric scaling of muscle CSA using body surface area (BSA), with a scaling exponent of 2/3 (i.e., corrected muscle CSA = uncorrected muscle CSA ÷ BSA2/3). BSA was calculated using the Mosteller formula [31]. Corrected muscle CSA showed no statistically significant residual correlation with either body mass index (BMI) or body weight.
Reproducibility measurements
Reproducibility for the quantitative analyses of the muscle CSA for each muscle group separately and all groups combined was calculated in a random sample of 12 OAI image data sets that were assessed 3 times by the same investigator. The coefficient of variation (CV) was determined using root-mean-square averages of standard deviations of repeated measurements as described previously [32]. Reproducibility for the semi-quantitative analyses of cartilage using the WORMS score and cartilage T2 were previously analyzed by our group [25]. Briefly, the WORMS analysis inter-observer agreement was 95.3%, and the intra-observer agreement was 95.4% and 95.1% respectively for the two observers. The inter-observer agreement has a Cohen’s kappa value of 0.67, and the intra-observer agreements had Cohen’s kappa values of 0.69 and 0.72, respectively. The CV for T2 quantification measurements was 1.17%.
Statistical Analysis
All statistical processing was performed with JMP software Version 8 (SAS Institute, Cary, NC). The level of significance was defined for all calculations as p < 0.05. Statistical significance of group differences was determined using Student’s t-test. Our primary outcome was cartilage T2 and our primary comparisons were between low and high VM and VL CSA groups, which were reported in Table 4. To control for multiple comparisons across genders and compartments, we give comparison-wise p-values but indicate which comparisons would be statistically significant at α=0.05 after applying a Bonferroni correction for all the comparisons within the table. Tables 5, 6, and 7 report comparisons for the ratio of VL to VM CSA, which were more exploratory. Within those tables we also used a Bonferroni correction to control for multiple testing. Correlation between muscle CSA and PASE, between CSA and cartilage T2, and between cartilage T2 and VL/VM ratio was analyzed using Pearson correlation coefficients. Association between cartilage T2 and VL/VM ratio was analyzed using up to second degree polynomial linear regression models. Residual vs. predicted plots were used to diagnose approximate normality, homogeneity of variance, and presence of outliers. Residuals are found to be approximately normal, homoscedastic, and without significant outliers. We conducted a sensitivity analysis for our primary comparisons to assess possible confounding due to different activity levels by comparing results adjusted and unadjusted for PASE.
Table 4.
Comparison of cartilage T2 values (compartment specific and combined mean T2) in subject groups (separated by gender) with high and low muscle cross-sectional area. VM=vastus medialis; VL=vastus lateralis. Numbers in parenthesis represent 95% confidence intervals. P represents comparison-wise p-values.
| Patellofemoral Compartment |
Medial Compartment |
Lateral Compartment |
Combined | ||
|---|---|---|---|---|---|
| Male (76) | Low | 44.26 | 44.18 | 43.19 | 43.88 |
| VM | (43.37 – 45.15) | (43.41 – 44.96) | (42.34 – 44.04) | (43.22 – 44.54) | |
| High | 45.90 | 45.30 | 44.16 | 45.12 | |
| VM | (44.89 – 46.92) | (44.36 – 46.25) | (43.14 – 45.19) | (44.29 – 45.96) | |
| P | 0.0081 | 0.034 | 0.071 | 0.010 | |
| Female (98) | Low | 44.49 | 45.86 | 44.66 | 44.98 |
| VM | (43.54 – 45.45) | (45.05 – 46.67) | (43.79 – 45.54) | (44.25 – 45.72) | |
| High | 44.42 | 45.28 | 43.87 | 44.49 | |
| VM | (43.55 – 45.29) | (44.58 – 45.98) | (42.96 – 44.77) | (43.81 – 45.16) | |
| p | 0.45 | 0.15 | 0.10 | 0.16 | |
| Male (76) | Low | 45.00 | 44.88 | 44.03 | 44.63 |
| VL | (44.03 – 45.97) | (43.94 – 45.82) | (43.07 – 44.98) | (43.79 – 45.48) | |
| High | 45.16 | 44.61 | 43.33 | 44.37 | |
| VL | (44.14 – 46.18) | (43.79 – 45.43) | (42.39 – 44.27) | (43.66 – 45.07) | |
| p | 0.41 | 0.33 | 0.29 | 0.31 | |
| Female (98) | Low | 45.23 | 46.09 | 45.28 | 45.51 |
| VL | (44.20 – 46.26) | (45.26 – 46.92) | (44.34 – 46.23) | (44.69 – 46.32) | |
| High | 43.73 | 45.05 | 43.27 | 44.02 | |
| VL | (43.01 – 44.45) | (44.40 – 45.71) | (42.52 – 44.02) | (43.49 – 44.54) | |
| p | 0.0096 | 0.025 | 0.0006* | 0.0014* |
Those marked with an asterisk are statistically significant at α=0.05 after Bonferroni correction.
Table 5.
Comparison of cartilage T2 values (compartment specific and combined mean T2) in subject groups (combined and separated by gender) with high and low vastus lateralis/vastus medialis cross-sectional area ratio (VL/VM). Numbers in parenthesis represent 95% confidence intervals. P represents comparison-wise p-values.
| Patellofemoral Compartment |
Medial Compartment |
Lateral Compartment |
Combined | ||
|---|---|---|---|---|---|
| All subjects combined (174) | Low | 45.43 | 45.62 | 44.53 | 45.17 |
| VL/VM | (44.78 – 46.08) | (45.02 – 46.22) | (43.86 – 45.20) | (44.62 – 45.71) | |
| High | (43.42 – 44.70) | 44.80 | 43.47 | 44.10 | |
| VL/VM | (44.27 – 45.33) | (42.87 – 44.07) | (43.65 – 44.56) | ||
| p | 0.0016* | 0.0210 | 0.01 | 0.0017* | |
| Male (76) | Low | 45.24 | 45.26 | 44.51 | 45.00 |
| VL/VM | (44.36 – 46.11) | (44.39 – 46.14) | (43.65 – 45.36) | (44.26 – 45.75) | |
| High | 44.93 | 44.22 | 42.85 | 44.00 | |
| VL/VM | (43.83 – 46.03) | (43.37 – 45.08) | (41.88 – 43.81) | (43.22 – 44.78) | |
| p | 0.33 | 0.045 | 0.0056 | 0.032 | |
| Female (98) | Low | 44.90 | 46.10 | 44.77 | 45.22 |
| VL/VM | (43.93 – 45.88) | (45.36 – 46.85) | (43.76 – 45.79) | (44.43 – 46.01) | |
| High | 44.04 | 45.04 | 43.74 | 44.26 | |
| VL/VM | (43.20 – 44.87) | (44.30 – 45.79) | (43.01 – 44.67) | (43.67 – 44.86) | |
| p | 0.088 | 0.023 | 0.050 | 0.028 |
Those marked with an asterisk are statistically significant at α=0.05 after Bonferroni correction.
Table 6.
Semi-quantitative MR morphological scores (WORMS and RECHT scores) in subjects (males and females combined, n=174) with low and high vastus lateralis/vastus medialis (VL/VM) ratio. Numbers in parenthesis represent 95% confidence intervals. P represents comparison-wise p-values.
| Low VL/VM | High VL/VM | P | |
|---|---|---|---|
| WORMS summation score | 18.68 (15.48 – 22.74) | 14.12 (10.54 – 16.78) | 0.032 |
| Menisci | 2.07 (1.42 – 2.71) | 1.33 (0.80 – 1.87) | 0.041 |
| Ligaments | 0.38 (0.19 – 0.56) | 0.46 (024 – 0.69) | 0.28 |
| Cartilage lesion | 4.80 (3.85 – 5.75) | 3.39 (2.53 – 4.24) | 0.015 |
| Bone marrow edema | 1.40 (0.99 – 1.82) | 0.99 (0.66 – 1.32) | 0.062 |
| Subarticular cysts | 0.11 (0.00 – 0.23) | 0.11 (0.02 – 0.19) | 0.46 |
| Osteophytes | 4.89 (3.50 – 6.30) | 3.66 (2.21 – 5.11) | 0.11 |
| Joint effusion | 0.51 (0.33 – 0.67) | 0.31 (0.17 – 0.45) | 0.04 |
| Loose bodies | 0.08 (0.01 – 0.15) | 0.01 (0.00 – 0.04) | 0.042 |
| Popliteal cysts | 0.53 (0.33 – 0.72) | 0.40 (0.23 – 0.56) | 0.16 |
| RECHT cartilage summation score | 4.33 (3.50 – 5.16) | 3.05 (2.32 – 3.77) | 0.011 |
Those marked with an asterisk are statistically significant at α=0.05 after Bonferroni correction.
Table 7.
Semi-quantitative MR morphological scores (WORMS and RECHT scores) in female subjects with low and high vastus lateralis/vastus medialis (VL/VM) ratio. Numbers in parenthesis represent 95% confidence intervals. P represents comparison-wise p-values.
| Low VL/VM | High VL/VM | P | |
|---|---|---|---|
| WORMS summation score | 21.14 (15.67 – 26.62) | 12.43 (8.41 – 16.45) | 0.0058 |
| Menisci | 2.12 (1.24 – 3.01) | 0.8 (0.34 – 1.25) | 0.041 |
| Ligaments | 0.41 (0.15 – 0.66) | 0.2 (0.04 – 0.37) | 0.090 |
| Cartilage lesion | 5.41 (3.96 – 6.86) | 3.0 (1.97 – 4.03) | 0.0039* |
| Bone marrow edema | 1.14 (0.70 – 1.59) | 0.69 (0.34 – 1.05) | 0.057 |
| Subarticular cysts | 0.08 (0.00 – 0.16) | 0.04 (0.00 – 0.10) | 0.19 |
| Osteophytes | 6.41 (4.04 – 8.78) | 4.44 (2.21 – 6.67) | 0.11 |
| Joint effusion | 0.51 (0.27 – 0.75) | 0.27 (0.10 – 0.43) | 0.045 |
| Loose bodies | 0.06 (0.0 – 0.15) | 0.02 (0.00 – 0.06) | 0.21 |
| Popliteal cysts | 0.43 (0.17 – 0.68) | 0.42 (0.20 – 0.63) | 0.47 |
| RECHT cartilage summation score | 4.57 (3.43 – 5.71) | 2.65 (1.76 – 3.54) | 0.025 |
Those marked with an asterisk are statistically significant at α=0.05 after Bonferroni correction.
Results
Patient characteristics including questionnaires and physical examination
Table 1 shows baseline participant characteristics, including eligibility risk factors, combined and by gender. While there were no gender related statistically significant differences in age, PASE values, KOOS scores, repeated chair stand pace and time required for 400-meter walk, women had statistically significantly lower height, BMI, and flexion and extension muscle strength compared to male subjects.
Table 1.
Participant characteristics. ± represents standard deviations.
| All | Male | Female | p | |
|---|---|---|---|---|
| Number | 174 | 76 | 98 | |
| Risk factors | ||||
| Knee injury in history | 50 | 27 | 23 | |
| Knee surgery in history | 21 | 15 | 6 | |
| Family history of knee replacement | 28 | 15 | 13 | |
| Heberden’s nodes in hands | 33 | 5 | 27 | |
| PASE scale | 199.21 ± 80.39 | 205.18 ± 77.65 | 194.57 ± 83.55 | 0.19 |
| PASE scale range | 27 – 378 | 27 – 378 | 27 – 371 | |
| Age | 50.55 ± 2.93 | 50.59 ± 2.87 | 50.52 ± 2.99 | 0.44 |
| Height (cm) | 169.74 ± 9.06 | 177.00 ± 6.71 | 164.12 ± 6.21 | <0.0001 |
| BMI | 23.88 ± 2.08 | 24.70 ± 1.59 | 23.25 ± 2.20 | <0.0001 |
| Repeated chair stand pace (stands/sec) | 0.61 ± 0.156 | 0.61 ± 0.6 | 0.61 ± 0.16 | 0.60 |
| 400-meter walk (sec) | 271.39 ± 30.53 | 271.06 ± 34.96 | 271.64 ± 26.80 | 0.55 |
| Right knee flexion maximal force (Newton) | 162.87 ± 68.16 | 191.24 ± 80.95 | 142.97 ± 47.97 | <0.0001 |
| Right knee extension maximal force (Newton) | 392.45 ± 112.82 | 457.15 ± 111.06 | 345.52 ± 88.64 | <0.0001 |
Gender differences in thigh muscle cross-sectional area measurements
As shown in Table 2, there is a statistically significant difference in total muscle CSA of the thigh (quadriceps + hamstring) between male and female subjects (mean 388.17 mm2 for male versus 313.10 mm2 for female, p = 0.0003). Similarly, statistically significant gender differences in muscle size are noted in all the muscle groups measured except vastus lateralis. After correcting for body size using BSA, no statistically significant gender difference in total muscle CSA (mean 248.14 for male versus 220.84 for female, p = 0.0744) was noted. Residual statistically significant gender difference in quadriceps CSA was noted, but to a lesser degree of statistical significance compared to before correction for BSA (p = 0.0273 after correction vs. 0.0003 before correction). Vastus medialis still demonstrated a statistically significant gender difference (mean 52.46 for male vs. 37.33 for female, p < 0.0001). Female subjects also had a significantly higher VL/VM ratio than male subjects (p < 0.0001).
Table 2.
Muscle cross-sectional area measurements with and without correction for body size using body surface area (BSA). Numbers in parenthesis represent 95% confidence intervals.
| All | Male | Female | p | |
|---|---|---|---|---|
| Number | 174 | 76 | 98 | |
| Area, uncorrected (mm2) | ||||
| Total | 345.89 | 388.17 | 313.10 | 0.0009 |
| (323.06 – 368.71) | (349.25 – 427.08) | (287.28 – 338.91) | ||
| Quadriceps | 189.40 | 216.702 | 168.24 | 0.0003 |
| (176.08 – 202.73) | (193.48 – 239.92) | (153.88 – 182.59) | ||
| Hamstring | 156.48 | 171.47 | 144.86 | 0.0055 |
| (146.46 – 166.50) | (154.74 – 188.19) | (132.92 – 156.80) | ||
| Vastus lateralis | 54.37 | 57.60 | 51.86 | 0.088 |
| (50.29 – 58.45) | (50.94 – 64.26) | (46.74 – 56.98) | ||
| Vastus medialis | 65.75 | 82.18 | 53.01 | <0.0001 |
| (61.15 – 70.36) | (74.62 – 89.73) | (48.67 – 57.36) | ||
| Area, Corrected by BSA2/3 | ||||
| Total | 232.77 | 248.14 | 220.84 | 0.074 |
| (218.13 – 247.41) | (223.79 – 272.49) | (202.95 – 238.73) | ||
| Quadriceps | 127.36 | 138.54 | 118.70 | 0.027 |
| (118.32 – 135.90) | (123.89 – 153.18) | (108.75 – 128.64) | ||
| Hamstring | 105.40 | 109.60 | 102.15 | 0.266 |
| (98.94 – 111.87) | (99.22 – 119.98) | (98.86 – 110.44) | ||
| Vastus lateralis | 36.75 | 36.92 | 36.61 | 0.91 |
| (34.04 – 39.46) | (32.69 – 41.15) | (33.03 – 40.20) | ||
| Vastus medialis | 43.94 | 52.46 | 37.33 | <0.0001 |
| (41.09 – 46.78) | (47.81 – 57.12) | (34.34 – 40.32) | ||
| VL/VM ratio | 0.89 | 0.71 | 1.02 | <0.0001 |
| (0.83 – 0.95) | (0.65 – 0.77) | (0.94 – 1.11) |
Muscle cross-sectional area in relation to clinical parameters of physical performance and activity
Both the male and female subjects were divided into two groups of equal size according to their uncorrected total muscle CSA. As shown in Table 3, male subjects in the large muscle size group showed statistically significant greater maximal knee flexion and extension forces compared to those with smaller muscle size. Female subjects with larger muscle size also demonstrated significantly higher maximal extension force, and higher but not significantly different mean maximal flexion force. In neither group was there a statistically significant difference in terms of 400-meter walk time or the pace of performing repeated chair stands. After normalization of muscle size using BSA, no statistically significant difference was found in any of the 4 parameters measured between male or female subjects with smaller muscle size and those with larger muscle size.
Table 3.
Total thigh muscle cross-sectional area (uncorrected) in relation to clinical parameters of physical performance.
| All | Low Muscle area | High Muscle area | p | |
|---|---|---|---|---|
| Male | 76 | 38 | 38 | |
| 400 meter walk (sec) | 270.25 | 271.83 | 0.42 | |
| (259.68 – 280.81) | (259.15 – 284.51) | |||
| Repeated chair stand (stands/sec) | 0.59 | 0.62 | 0.19 | |
| (0.55 – 0.63) | (0.56 – 0.68) | |||
| Maximal extension force (Newton) | 428.19 | 494.10 | 0.0077 | |
| (392.19 – 464.19) | (453.99 – 534.22) | |||
| Maximal flexion force (Newton) | 173.78 | 213.52 | 0.025 | |
| (148.47 – 199.09) | (181.95 – 245.08) | |||
| Female | 98 | 49 | 49 | |
| 400 meter walk (sec) | 270.04 | 273.76 | 0.25 | |
| (262.43 – 277.64) | (265.68 – 281.84) | |||
| Repeated chair stand (stands/sec) | 0.59 | 0.63 | 0.12 | |
| (0.55 – 0.64) | (0.58 – 0.68) | |||
| Maximal extension force (Newton) | 325.70 | 361.41 | 0.025 | |
| (304.41 – 346.98) | (332.03 – 390.79) | |||
| Maximal flexion force (Newton) | 136.46 | 147.96 | 0.13 | |
| (122.55 – 150.36) | (132.93 – 162.98) |
Subjects with higher PASE values had larger mean total muscle CSA and higher mean CSA (both corrected and uncorrected) of all the 4 measured muscle groups. However, this difference was not statistically significant. In addition, we did not find a statistically significant correlation of muscle CSA (both corrected and uncorrected) or VL/VM ratio with PASE. Results after adjusting for PASE in all the CSA analyses were virtually the same as the unadjusted results, so we presented the unadjusted results.
Association of VL/VM ratio with cartilage T2 values
To study the relationship between vasti muscles and cartilage T2, we first divided female and male subjects separately into equal groups according to their vastus medialis CSA, we found that male subjects in the high vastus medialis group demonstrated a trend of having higher mean T2 values (individual compartments and all compartments combined) (Table 4), although no statistical significance was detected after applying Bonferroni correction. No statistically significant difference was found in female subjects. On the other hand, when vastus lateralis CSA was used to equally divide the study subjects, we found significantly lower T2 values in female subjects with larger vastus lateralis but no statistically significant difference in male subjects. (Table 4).
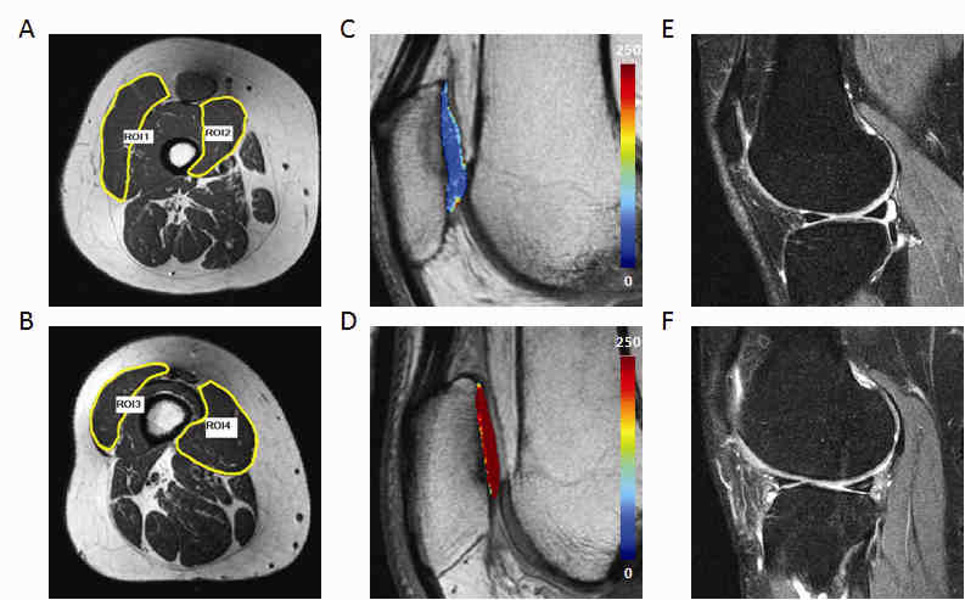
Next, we combined male and female datasets and divided subjects into two groups of equal size according to their vastus lateralis/medialis CSA ratio (VL/VM ratio). As shown in Table 5, there is a statistically significant difference in mean cartilage T2 between subjects with low VL/VM ratio and those with high ratio (mean 45.17 ± 2.52 versus 44.10 ± 2.12, p = 0.0017). Representative thigh muscle images with corresponding cartilage T2 color maps and MR morphological images from participants with low and high VL/VM ratios are shown in Figure 1. Bivariate fit of cartilage mean T2 (all compartments combined) by VL/VM ratio using a second degree polynomial regression model with VL/VM as the predictor and mean T2 as the response demonstrated a significant curvilinear relationship (T2mean = 45.96 − 1.67 × VL/VM ratio + 0.97 × (VL/VM ratio − 0.89)2, p = 0.0082). Particularly significant differences in T2 values were noted in the patellofemoral compartment. We also analyzed male and female subjects separately using their VL/VM ratio (Table 5). Although no statistical significance was noted after Bonferroni correction was applied, a consistent trend showing that subjects with higher VL/VM ratio have lower mean T2 values was evident in all compartments analyzed (Table 5).
Figure 1.
Representative images of subjects with significant difference in vastus lateralis volume/vastus medialis cross-sectional area ratio showing difference in muscle size (A, B), cartilage T2 values (C, D, mean T2 values of 43.4 and 45.8 respectively ), and MR morphological abnormalities (E, F). A, C, and E are from a subject with high VL/VM ratio (1.72) whereas B, D, and F are from a subject with low VL/VM ratio (0.58). Note that relatively normal morphology is demonstrated in E, whereas tear of the posterior horn of the lateral meniscus and full-thickness tear of the lateral femoral condyle cartilage are observed in F. ROI 1 and 2 represent cross-sectional area of vastus lateralis and medialis of the subject with high VL/VM ratio (119.38 and 69.50 mm2 respectively). ROI 3 and 4 represent cross-sectional area of vastus lateralis and medialis of the subject with low VL/VM ratio (30.45 and 52.46 mm2 respectively).
Association of VL/VM ratio with knee morphological abnormalities
We next analyzed the differences in MR morphological changes between these two groups. We identified a consistent trend showing that subjects with higher VL/VM ratio have lower morphological abnormalities scores (both WORMS summation scores and individual abnormality WORMS scores), although no statistical significance was noted after applying Bonferroni correction (Table 6). The Recht scoring system was also used to compare cartilage abnormalities and a similar non-statistically significant trend was noted. We further analyzed males and females separately for the differences in WORMS scores between subjects with higher VL/VM ratio and those with lower ratio. No statistically significant differences in WORMS scores (summation score and individual component scores) was found in males. In females, however, subjects with high VL/VM ratio showed significantly lower meniscus and cartilage WORMS scores even after application of Bonferroni correction (Table 7).
Reproducibility
The CV for total thigh muscle CSA measurements (quadriceps and hamstring combined) was 0.72%. The CV for CSA measurements of quadriceps, hamstring, vastus medialis, and vastus lateralis were 0.92%, 0.96%, 1.34%, and 1.67% respectively.
Discussion
In our study, we investigated vastus lateralis and vastus medialis CSA in relation to 3T MRI knee morphological abnormalities and T2 relaxation time in a cohort of asymptomatic, middle-aged subjects from the OAI incidence cohort. Our results showed a statistically significant reduction in gender difference in muscle CSA after correction for body size using BSA, suggesting that the statistically significant gender difference in muscle size is at least in part related to gender difference in body size. Interestingly, female subjects were found to have significantly smaller vastus medialis CSA even after correction for body size, and significantly higher VL/VM ratio, which are consistent with findings of Berry et al. [33] We used BSA to normalize muscle CSA as compared to other descriptors of body size such as BMI and body weight because it is less affected by abnormal adipose mass and has been widely used for cardiac muscle volume normalization [34, 35]. Although subjects with higher PASE score demonstrated larger mean muscle size, there was no statistically significant correlation between these two variables. This could be related to the fact that PASE evaluates physical activities within the past 7 days, whereas muscle volume change involves protein biosynthesis or degradation and reflects changes which have been ongoing over period of months to years. Higher level of physical activity has been associated with higher knee cartilage T2 values [25] and higher CSA of vastus medialis [33]. However, in our study we did not find statistically significant correlation between PASE scores and T2 values or VL/VM ratio.
The vasti muscles are very important in maintaining rotational stabilization of the knee and in preventing knee injuries, especially ligamentous ruptures [36]. Anatomic and electromyographic analyses showed that vastus medialis and lateralis have a synergistic function with regard to the extension of the knee but an antagonistic function in stabilizing the rotation of the femur against the tibia. However, the role of vastus lateralis and medialis CSA in early or preclinical OA is not well understood. Although some previous studies suggested that vastus muscle imbalance and decreased vastus medialis/vastus lateralis electromyographic activity ratio are associated with increased risk of PFPS [8], there is no reliable evidence to suggest that PFPS leads to patellofemoral osteoarthritis. On the other hand, Kannus et al. showed in a prospective study that in most of the patients with PFPS, the condition did not lead to patellofemoral osteoarthritis [37]. While debates exist as to whether vastus lateralis or medialis can be preferentially activated, physiotherapy aimed to restore VL/VM balance has been shown to be efficacious in PFPS patients [8]. Moreover, recent studies suggested that physiotherapy exercises such as open kinetic chain knee extension exercise and double leg semisquat exercise without hip adduction could produce greater activation of vastus lateralis relative to vastus medialis [9, 38]. Whether exercises that preferentially strengthen vastus lateralis are beneficial to individuals with early OA remains to be investigated.
Our results suggested that VL/VM ratio is associated with OA related cartilage T2 changes and morphological abnormalities. This is in keeping with findings of Berry et al. which showed that increased CSA of the vastus medialis is associated with an increased risk of patella cartilage defects [33, 39]. However, the mechanism underlying association between VL/VM ratio and OA remains to be elucidated. Interestingly, Sogabe et al. reported that healthy male individuals with genu varum had significantly larger VM/VL CSA ratio compared to controls [40]. Lewek et al. found significantly greater vastus medialis-medial gastrocnemius co-contraction in patients with medial compartment OA and genu varum [41]. Genu varum is positively correlated with larger medial compartment load and higher external knee adduction moment [42, 43]. It has been proposed in the pathogenesis of knee OA that the external adduction moment applied about the knee influences disease initiation, severity, and progression [44–46]. The results for the VL/VM ratio were more exploratory and to further evaluate its role in the pathogenesis of OA, future studies of the relation between VL/VM balance and knee alignment and/or adduction moment will be needed.
There is increasing evidence to suggest a practically significant correlation between cartilage T2 values and MRI morphologic abnormalities. Stahl et al. showed in a small sample study, that T2 relaxation times were significantly higher in the tibio-femoral cartilage of patients with early knee OA compared to healthy controls [47]. In another study with asymptomatic subjects, Stahl et al. found [48] a statistically significant difference in T1rho and T2 relaxation time in active subjects with and without cartilage defects. Recently, Stehling et al. also found an association between T2 and prevalent cartilage lesions in OAI subjects with higher PASE score [25]. In our study, we found that subjects with higher VL/VM ratio have not only statistically significantly lower cartilage T2 values, but also significantly less morphological abnormalities associated with OA.
One limitation of our study is that only standardized axial T1W images through the right mid thigh region were used for segmentation of the thigh muscle groups, which allowed for evaluation of muscle size using CSA but not muscle volume. Various methods have been described to define the mid thigh region [33, 49, 50]. In our study, the mid thigh region was arbitrarily defined as 15 cm above the upper border of patella according to OAI standard protocol. This definition allowed for ease and consistency in performing MR examinations in approximately 5000 participants of OAI. However, factors such as patella location, height, and quadriceps length were not taken into consideration. Another limitation of our study is that while subcutaneous and peripheral fat was excluded during muscle segmentation, fatty infiltration within each muscle group was not evaluated separately. Therefore, variation in intramuscular fat was not accounted for when calculating muscle CSA. Still another limitation of our study is that we only obtained T2 maps of the right knee, which may not be the dominant knee for all subjects. However, data about leg dominance was not available from OAI database.
Identification of OA risk factors especially modifiable risk factors is important in understanding OA pathogenesis and prevention of OA development and progression. Our data showed that higher VL/VM ratio is associated with statistically significantly lower cartilage T2 values and less morphological changes detected by MRI at 3T, suggesting that vastus lateralis/medialis balance may play an important role in the pathogenesis of OA and that people with relatively lower VL/VM ratio may be at increased risk for developing OA. In the future, a longitudinal study will be necessary to compare the progression of cartilage T2 and morphological abnormalities between subjects with low VL/VM ratio and those with high ratio and help further define the role of vastus lateralis/medialis balance in the pathogenesis of OA.
Acknowledgements
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Drs. Judong Pan and Thomas M. Link both had full access to all of the study data and assume responsibility for its integrity and the accuracy of the data analysis.
Conception and design: Link TM.
Analysis and interpretation of the data: Pan J, Stehling C, Muller-Hocker C, and Schwaiger BJ, McCulloch CE.
Drafting of the article: Pan J.
Critical revision of the article for important intellectual content: Link TM and Nevitt MC.
Final approval of the article: All authors.
Provision of study materials or patients: Lynch J.
Statistical analysis: Pan J and Stehling C.
Collection and assembly of data: Pan J and Stehling C.
Competing Interest Statement
Nothing to declare. There is no conflict of interest and the paper has not been submitted elsewhere.
References
- 1.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2008;34:731–754. doi: 10.1016/j.rdc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis--a biomechanical perspective. J Sci Med Sport. 2004;7:347–357. doi: 10.1016/s1440-2440(04)80030-6. [DOI] [PubMed] [Google Scholar]
- 3.Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171–175. doi: 10.1002/jor.1100180202. [DOI] [PubMed] [Google Scholar]
- 4.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hootman J, Fitzgerald S, Macera C. Lower extremity muscle strength and risk of self-reported hip or knee osteoarthritis. J Phys Act Health. 2004;1:321–330. [Google Scholar]
- 8.Fagan V, Delahunt E. Patellofemoral pain syndrome: a review on the associated neuromuscular deficits and current treatment options. Br J Sports Med. 2008;42:789–795. doi: 10.1136/bjsm.2008.046623. [DOI] [PubMed] [Google Scholar]
- 9.Irish SE, Millward AJ, Wride J, Haas BM, Shum GL. The effect of closed-kinetic chain exercises and open-kinetic chain exercise on the muscle activity of vastus medialis oblique and vastus lateralis. J Strength Cond Res. 24:1256–1262. doi: 10.1519/JSC.0b013e3181cf749f. [DOI] [PubMed] [Google Scholar]
- 10.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32:615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 11.Jaric S. Role of body size in the relation between muscle strength and movement performance. Exerc Sport Sci Rev. 2003;31:8–12. doi: 10.1097/00003677-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 13.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17:1135–1146. doi: 10.1007/s00330-006-0453-5. [DOI] [PubMed] [Google Scholar]
- 14.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qvist P, Christiansen C, Karsdal MA, Madsen SH, Sondergaard BC, Bay-Jensen AC. Application of biochemical markers in development of drugs for treatment of osteoarthritis. Biomarkers. 15:1–19. doi: 10.3109/13547500903295873. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23:S148–S153. [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 18.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80:163–166. doi: 10.2466/pms.1995.80.1.163. [DOI] [PubMed] [Google Scholar]
- 22.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 23.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 28.Recht MP, Piraino DW, Paletta GA, Schils JP, Belhobek GH. Accuracy of fat-suppressed three-dimensional spoiled gradient-echo FLASH MR imaging in the detection of patellofemoral articular cartilage abnormalities. Radiology. 1996;198:209–212. doi: 10.1148/radiology.198.1.8539380. [DOI] [PubMed] [Google Scholar]
- 29.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 30.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 31.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 32.Beneke R, Neuerburg J, Bohndorf K. Muscle cross-section measurement by magnetic resonance imaging. Eur J Appl Physiol Occup Physiol. 1991;63:424–429. doi: 10.1007/BF00868073. [DOI] [PubMed] [Google Scholar]
- 33.Berry PA, Teichtahl AJ, Galevska-Dimitrovska A, Hanna FS, Wluka AE, Wang Y, et al. Vastus medialis cross-sectional area is positively associated with patella cartilage and bone volumes in a pain-free community-based population. Arthritis Res Ther. 2008;10:R143. doi: 10.1186/ar2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuspidi C, Meani S, Negri F, Giudici V, Valerio C, Sala C, et al. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens. 2009;23:728–734. doi: 10.1038/jhh.2009.16. [DOI] [PubMed] [Google Scholar]
- 35.Sardinha LB, Silva AM, Minderico CS, Teixeira PJ. Effect of body surface area calculations on body fat estimates in non-obese and obese subjects. Physiol Meas. 2006;27:1197–1209. doi: 10.1088/0967-3334/27/11/012. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt O, Mittelmeier H. The biomechanical significance of the vastus medialis and lateralis muscles (author's transl) Arch Orthop Trauma Surg. 1978;91:291–295. doi: 10.1007/BF00389612. [DOI] [PubMed] [Google Scholar]
- 37.Kannus P, Natri A, Paakkala T, Jarvinen M. An outcome study of chronic patellofemoral pain syndrome. Seven-year follow-up of patients in a randomized, controlled trial. J Bone Joint Surg Am. 1999;81:355–363. doi: 10.2106/00004623-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Coqueiro KR, Bevilaqua-Grossi D, Berzin F, Soares AB, Candolo C, Monteiro-Pedro V. Analysis on the activation of the VMO and VLL muscles during semisquat exercises with and without hip adduction in individuals with patellofemoral pain syndrome. J Electromyogr Kinesiol. 2005;15:596–603. doi: 10.1016/j.jelekin.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Berry PA, Hanna FS, Teichtahl AJ, Wluka AE, Urquhart DM, Bell RJ, et al. Vastus medialis cross-sectional area is associated with patella cartilage defects and bone volume in healthy women. Osteoarthritis Cartilage. 2008;16:956–960. doi: 10.1016/j.joca.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Sogabe A, Mukai N, Miyakawa S, Mesaki N, Maeda K, Yamamoto T, et al. Influence of knee alignment on quadriceps cross-sectional area. J Biomech. 2009;42:2313–2317. doi: 10.1016/j.jbiomech.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 44.Amin S, Luepongsak N, McGibbon CA, LaValley MP, Krebs DE, Felson DT. Knee adduction moment and development of chronic knee pain in elders. Arthritis Rheum. 2004;51:371–376. doi: 10.1002/art.20396. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 47.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Stahl R, Luke A, Ma CB, Krug R, Steinbach L, Majumdar S, et al. Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects-a 3.0 T magnetic resonance imaging study. Skeletal Radiol. 2008;37:627–638. doi: 10.1007/s00256-008-0491-y. [DOI] [PubMed] [Google Scholar]
- 49.Segal NA, Glass NA, Baker JL, Torner JC. Correcting for fat mass improves DXA quantification of quadriceps specific strength in obese adults aged 50–59 years. J Clin Densitom. 2009;12:299–305. doi: 10.1016/j.jocd.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]