Abstract
Intravital microscopy encompasses various optical microscopy techniques aimed at visualizing biological processes in live animals. In the last decade, the development of non-linear optical microscopy resulted in an enormous increase of in vivo studies, which have addressed key biological questions in fields such as neurobiology, immunology and tumor biology. Recently, few studies have shown that subcellular processes can be imaged dynamically in the live animal at a resolution comparable to that achieved in cell cultures, providing new opportunities to study cell biology under physiological conditions. The overall aim of this review is to give the reader a general idea of the potential applications of intravital microscopy with a particular emphasis on subcellular imaging. An overview of some of the most exciting studies in this field will be presented using resolution as a main organizing criteria. Indeed, first we will focus on those studies in which organs where imaged at the tissue level, then on those focusing on single cells imaging, and finally on those imaging subcellular organelles and structures.
Keywords: Intravital microscopy, non-linear microscopy, live animal imaging, membrane traffic
Introduction
In the last two decades the field of mammalian cell biology has developed extraordinarily, and this is in part due to the advancements in the ability to image almost any cellular process in cultured cells. Indeed, the level of resolution currently achieved allows from imaging the movements of a single cell to tracking subcellular organelles or even single molecules. In addition, cell cultures are extremely flexible systems that offer the advantage of being easily manipulated both pharmacologically and genetically, thus enabling the acquisition of detailed molecular information about the machinery regulating the process of interest. However, a major limitation of in vitro models is that they do not always reconstitute the tissue environment as found in animal tissues under physiological conditions. For example, cell cultured on plastic or glass surfaces, which are the most utilized experimental models in cell biology, lack the three-dimensional organization, which is crucial for many cellular processes (Cukierman et al., 2001). On the other hand, three-dimensional cell culture models, which are now widely adopted and utilize various purified components of the extracellular matrix, lack most of the key components that play an active role in the environment of any native tissue, such as other cell types and signaling molecules (Cukierman et al., 2001; Ghajar and Bissell, 2008; Xu et al., 2009). To overcome these limitations, a large effort has been directed towards developing techniques and tools to image and study cellular events in living animals, with the goal of achieving the same depth of analysis that is currently available for in vitro models. Although in live animals organs have been imaged since the early 1930’s (Beck and Berg, 1931), the major breakthroughs in this field occurred in the last decade with the improvement of conventional microscopes and more importantly with the development of microscopes based on the “nonlinear” excitation of the specimen, which has opened the door to deep tissue imaging (Helmchen and Denk, 2005; Mertz, 2004; Zipfel et al., 2003b).
The focus of this review is primarily on intravital microscopy (IVM), which includes all those optical microscopy techniques that are utilized to perform kinetic and functional studies in living animals. Although IVM has been utilized also in smaller organisms such as drosophila, zebra fish, and C. elegans (Gualda et al., 2008; O’Brien et al., 2009; Vinegoni et al., 2009; Yaniv et al., 2006), we will discuss primarily those studies performed in small rodents. First, we will present a brief overview of some of the techniques based on non-linear optical microscopy, suggesting more specialized articles to the reader for the technical details. Next, we will review how IVM has been applied to the imaging of biological processes at the level of either tissues or single cells, leading to tremendous advancements in fields such as neurobiology, cancer biology and immunology. Finally, we will review recent studies in which IVM has been utilized to image processes at a subcellular level, discussing how this area has opened a new era of investigations in cell biology.
Non-linear Optical Microscopy
Non-linear optical microscopy techniques generate contrast by using higher-order interactions between light and biological matter. Processes whose dependence from the incident light is nonlinear typically involve the absorption or the scattering and recombination of two or more photons by the specimen (Campagnola and Loew, 2003; Helmchen and Denk, 2005; Mertz, 2004; Oheim et al., 2006; Rubart, 2004; So et al., 2000; Stutzmann and Parker, 2005; Svoboda and Yasuda, 2006; Zipfel et al., 2003b).
Two- and Three-Photon Microscopy
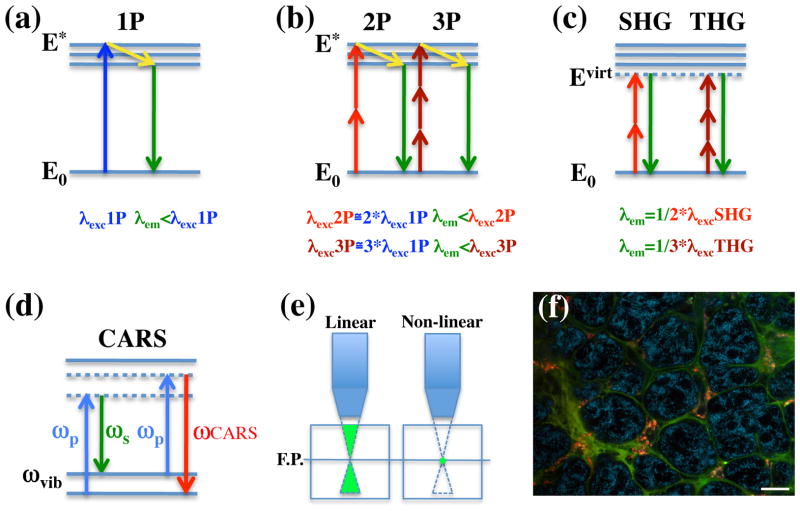
The theoretical formulation of multi-photon excitation was proposed for the first time by Maria Goppert-Mayer in 1931 (Göppert-Mayer, 1931). However, it took 30 years to be experimentally proven with the invention of the first laser, and almost sixty years for the first multi-photon microscope to be built (Denk et al., 1990). Two- (three-) photon excitation is based on the fact that a molecule of a fluorophore can be excited by the almost simultaneous absorption (between atto- and femto-seconds, 10−18-10−15 sec) of two (three) photons that have half (a third) of the energy that would be required to fill the gap between two of its energetic levels (Fig. 1a and 1b) (Helmchen and Denk, 2005; Zipfel et al., 2003b). This implies that multi-photon excitation requires near infrared light (NIR) or infrared light (IR) that have the ability to penetrate biological tissues deeper than UV or visible light, making it the ideal choice for deep tissue imaging (Oheim et al., 2001). Indeed, whereas in single confocal microscopy biological specimen can be imaged up to a depth of 50–60 μm, in multi-photon microscopy (MPM) this range can be extended up to 1 mm either using tissues that exhibit a lower light scattering such as the brain (Theer et al., 2003) or by utilizing longer excitation wavelengths (Andresen et al., 2009; Kobat et al., 2009). The probability of multi-photon transitions to occur is extremely low and requires very high light intensities concentrated in space and in time. This is achieved by using lasers that emit NIR/IR light in very short pulses (typically in the order of 100 fs) at high repetition rates (80–100 MHz), and by using high numerical aperture lenses that focus the light to the excitation spot. In ideal conditions the absorption and the emission occur in a very small volume (1 fl) (Helmchen and Denk, 2005; Zipfel et al., 2003b) reducing significantly both photo-toxicity and photo-bleaching. Furthermore, this avoids the issue of off-focus emission, which in confocal microscopy is eliminated through the use of a pinhole (Fig. 1d). Finally, another important feature of multi-photon excitation is that for all the fluorophores characterized so far, the absorption spectra are much broader than in single photon excitation. This enables the imaging of multiple fluorophores using a single excitation wavelength (Fig. 1f) (Bestvater et al., 2002; Spiess et al., 2005).
Figure 1. Non linear optical microscopy.
a–d Jablonski diagram illustrating (a) single photon (1P), (b) two-photon (2P) and three-photon (3P) excitation, (c) second (SHG) and third (THG) harmonic generation, and, (d) Coherent Anti-Stokes Raman Spectroscopy (CARS). a,b - In both single and MP microscopy, the emitted photons have a lower energy than the sum of the incident ones, due to some energy loss (yellow arrow). c – In SHG and THG the incident photons are scattered and recombine in a single one, without energy loss. d- In CARS microscopy two beams are used: the pump (ωp) and the stokes (ωs). When they are tuned to match a vibrational energy gap (ωvib), a strong anti-stokes signal is generated (ωCARS). Note that both in the harmonic emission and in CARS no electronic transitions occur. e) Non-linear emission occurs at the focal spot. f) Multiple fluorophores can be imaged using a single excitation wavelength. Alexa 488-dextran transferrin (green) and Texas Red–dextran (red) were injected in the submandibular glands of male rats and internalized into endosomal vesicles by fibroblast located in the stroma. After 1 hour, the glands were imaged by MPM using 750 nm as excitation wavelength. The endogenous fluorescence highlights the acinar structures (cyan). Note that both transferrin and dextran bind to the extracellular matrix surrounding the acini. Scale bar - 20 μm
Second and Third Harmonic Generation Microscopy
Second harmonic generation (SHG) and third harmonic generation (THG) are processes that do not involve any energy absorption since the incident photons are scattered, recombining into a single photon in a process without energy loss (Fig. 1c). For this reason they are suitable for imaging biological specimen with even lower photo-toxicity than MPM (Campagnola and Loew, 2003). Although the major harmonic signals are produced in the forward direction and thus more suited for imaging slices, the backward scattering signal is still sufficient for imaging thick tissue in live organisms. Several molecules are able to generate harmonic signals, especially when assembled in highly ordered structures. Among them are collagen, microtubules, and muscle myosin (Campagnola and Loew, 2003; Zipfel et al., 2003a; Zoumi et al., 2002). Recently, lipids forming lipid bodies have also been successfully imaged in various living organisms by using THG (Debarre et al., 2006). Due to the different nature of the harmonic emission, SHG and THG are often combined with MPM, expanding the repertoire of information that can be acquired. Recently, using spectral un-mixing techniques, up to six intrinsic signals coming from both multi-photon and harmonic emissions were resolved, providing very detailed information about the architecture of the skin in live nude mice (Radosevich et al., 2008).
Coherent Anti-Stokes Raman Scattering (CARS)
CARS microscopy is based on the use of two laser pulses (the pump and the Stokes) that are tuned to match the energetic gap between two vibrational levels in the molecule of interest (Muller and Zumbusch, 2007) (Fig. 1d). Under these conditions a strong anti-Stokes signal is emitted, which generates the contrast to image the specimen. One of the major applications of CARS microscopy comes from tuning the wavelengths to the CH2 vibrational bands that enables imaging various molecules such as myelin fibers, and lipids, either within the membrane bilayer or in intracellular storage compartments. Furthermore, CARS has been utilized to image arterial walls and atherosclerotic lesions (Evans et al., 2005; Fu et al., 2008; Wang et al., 2009). Although the use of CARS in live animals has been very limited thus far, this is a very promising technique that has a strong potential for live animal studies especially when complemented with MPM and SHG.
Fluorescence Lifetime Imaging (FLIM)
FLIM is based on the measurement of the lifetime that a given molecule spends in an excited state. This parameter is a characteristic of each molecule, and does not depend on its concentration. Since the lifetime is sensitive to modifications of the environment, FLIM has become a powerful tool in the quantitative analysis of cellular parameters such as pH, oxygen levels, ions concentration, and the metabolic state of various biomolecules. When FLIM is combined with multi-photon excitation and harmonic generation, it becomes suitable for deep tissue imaging, providing valuable information on the tissue microenvironment (Levitt et al., 2009; Niesner et al., 2008; Provenzano et al., 2009).
Imaging tissue architecture and function in vivo
Intrinsic or endogenous fluorescence
Several endogenous molecules are excited using either non-linear optical techniques or single photon microscopy, providing valuable information on the tissue architecture without the need for exogenous labeling (Zipfel et al., 2003a). Although several studies have been performed utilizing endogenous emissions in explanted tissues, only few have been carried out in live animals. One of the molecules that has been exploited for this purpose is NAD(P)H that emits in the visible range upon either single photon (360 nm) or two-photon excitation (710–760 nm). Although its two-photon cross section is very low, its abundance within the cell makes it a suitable endogenous label for both metabolic and structural studies (Fig. 2a–i). Changes in the levels of NAD(P)H were measured in live mice during ischemia and reperfusion in the jejunum (Guan et al., 2009), microcirculatory failure in the liver (Paxian et al., 2004) or in the kidney after LPS-induced sepsis (Wu et al., 2007), providing novel data on the metabolic state of the tissue under pathological conditions. Recently, levels of NAD(P)H were measured in the skin and in the liver using FLIM (Roberts et al., 2008). NAD(P)H is distributed both in the cytoplasm and in the mitochondria, and at a relatively low magnification, its signal highlights the details of the architecture of various tissues. For example, in the brain, the astrocytes show a characteristic NAD(P)H pattern that distinguishes them from other cell populations (Kasischke et al., 2004); in skeletal muscles of both live animals and humans the architecture and the contractions of the sarcomere have been imaged taking advantage of the high concentration of mitochondria along the sarcomere Z-discs (Llewellyn et al., 2008; Rothstein et al., 2005); in the salivary glands of both live rats and mice, the architecture of the acini have been resolved by exciting the intrinsic emission of NAD(P)H (Fig. 2i and 2i′); and the fine details of the structure of the large ducts, which are highly enriched in mitochondria on the basolateral pole of the epithelium, have also been imaged at a level of resolution almost comparable to that obtained by classical immunohistochemistry (Masedunskas and Weigert, 2008; Sramkova et al., 2009) (Fig. 2i′, 2i″ and 2j). Another molecule whose intrinsic fluorescence has been exploited for in vivo imaging is collagen, which when arranged in fibers generates a strong SHG signal (Campagnola and Loew, 2003; Zoumi et al., 2002). Due to its very low turnover and stability, several studies have been focusing on analyzing the architecture of collagen fibers in various explanted organs under both physiological and pathological conditions (Cox et al., 2003; Megens et al., 2007; Morishige et al., 2006; Pena et al., 2007; Schenke-Layland et al., 2008). Moreover, imaging collagen fibers in live animals has proven to be a valuable reference point within the tissue, particularly in the context of tumor migration and invasion where an important issue is to correctly locate and orient tumors that are repeatedly imaged over long period of times (Brown et al., 2003; Perentes et al., 2009; Wyckoff et al., 2007). Furthermore, imaging the organization of collagen fibers has been extremely valuable in studies related to skin diseases both in live rodents and in patients (Konig et al., 2007). Finally, in order to highlight various structural features in live animals, other molecules have been imaged by using different modalities, such as elastin in the skin (TPM), myosin fibers in the skeletal muscle (SHG), myelin fibers in the corpus callosum (CARS) or lipid-enriched structures (CARS) (Evans et al., 2005; Fu et al., 2008; Konig et al., 2007; Llewellyn et al., 2008).
Figure 2. Imaging the architecture of the tissues in live animals.
a–i Excitation of intrinsic fluorescence to image tissue architecture. Rats were anesthetized and various organs such as liver (a), kidney (b), brain cortex (c), skeletal muscle (d), epididymis (f), bladder (g), prostate (h) and lacrimal glands (i) were imaged at a low magnification by using 740 nm as excitation wavelength. Scale bar - 100 μm. (i) The submandibular glands were imaged at a higher magnification (i) and details of the structure the acini (i′) and the large striated ducts (i″) are compared with the classical H&E staining (j). Scale bars - 10 μm. k–m Imaging the vasculature in live animal. Texas-Red dextran was systemically injected in anesthetized rats and the liver (k), the kidney (l) and the brain cortex (m) were imaged using 740 nm (k,l) or 920 nm (m) as excitation wavelength. n- Vasculature and salivary ducts in live animals. FITC dextran was injected systemically in anesthetized rats, whereas Texas-Red dextran was injected into the Wharton’s duct as described in Sramkova et al. 2009. The salivary glands were imaged by MPM using 920 nm as excitation wavelength. Scale bars 20 μm.
These examples show that the use of intrinsic fluorescence in a given tissue can be a powerful tool not only for basic research but also for diagnostic purposes, since it does not require the use of exogenous labeling (Konig et al., 2007). Hence, characterizing the patterns of intrinsic fluorescence generated in different tissues is a crucial direction to explore and develop further.
Exogenous labeling of the tissues
Another approach to image tissue architecture and function is to either administer exogenous dyes or to genetically introduce fluorescent proteins selectively targeted to the tissue of interest (Fig. 2k–n). For example, systemic injections of fluorescently labeled bovine serum albumin (BSA) or dextrans of different sizes have enabled studying and measuring both glomerular filtration and tubular reabsorption in the kidney (Kang et al., 2006; Yu et al., 2005, 2007) (Fig. 2l and supplementary movie 2). In the pancreas of live mice, by imaging with a millisecond temporal resolution, blood flow patterns were determined in the islet vasculature bed (Nyman et al., 2008). This approach has been extensively used in neuroscience where vasculature flow has been imaged and measured either in normal conditions or under acute ischemic damage in the brain cortex (Levene et al., 2004; Theer et al., 2003; Zhang and Murphy, 2007) (Fig. 2m) or in the olfactory bulb (Chaigneau et al., 2003; Chaigneau et al., 2007; Stefanovic et al., 2008). Imaging the vasculature both acutely and chronically has been an extremely important tool in the context of cancer biology to address key questions such as the contribution of the local microenvironment to tumor-induced angiogenesis (Fukumura and Jain, 2008; Koehl et al., 2009), tumor-induced vascular permeability (Gavard et al., 2009), and to follow the bio-distribution of drugs or other molecules in the tumoral mass (Bhirde et al., 2009; Smith et al., 2008). Moreover, exogenous dyes can also be locally administered in different tissues to highlight various structural features. For example, sulforhodamine B has been injected into the muscles of mice to image elastin fibers, or to selectively stain the astrocytes in the brain (Ricard et al., 2007; Verant et al., 2008); dextran has been injected into the salivary ducts of rats to image dynamically the ductal system (Masedunskas and Weigert, 2008; Sramkova et al., 2009) (Fig. 2n and supplementary movie 3); curcumin and metoxy-04 has been injected to label amyloid plaques in tg2576 mice, a model for Alzheimer’s disease (Garcia-Alloza et al., 2007; Spires et al., 2005); and Ru(phen3)2+ has been used to image the level of oxygen in the liver (Paxian et al., 2004). Finally, significant information about the architecture of tissues in vivo has been generated through the use of transgenic models expressing fluorescent reporters under the control of specific tissue promoters. The field of neuroscience has pioneered this approach with the generation of mice with specific neuronal populations expressing GFP or YFP, and recently, using a combinatorial strategy, the so-called “brainbow” mice were generated in which neurons are labeled with different colors, providing an experimental tool to analyze the neuronal circuitry (Livet et al., 2007; Svoboda and Yasuda, 2006). The use of fluorescent transgenic models is now rapidly expanding to address biological questions in other fields. For example, mice have been generated to image the pancreatic beta cells (Nyman et al., 2008), the endothelium in various organs such as the kidney and the spleen, or in the presence of implanted tumors (Grayson et al., 2003; Hillen et al., 2008; Sutton et al., 2003), and many more are becoming available.
Imaging single cells in vivo
The ability to follow over time the fate of single cells within a given organ in live animals has contributed to major breakthroughs in fields such as cancer biology, immunology, microbiology, and recently in stem cell research. In cancer biology several experimental systems have been developed to track the motility of cancer cells within a tumor in vivo. For example, mammary tumors have been imaged in situ in mice models highlighting the role of the macrophages during the intravasation process (Wang et al., 2007; Wyckoff et al., 2007), and the migration of highly invasive melanomas have been tracked dynamically, leading to the determination of interesting correlations between the differentiation state of the cells and their migration ability (Pinner et al., 2009; Pinner and Sahai, 2008). Notably, a lot of effort has been also placed to perform long term imaging of tumors in the same animal in order to provide valuable information on the invasive process of slowly migrating tumors. This has become possible by the development of procedures to install optical windows in various areas of the body. Two successful examples are the dorsal skin chamber, installed in the back of immunocompromised mice, and the optical window installed in the mammary fat pad (Alexander et al., 2008; Gligorijevic et al., 2009; Kedrin et al., 2008). These techniques have been combined with the use of novel fluorescent proteins that can be either photo-activated or photo-switched (Gligorijevic et al., 2009; Lippincott-Schwartz and Patterson, 2008; Patterson and Lippincott-Schwartz, 2002), and have provided a plethora of novel information about the modality of migration of invasive cells in vivo and their relationship with the local microenvironment, particularly the vasculature and the lymphatic system.
Imaging the cells of the immune system in a live animal has revealed novel aspects of the dynamics of cellular immunity. Most of the experimental systems are based on transferring of fluorescently labeled isolated cells into recipient animals. From the first pioneering studies looking at the movements of B lymphocytes and T cells in the intact lymph-nodes (Bousso and Robey, 2003; Mempel et al., 2004; Miller et al., 2002; Stoll et al., 2002), a number of immunological questions have been addressed, spanning from T Cell activation (Hickman et al., 2008; Miller et al., 2004), the formation of mycobacterium-induced granulomas in the liver (Egen et al., 2008), T cell infiltration and elimination of solid tumors (Boissonnas et al., 2007; Breart et al., 2008), migration of dendritic cells (Roediger et al., 2008), and the extrafollicular activation of B cells (Qi et al., 2006). For a more complete overview of these processes we suggest more focused reviews (Cahalan and Parker, 2008; Germain et al., 2005; Hickman et al., 2009; Nitschke et al., 2008). Another field that has benefited from the development of IVM microscopy is the biology of pathogen infection. One of the first studies conducted to image the progression of bacterial infections in live tissue was performed in the kidney, where the proliferation of a GFP-expressing uro-pathogenic Escherichia Coli was studied (Mansson et al., 2007). Several other studies were performed using different approaches and strategies to image either later stages of the infectious process as in the case of the Staphylococcus aureus and the Borrelia burgdorferi (Laschke et al., 2005; Norman et al., 2008) or focusing on the site of the infection as in the case of the Leishmania major, where the interaction with CD4+ T cells was analyzed (Filipe-Santos et al., 2009).
Finally, IVM has been recently utilized in stem cell research to track individual hematopoietic stem cells over time in the calvarium bone marrow of living mice, thus opening the field to new and exciting discoveries (Lo Celso et al., 2009).
Imaging in vivo at the subcellular level: a new approach to cell biology
In a live animal, the major challenge in performing imaging at a subcellular level is represented by the motion artifacts due to the respiration and the heartbeat. The use of stereotactic devices that completely immobilize the head of the animal has been instrumental in achieving this high level of resolution in the brain. Indeed, the first structures that were resolved in vivo at a submicron resolution were the dendritic spines that can be imaged for over a month in transgenic mice expressing YFP or GFP in a subset of layer V pyramidal neurons (Mizrahi et al., 2004; Pan and Gan, 2008; Svoboda and Yasuda, 2006). In the last few years, both surgical procedures and novel devices ensuring the stabilization of the organ of interest have been developed. The first application of IVM for the imaging of fast moving intra-cellular organelles in organs other than the brain was pioneered by the Molitoris group who has studied the internalization of fluorescently labeled dextrans and folic acid in the externalized kidney, establishing also a methodology to extract quantitative information (Dunn et al., 2002; Sandoval et al., 2004; Sandoval and Molitoris, 2008). More recently, the endocytosis of different systemically injected fluorescent molecules and their trafficking through the endosomal and the lysosomal system has been imaged in the submandibular glands of live rats at a much higher resolution than previously reported (Masedunskas and Weigert, 2008). In this study, the use of a custom-made holder designed to stabilize the externalized glands has enabled to continuously follow the fate of the injected molecules from the internalization at the plasma membrane to and throughout the endosomal system for a long period of time (Fig. 3a and supplementary movie 4). Remarkably, intracellular events such as endosomal or lysosomal fusion were imaged at a resolution comparable to that achieved in cell cultures (Fig 3b. and supplementary movie 5). Using a similar approach, compensatory endocytosis, a process of membrane retrieval that is associated with agonist-induced secretory granule exocytosis was also imaged in the acini of the salivary glands of live rats (Sramkova et al., 2009). Another tool that has increased the ability to detect small organelles in living animals are the quantum-dots (Qdots), which are semiconductor nanocrystals encapsulated with biopolymers that exhibit a very bright and stable fluorescence, and can be coupled to any biological molecule (Li et al., 2007; Lidke et al., 2004; Michalet et al., 2005). Qdots internalization has been shown in dendritic cells in the lymph-nodes of live mice (Sen et al., 2008), whereas Qdots conjugated to nanotubes linked to EGF have been imaged during their internalization in head and neck tumor cells transplanted in the back of immunocompromised mice (Bhirde et al., 2009). Exocytosis is another subcellular process that has been imaged in the kidney, where the release of renin from the granular cells of the glomeruli was studied by using quinacrine to label a population of secretory granules (Toma et al., 2006). Other subcellular compartments, such as mitochondria, were imaged dynamically in live animals either in the liver during ischemia-reperfusion using Rhodamine123 (Zhong et al., 2008), or in the kidney using tetra-methyl rhodamine methyl ester (TMRM) (Hall et al., 2009) (Fig. 3b and supplementary movie 5).
Figure 3. Imaging subcellular structures in live animals.
a) Endocytosis of fluorescently labeled dextrans in the salivary glands of live rats. Anesthetized rats were injected with Hoechst to label the nuclei (blue), and imaged in time-lapse by using two-photon microscopy. After 2:30 minutes, a 500 kDa FITC-dextran was injected to label the vasculature (green) and after 6:00 min a 70 kDa Texas-red dextran was injected to image the endocytic process. Endocytic structures appeared right after the injection and they increased in number and in size over time (see supplementary movie 4). Excitation wavelength 820 nm. Scale bar - 20 μm. b) Imaging lysosomal fusion in a live animal. Rats were injected with Alexa 488 dextran (green) and Mitotracker (red) and after 4 hours the submandibular glands were imaged in time-lapse by using single photon confocal microscopy. Two lysosomal structures were caught during a fusion event (inset). Note the dynamics of both the lysosomes and the mitochondria in supplementary movie 5. Scale bar- 5 μm. c-e Gene transduction in live animal. The acinar cells of the salivary glands of live rats were transduced by using plasmid DNA encoding for different genes as described in Sramkova et al., 2009. c) Cell expressing TGN38-mCherry, which show the typical TGN ribbon-like structure (red, arrows), and the water channel Aquaporin5-YFP (arrowheads), localized both at the apical plasma membrane and in vesicular structures (arrowheads). d) Cell expressing Life Act-GFP to label F-actin (Riedl et al., 2008). Note the enrichment of F-actin at the apical pole of the plasma membrane. e) Cell expressing LifeAct-GFP (green) and TGN-mCherry (red, arrow). Texas red dextran was also injected systemically in the rat and appeared in a blood vessel (arrowheads, supplementary movie 6). Scale bars - 5 μm.
A major breakthrough that has allowed extending IVM to many other areas of cell biology is the ability to rapidly transduce fluorescently tagged genes in specific cell populations of the organ of interest. One of the first studies in this direction was aimed at studying the actin cytoskeleton and was performed in the endothelial cells of the kidney that were transduced with either GFP-actin or GFP-cofilin by using micro-puncture techniques and adenoviral vectors (Ashworth and Tanner, 2006; Tanner et al., 2005). The skeletal muscle in live mice is also an organ suitable to transduce genes and has been exploited to study various cellular functions. For example, the activity of the protease calpain was measured by using the fluorescence resonance energy transfer (FRET) signal generated by a calpain sensor (Stockholm et al., 2005); the calcium sensor Cameleon was transduced to image and measure the change in calcium levels in the sacroplasmic reticulum; whereas the cAMP sensor Epac was utilized to look at changes in cAMP levels during beta-adrenergic stimulation (Rudolf et al., 2006). Finally, the dynamics of the translocation of the glucose transporter GLUT4 to the sarcolemma in response to insulin was analyzed with respect to the metabolism of phosphoinositides (Lauritzen et al., 2006). Recently, using the salivary glands as a model organ and plasmid DNA, it was shown that genes can be selectively targeted and robustly expressed in the different subpopulations of cells forming the parenchyma of the salivary glands (Sramkova et al., 2009) (Fig. 3c–e and supplementary movie 6). By using high resolution TPM, different subcellular organelles were imaged dynamically such as clathrin-coated vesicles, the trans-Golgi network (Fig. 3c and 3e), and early endosomal compartments, with a resolution comparable to that achieved in cell culture by single photon confocal microscopy. Furthermore, the dynamics of the actin cytoskeleton (Fig. 3d, 3e and supplementary movie 6) and the distribution of the water channel Aquaporin 5 (Fig. 3c) at the plasma membrane was also analyzed, showing that this technology can be applied to address and study different cellular processes.
Finally, we would like to highlight the fact that when the cells of interest are located in the first 30–50 μm from the surface of the organ and the labeled fluorophores that are utilized are particularly bright, single photon confocal microscopy can also be utilized, enabling to perform imaging at a much higher resolution (compare fig 3a and 3b, and supplementary movies 4 and 5).
Outlook
In conclusion, IVM is a powerful approach that is now mature to be fully exploited in many different areas of cell biology to answer very specific questions in the proper physiological context. In terms of improving the imaging of subcellular structures, we envision that the future directions should focus on refining surgical procedures, developing tools to minimize the motion of the organs without compromising their physiology, routinely introducing miniaturized lenses and micro-endoscopes that can be properly oriented in the tissue, and developing novel fluorophores specifically designed for deep tissue imaging. Furthermore, the generation of more sophisticated molecular and genetic tools will increase the repertoire of intracellular processes that can be imaged and provide effective tools to dissect the molecular machinery regulating the process of interest. This is clearly the beginning of a new era of novel and exciting discoveries in the biomedical field.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research. We apologize to those whose work could not be cited due to space limitations. We would like to thank Dr. Silvio Gutkind, Dr. Julie Donaldson and Dr. Omayma Al-Awar for critical reading of the manuscript and all the members of the Oral and Pharyngeal Cancer Branch for invaluable assistance.
Bibliography
- Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20:54–62. doi: 10.1016/j.copbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Ashworth SL, Tanner GA. Fluorescent labeling of renal cells in vivo. Nephron Physiol. 2006;103:p91–96. doi: 10.1159/000090626. [DOI] [PubMed] [Google Scholar]
- Beck JS, Berg BN. The Circulatory Pattern in the Islands of Langerhans. Am J Pathol. 1931;7:31–36. 31. [PMC free article] [PubMed] [Google Scholar]
- Bestvater F, Spiess E, Stobrawa G, Hacker M, Feurer T, Porwol T, Berchner-Pfannschmidt U, Wotzlaw C, Acker H. Two-photon fluorescence absorption and emission spectra of dyes relevant for cell imaging. J Microsc. 2002;208:108–115. doi: 10.1046/j.1365-2818.2002.01074.x. [DOI] [PubMed] [Google Scholar]
- Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21:1356–1360. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knopfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, Kable E, Jones A, Fraser I, Manconi F, Gorrell MD. 3-dimensional imaging of collagen using second harmonic generation. J Struct Biol. 2003;141:53–62. doi: 10.1016/s1047-8477(02)00576-2. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Debarre D, Supatto W, Pena AM, Fabre A, Tordjmann T, Combettes L, Schanne-Klein MC, Beaurepaire E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat Methods. 2006;3:47–53. doi: 10.1038/nmeth813. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CL, Potma EO, Puoris’haag M, Cote D, Lin CP, Xie XS. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc Natl Acad Sci U S A. 2005;102:16807–16812. doi: 10.1073/pnas.0508282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O, Pescher P, Breart B, Lippuner C, Aebischer T, Glaichenhaus N, Spath GF, Bousso P. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe. 2009;6:23–33. doi: 10.1016/j.chom.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Fu Y, Huff TB, Wang HW, Wang H, Cheng JX. Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt Express. 2008;16:19396–19409. doi: 10.1364/oe.16.019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. 2008;116:695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29:2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN, Castellino F, Chieppa M, Egen JG, Huang AY, Koo LY, Qi H. An extended vision for dynamic high-resolution intravital immune imaging. Semin Immunol. 2005;17:431–441. doi: 10.1016/j.smim.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorijevic B, Kedrin D, Segall JE, Condeelis J, van Rheenen J. Dendra2 photoswitching through the Mammary Imaging Window. J Vis Exp. 2009 doi: 10.3791/1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göppert-Mayer M. Uber elementarakte mit zwei quantensprungen. Ann Phys (Leipzig) 1931;5:273–294. [Google Scholar]
- Grayson MH, Hotchkiss RS, Karl IE, Holtzman MJ, Chaplin DD. Intravital microscopy comparing T lymphocyte trafficking to the spleen and the mesenteric lymph node. Am J Physiol Heart Circ Physiol. 2003;284:H2213–2226. doi: 10.1152/ajpheart.00999.2002. [DOI] [PubMed] [Google Scholar]
- Gualda EJ, Filippidis G, Voglis G, Mari M, Fotakis C, Tavernarakis N. In vivo imaging of cellular structures in Caenorhabditis elegans by combined TPEF, SHG and THG microscopy. J Microsc. 2008;229:141–150. doi: 10.1111/j.1365-2818.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- Guan Y, Worrell RT, Pritts TA, Montrose MH. Intestinal ischemia-reperfusion injury: reversible and irreversible damage imaged in vivo. Am J Physiol Gastrointest Liver Physiol. 2009;297:G187–196. doi: 10.1152/ajpgi.90595.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Unwin RJ, Parker N, Duchen MR. Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol. 2009;20:1293–1302. doi: 10.1681/ASN.2008070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Hickman HD, Bennink JR, Yewdell JW. Caught in the act: intravital multiphoton microscopy of host-pathogen interactions. Cell Host Microbe. 2009;5:13–21. doi: 10.1016/j.chom.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- Hillen F, Kaijzel EL, Castermans K, oude Egbrink MG, Lowik CW, Griffioen AW. A transgenic Tie2-GFP athymic mouse model; a tool for vascular biology in xenograft tumors. Biochem Biophys Res Commun. 2008;368:364–367. doi: 10.1016/j.bbrc.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobat D, Durst ME, Nishimura N, Wong AW, Schaffer CB, Xu C. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt Express. 2009;17:13354–13364. doi: 10.1364/oe.17.013354. [DOI] [PubMed] [Google Scholar]
- Koehl GE, Gaumann A, Geissler EK. Intravital microscopy of tumor angiogenesis and regression in the dorsal skin fold chamber: mechanistic insights and preclinical testing of therapeutic strategies. Clin Exp Metastasis. 2009;26:329–344. doi: 10.1007/s10585-008-9234-7. [DOI] [PubMed] [Google Scholar]
- Konig K, Ehlers A, Riemann I, Schenkl S, Buckle R, Kaatz M. Clinical two-photon microendoscopy. Microsc Res Tech. 2007;70:398–402. doi: 10.1002/jemt.20445. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Kerdudou S, Herrmann M, Menger MD. Intravital fluorescence microscopy: a novel tool for the study of the interaction of Staphylococcus aureus with the microvascular endothelium in vivo. J Infect Dis. 2005;191:435–443. doi: 10.1086/427193. [DOI] [PubMed] [Google Scholar]
- Lauritzen HP, Ploug T, Prats C, Tavare JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes. 2006;55:1300–1306. doi: 10.2337/db05-1216. [DOI] [PubMed] [Google Scholar]
- Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol. 2004;91:1908–1912. doi: 10.1152/jn.01007.2003. [DOI] [PubMed] [Google Scholar]
- Levitt JA, Matthews DR, Ameer-Beg SM, Suhling K. Fluorescence lifetime and polarization-resolved imaging in cell biology. Curr Opin Biotechnol. 2009;20:28–36. doi: 10.1016/j.copbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Li ZB, Cai W, Chen X. Semiconductor quantum dots for in vivo imaging. J Nanosci Nanotechnol. 2007;7:2567–2581. doi: 10.1166/jnn.2007.628. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, Jares-Erijman EA, Jovin TM. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Patterson GH. Fluorescent proteins for photoactivation experiments. Methods Cell Biol. 2008;85:45–61. doi: 10.1016/S0091-679X(08)85003-0. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Llewellyn ME, Barretto RP, Delp SL, Schnitzer MJ. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature. 2008;454:784–788. doi: 10.1038/nature07104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson LE, Melican K, Boekel J, Sandoval RM, Hautefort I, Tanner GA, Molitoris BA, Richter-Dahlfors A. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol. 2007;9:413–424. doi: 10.1111/j.1462-5822.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megens RT, Reitsma S, Schiffers PH, Hilgers RH, De Mey JG, Slaaf DW, oude Egbrink MG, van Zandvoort MA. Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J Vasc Res. 2007;44:87–98. doi: 10.1159/000098259. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Mertz J. Nonlinear microscopy: new techniques and applications. Curr Opin Neurobiol. 2004;14:610–616. doi: 10.1016/j.conb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci. 2004;24:3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg. 2006;32:1784–1791. doi: 10.1016/j.jcrs.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Zumbusch A. Coherent anti-Stokes Raman Scattering Microscopy. Chemphyschem. 2007;8:2156–2170. doi: 10.1002/cphc.200700202. [DOI] [PubMed] [Google Scholar]
- Niesner RA, Andresen V, Gunzer M. Intravital two-photon microscopy: focus on speed and time resolved imaging modalities. Immunol Rev. 2008;221:7–25. doi: 10.1111/j.1600-065X.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Nitschke C, Garin A, Kosco-Vilbois M, Gunzer M. 3D and 4D imaging of immune cells in vitro and in vivo. Histochem Cell Biol. 2008;130:1053–1062. doi: 10.1007/s00418-008-0520-x. [DOI] [PubMed] [Google Scholar]
- Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4:e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest. 2008;118:3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien GS, Rieger S, Martin SM, Cavanaugh AM, Portera-Cailliau C, Sagasti A. Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos. J Vis Exp. 2009 doi: 10.3791/1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oheim M, Beaurepaire E, Chaigneau E, Mertz J, Charpak S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J Neurosci Methods. 2001;111:29–37. doi: 10.1016/s0165-0270(01)00438-1. [DOI] [PubMed] [Google Scholar]
- Oheim M, Michael DJ, Geisbauer M, Madsen D, Chow RH. Principles of two-photon excitation fluorescence microscopy and other nonlinear imaging approaches. Adv Drug Deliv Rev. 2006;58:788–808. doi: 10.1016/j.addr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pan F, Gan WB. Two-photon imaging of dendritic spine development in the mouse cortex. Dev Neurobiol. 2008;68:771–778. doi: 10.1002/dneu.20630. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- Paxian M, Keller SA, Cross B, Huynh TT, Clemens MG. High-resolution visualization of oxygen distribution in the liver in vivo. Am J Physiol Gastrointest Liver Physiol. 2004;286:G37–44. doi: 10.1152/ajpgi.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pena AM, Fabre A, Debarre D, Marchal-Somme J, Crestani B, Martin JL, Beaurepaire E, Schanne-Klein MC. Three-dimensional investigation and scoring of extracellular matrix remodeling during lung fibrosis using multiphoton microscopy. Microsc Res Tech. 2007;70:162–170. doi: 10.1002/jemt.20400. [DOI] [PubMed] [Google Scholar]
- Perentes JY, McKee TD, Ley CD, Mathiew H, Dawson M, Padera TP, Munn LL, Jain RK, Boucher Y. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat Methods. 2009;6:143–145. doi: 10.1038/nmeth.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner S, Jordan P, Sharrock K, Bazley L, Collinson L, Marais R, Bonvin E, Goding C, Sahai E. Intravital imaging reveals transient changes in pigment production and Brn2 expression during metastatic melanoma dissemination. Cancer Res. 2009;69:7969–7977. doi: 10.1158/0008-5472.CAN-09-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner S, Sahai E. Imaging amoeboid cancer cell motility in vivo. J Microsc. 2008;231:441–445. doi: 10.1111/j.1365-2818.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Keely PJ. Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin Exp Metastasis. 2009;26:357–370. doi: 10.1007/s10585-008-9204-0. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Radosevich AJ, Bouchard MB, Burgess SA, Chen BR, Hillman EM. Hyperspectral in vivo two-photon microscopy of intrinsic contrast. Opt Lett. 2008;33:2164–2166. doi: 10.1364/ol.33.002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard C, Vial JC, Douady J, van der Sanden B. In vivo imaging of elastic fibers using sulforhodamine B. J Biomed Opt. 2007;12:064017. doi: 10.1117/1.2821421. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MS, Roberts MJ, Robertson TA, Sanchez W, Thorling C, Zou Y, Zhao X, Becker W, Zvyagin AV. In vitro and in vivo imaging of xenobiotic transport in human skin and in the rat liver. J Biophotonics. 2008;1:478–493. doi: 10.1002/jbio.200810058. [DOI] [PubMed] [Google Scholar]
- Roediger B, Ng LG, Smith AL, Fazekas de St Groth B, Weninger W. Visualizing dendritic cell migration within the skin. Histochem Cell Biol. 2008;130:1131–1146. doi: 10.1007/s00418-008-0531-7. [DOI] [PubMed] [Google Scholar]
- Rothstein EC, Carroll S, Combs CA, Jobsis PD, Balaban RS. Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters. Biophys J. 2005;88:2165–2176. doi: 10.1529/biophysj.104.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubart M. Two-photon microscopy of cells and tissue. Circ Res. 2004;95:1154–1166. doi: 10.1161/01.RES.0000150593.30324.42. [DOI] [PubMed] [Google Scholar]
- Rudolf R, Magalhaes PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol. 2004;287:C517–526. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- Sandoval RM, Molitoris BA. Quantifying endocytosis in vivo using intravital two-photon microscopy. Methods Mol Biol. 2008;440:389–402. doi: 10.1007/978-1-59745-178-9_28. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Xie J, Angelis E, Starcher B, Wu K, Riemann I, MacLellan WR, Hamm-Alvarez SF. Increased degradation of extracellular matrix structures of lacrimal glands implicated in the pathogenesis of Sjogren’s syndrome. Matrix Biol. 2008;27:53–66. doi: 10.1016/j.matbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D, Deerinck TJ, Ellisman MH, Parker I, Cahalan MD. Quantum dots for tracking dendritic cells and priming an immune response in vitro and in vivo. PLoS One. 2008;3:e3290. doi: 10.1371/journal.pone.0003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 2008;8:2599–2606. doi: 10.1021/nl080141f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So PT, Dong CY, Masters BR, Berland KM. Two-photon excitation fluorescence microscopy. Annu Rev Biomed Eng. 2000;2:399–429. doi: 10.1146/annurev.bioeng.2.1.399. [DOI] [PubMed] [Google Scholar]
- Spiess E, Bestvater F, Heckel-Pompey A, Toth K, Hacker M, Stobrawa G, Feurer T, Wotzlaw C, Berchner-Pfannschmidt U, Porwol T, Acker H. Two-photon excitation and emission spectra of the green fluorescent protein variants ECFP, EGFP and EYFP. J Microsc. 2005;217:200–204. doi: 10.1111/j.1365-2818.2005.01437.x. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. Am J Physiol Cell Physiol. 2009;297:C1347–1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholm D, Bartoli M, Sillon G, Bourg N, Davoust J, Richard I. Imaging calpain protease activity by multiphoton FRET in living mice. J Mol Biol. 2005;346:215–222. doi: 10.1016/j.jmb.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Parker I. Dynamic multiphoton imaging: a live view from cells to systems. Physiology (Bethesda) 2005;20:15–21. doi: 10.1152/physiol.00028.2004. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Tanner GA, Sandoval RM, Molitoris BA, Bamburg JR, Ashworth SL. Micropuncture gene delivery and intravital two-photon visualization of protein expression in rat kidney. Am J Physiol Renal Physiol. 2005;289:F638–643. doi: 10.1152/ajprenal.00059.2005. [DOI] [PubMed] [Google Scholar]
- Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt Lett. 2003;28:1022–1024. doi: 10.1364/ol.28.001022. [DOI] [PubMed] [Google Scholar]
- Toma I, Kang JJ, Peti-Peterdi J. Imaging renin content and release in the living kidney. Nephron Physiol. 2006;103:p71–74. doi: 10.1159/000090622. [DOI] [PubMed] [Google Scholar]
- Verant P, Ricard C, Serduc R, Vial JC, van der Sanden B. In vivo staining of neocortical astrocytes via the cerebral microcirculation using sulforhodamine B. J Biomed Opt. 2008;13:064028. doi: 10.1117/1.3041163. [DOI] [PubMed] [Google Scholar]
- Vinegoni C, Razansky D, Pitsouli C, Perrimon N, Ntziachristos V, Weissleder R. Mesoscopic fluorescence tomography for in-vivo imaging of developing Drosophila. J Vis Exp. 2009 doi: 10.3791/1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Fu Y, Huff TB, Le TT, Wang H, Cheng JX. Chasing lipids in health and diseases by coherent anti-Stokes Raman scattering microscopy. Vib Spectrosc. 2009;50:160–167. doi: 10.1016/j.vibspec.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE, Condeelis JS. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292:F261–268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- Yu W, Sandoval RM, Molitoris BA. Quantitative intravital microscopy using a Generalized Polarity concept for kidney studies. Am J Physiol Cell Physiol. 2005;289:C1197–1208. doi: 10.1152/ajpcell.00197.2005. [DOI] [PubMed] [Google Scholar]
- Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 2007;292:F1873–1880. doi: 10.1152/ajprenal.00218.2006. [DOI] [PubMed] [Google Scholar]
- Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5:e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, Kim I, Wright GL, Lemasters JJ. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G823–832. doi: 10.1152/ajpgi.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003a;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003b;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci U S A. 2002;99:11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.