LETTER TO THE EDITOR
Follicular lymphoma (FL) is one of the most common forms of low-grade non-Hodgkin lymphoma in the Western hemisphere. It usually follows an indolent course. However, 25-60% of cases may transform to aggressive diffuse large B cell lymphomas (DLBCL). FLs are characterized by the t(14;18)(q32;q21) translocation, which results in the bcl-2 oncogene on chromosome 18 being joined to the immunoglobulin heavy-chain gene (IgH) on chromosome 14 (1). This leads to over-expression of a chimerical bcl-2/IgH transcript in FL cells. Since BCL-2 is an anti-apoptotic protein, it provides a constitutive survival signal. When these lymphocytes acquire additional mutations in genes such as p53, they transform to DLBCL (2, 3).
Although FL is sensitive to some chemotherapeutic agents, relapse is common and transformation to DLBCL is considered incurable with conventional chemotherapy. Current attempts to treat FL include rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The addition of rituximab to CHOP was found to be more beneficial than CHOP alone, and prolonged remission (4). Despite these promising results, only a small minority of patients achieves complete remission, and virtually all patients eventually relapse even after combination therapy. Hence, novel therapeutic agents are urgently needed to improve patient outcomes.
The PI3K/Akt/mTOR pathway plays a critical role in cell survival and proliferation, and is often activated in many types of cancer. PI3K is activated upon growth factor binding to their cognate receptors. Activated PI3K leads to the activation of PI3K-dependent kinase, PDK1, which phosphorylates and activates Akt (5). Phosphorylation of Akt at both Serine 473(S473) and Threonine 308(T308) is required for full activation.
Akt activates mTOR by inhibiting tuberous sclerosis complex 2 (TSC2), which heterodimerizes with TSC1, thereby inactivating Rheb and mTOR. mTOR associates with Raptor (mTORC1 complex) to phosphorylate the p70 S6 kinase (S6K), which in turn phosphorylates the S6 ribosomal protein (S6), as well as the eIF4E inhibitor, 4E-BP1, leading to protein translation. Additionally, mTOR associates with Rictor (mTORC2 complex) and functions in a feedback loop to phosphorylate and activate Akt at Ser473 (5). Akt can also upregulate Bcl-2 expression through cAMP-response element-binding protein. Thus, activation of the PI3K/Akt/mTOR pathway leads to protein synthesis and cell proliferation, as well as cell survival.
FL displays phosphorylated and activated Akt and mTOR kinases (6, 7). Downstream targets of mTOR, such as S6K and 4E-BP1, are also phosphorylated. These are sensitive to the single mTOR inhibitor, rapamycin (6, 7). Activation of the PI3K/Akt/mTOR pathway is required for FL growth as rapamycin inhibits the growth of FL cell lines in vitro, and in xenograft models (8). However, rapamycin studies with other cancers have yielded mixed results because rapamycin inhibits mTORC1, but not mTORC2, and recent literature suggests there is increased mTORC2-mediated phosphorylation of Akt in the presence of rapamycin, which may compensate for mTORC1 inhibition by rapamycin (9).
Since FL display an activated PI3K/Akt/mTOR pathway (6, 7), we investigated whether NVP-BEZ235, a dual PI3K and mTOR inhibitor, would be effective in inhibiting FL proliferation. NVP-BEZ235 inhibits the kinase activity of PI3K and mTOR by binding, reversibly and competitively, to their ATP-binding cleft (10). We first tested the ability of NVP-BEZ235 to inhibit the growth of the transformed FL cell lines, SUDHL16, FL-18, SUDHL4, and K422. Cells were treated with either 25 or 50nM NVP-BEZ235 for three days and MTS readings were taken every 24 hours. Each experiment was done in triplicate and standard deviation was calculated. Both these drug doses substantially inhibited cell growth of K422, SUDHL16 and FL-18 (Fig. 1a). Similar results were obtained with SUDHL4 (data not shown). The downstream targets of PI3K, including Akt, mTOR, and S6K, have previously been reported to be activated and phosphorylated in FL (6, 7). We also found that these kinases were phosphorylated in all the transformed FL cell lines: SUDHL4, K422, SUDHL16, and FL-18 (Fig. 1b). Dual inhibition of PI3K and mTOR with NVP-BEZ235 for either 16 or 24 hours inhibited Akt, mTOR and S6K phosphorylation in all cell lines. Reduced phosphorylation of S6K indicates that NVP-BEZ235 inhibited protein synthesis, while reduced phosphorylation of Akt at S473 suggests that mTORC2 feedback phosphorylation of Akt was also inhibited. Additionally, we performed an IC50 dose experiment on the FL-18 cell line. The IC50 of NVP-BEZ235 against the FL-18 cell line was 6.59 nM ± 0.41 nM (Fig. 2a). The inhibition of cell proliferation was due to increased apoptosis since the NVP-BEZ235-treated cells showed 1.6 to 2 fold increase of caspase-3 activation after 24 hours incubation with the drug, compared to cells treated with vehicle (DMSO) alone (Fig. 2b).
Figure 1. NVP-BEZ235 inhibits the growth of various follicular lymphoma cell lines by targeting the PI3K-Akt-mTOR-S6K pathway.
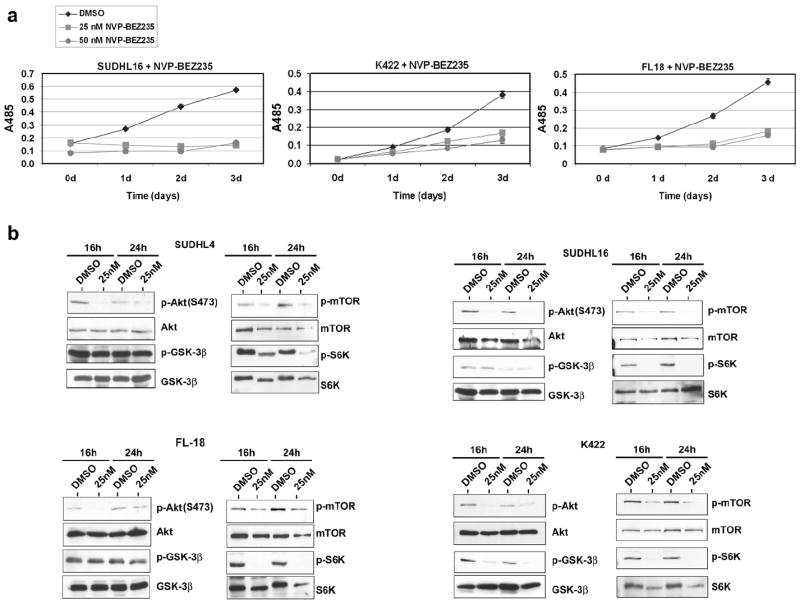
a. Follicular lymphoma cell lines SUDHL16, K422, and FL-18 (13) were treated with either DMSO or NVP-BEZ235 (25 nM or 50 nM) for the indicated times. MTS readings were taken at 0, 1, 2 and 3 days using the Cell Titer 96® AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. Absorbance was measured at 485 nm. The absorbance at 485 nm is depicted on the y-axis and time in days is shown on the x-axis. All samples were done in triplicate and standard deviation for each time point is shown. b. SUDHL4, SUDHL16, FL-18 and K422, were treated with 25nM NVP-BEZ235 for either 16 or 24 hours. Cells were harvested, lysed, and immunoblots were performed for total Akt, mTOR, GSK3β, and S6K, and their phosphorylated forms: p-Akt(Ser473), p-mTOR(Ser2448), p-GSK3b(Ser9) and p-S6K(Thr421/Ser424). The phosphorylation sites are indicated in parentheses. All antibodies were purchased from Cell Signaling.
Figure 2. Treatment with NVP-BEZ235 inhibits tumor growth in FL mouse xenograft model by targeting the PI3K-Akt-mTOR-S6K pathway.
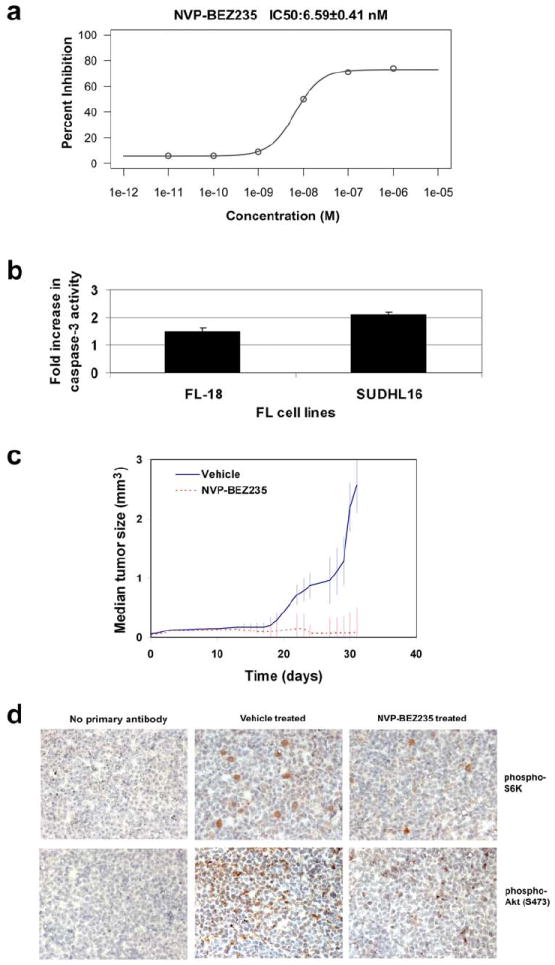
a. FL-18 cells were treated with various concentrations of NVP-BEZ235 for 72 hours. The IC50 for FL-18 growth inhibition was calculated at 6.59nM. b. FL-18 and SUDL16 cells were treated with either DMSO or 25 nM NVP-BEZ235 for 24 hours after which cells were harvested and subjected to a caspase-3 activation assay (Promega). The increase in caspase-3 activation in the NVP-BEZ235-treated cells compared to cells incubated with vehicle control is shown on the Y-axis. c. Treatment of FL-18 tumors with NVP-BEZ235 suppressed tumor growth in vivo. Tumor volume is depicted on the y-axis and time in days is shown on the x-axis. All animal procedures were performed in accordance with the UNC-CH IACUC. Briefly, NOD.CB17-Prkdcscid/J or 4-5-week-old athymic nude-Foxn1ˆnu female mice were injected with anti-asialo GM1 antibody (Wako) intraperitoneally. Twenty four hours later, the mice were subcutaneously injected 1×107 or 1×106 FL-18 cells per mouse. Treatment with NVP-BEZ235 or vehicle was started upon the development of palpable tumors. NVP-BEZ235 was dissolved in a 1:9 v/v mixture of 1-methyl-2-pyrrolidone (Fluka) and polyethylene glycol 300 (Sigma). A dose of 45 mg/kg or equal volume of the vehicle was administered orally 5-6 days per week as previously described (11). A total of eighteen mice were treated with NVP-BEZ235, and seventeen mice were treated with vehicle. Tumor volume (LxWxD) was calculated daily and animals were sacrificed when the tumor volumes of control mice reached the maximum allowed by IACUC. Tumors were excised from mice, fixed and sectioned. d. Immunohistochemistry of tumors from vehicle- and NVP-BEZ235-treated mice. Tumors were fixed, sectioned, and stained with the antibodies against phospho-S6K and phospho-Akt (S473) described previously (10). Images are shown at 400X magnification. Sections stained without primary antibody served as a negative control.
The anti-tumor activity of NVP-BEZ235 was studied in a mouse xenograft model established by subcutaneously injecting NOD.CB17- Prkdcscid/J or 4-5-week-old athymic nude-Foxn1ˆnu female mice with 1×107 or 1×106 FL-18 cells. Upon development of palpable tumors, the animals were split into two groups and treated 5-6 days a week with either vehicle or 45 mg/kg NVP-BEZ235 via oral gavage as previously reported (11). Treatment of FL-18 tumors with NVP-BEZ235 suppressed tumor growth in vivo to a significant degree (Fig. 2c) with p≤0.05, as calculated by Linear mixed-effects model. Prior to termination of the experiment, the mice in the vehicle-treated group weighed an average of 22.56±2.24 gm per mouse, and the mice in the NVP-BEZ235-treated group weighed an average of 22.50±2.33 gm per mouse (data not shown). Thus, minimal toxicity was seen in the drug-treated mice compared to the control group. The mice were sacrificed after maximal tumor size was reached in the control group. Tumors were excised, sectioned, and subjected to immunohistochemical analysis. We found reduced phosphorylated levels of the downstream effectors of PI3K and mTOR, i.e. Akt and S6K, respectively, in NVP-BEZ235-treated mice compared to vehicle-control treated mice (Fig. 2d). This suggests that the drug was inhibiting its targets effectively.
In conclusion, although rapamycin and its derivatives appear to be efficacious against FL (6-8), they only inhibit mTORC1, but not mTORC2 activity. Hence, a dual PI3K/mTOR inhibitor like NVP-BEZ235 may prove more effective in the clinic than single mTOR inhibitors. Additionally, the IC50 for NVP-BEZ235 (6.59nM) was much lower than the IC50 for solid tumors, which ranged from 15 to 20nM (12). Hence, our data suggest that NVP-BEZ235 has significant potential for treatment of FL.
Acknowledgments
This work was supported by NIH grant RO1-CA096500 and UCRF funding to BD, and funding from the Leukemia and Lymphoma society (6021) and the AIDS malignancy consortium to DD. BD is a Leukemia & Lymphoma Society Scholar and a Burroughs Wellcome Fund Investigator in Infectious Disease. We would like to acknowledge Novartis for providing us with NVP-BEZ235. We thank Charlene Ross and the Animal Studies core for their help. We thank Drs. Thomas Shea and Kristy Richards as well as members of the Damania and Dittmer labs for helpful discussions.
Footnotes
Conflict of interest The authors declare no conflict of interest
References
- 1.Yunis JJ, Oken MM, Kaplan ME, Ensrud KM, Howe RR, Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin’s lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- 2.Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993 Oct 15;82(8):2289–2295. [PubMed] [Google Scholar]
- 3.Sander CA, Yano T, Clark HM, Harris C, Longo DL, Jaffe ES, et al. p53 mutation is associated with progression in follicular lymphomas. Blood. 1993 Oct 1;82(7):1994–2004. [PubMed] [Google Scholar]
- 4.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005 Dec 1;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 5.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zha H, Raffeld M, Charboneau L, Pittaluga S, Kwak LW, Petricoin E, 3rd, et al. Similarities of prosurvival signals in Bcl-2-positive and Bcl-2-negative follicular lymphomas identified by reverse phase protein microarray. Lab Invest. 2004 Feb;84(2):235–244. doi: 10.1038/labinvest.3700051. [DOI] [PubMed] [Google Scholar]
- 7.Gulmann C, Espina V, Petricoin E, 3rd, Longo DL, Santi M, Knutsen T, et al. Proteomic analysis of apoptotic pathways reveals prognostic factors in follicular lymphoma. Clin Cancer Res. 2005 Aug 15;11(16):5847–5855. doi: 10.1158/1078-0432.CCR-05-0637. [DOI] [PubMed] [Google Scholar]
- 8.Ackler S, Xiao Y, Mitten MJ, Foster K, Oleksijew A, Refici M, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008 Oct;7(10):3265–3274. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 9.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004 Nov;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 10.Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, et al. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007 Mar 1;109(5):2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 Jul;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenitoba-Johnson KS, Jenson SD, Abbott RT, Palais RA, Bohling SD, Lin Z, et al. Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7259–7264. doi: 10.1073/pnas.1137463100. [DOI] [PMC free article] [PubMed] [Google Scholar]


