Abstract
Bioluminescence imaging (BLI) has emerged during the past five years as the preeminent method for rapid, cheap, facile screening of tumor growth and spread in mice. Both subcutaneous and orthotopic tumor models are readily observed with high sensitivity and reproducibility. User friendly commercial instruments exist and increasingly luciferase expressing tumor cells are available in academic institutions or commercially. There is an increasing literature on routine use of BLI for assessing chemotherapeutic efficacy, drug combinations, dosing and timing. In addition, BLI may be applied to more sophisticated questions of molecular biology by including specific promoter sequences. This chapter will describe routine methods used to support multiple investigators in our small animal imaging resource.
Keywords: Luciferase, luciferin, charged-coupled device cameras (CCD), Igor Pro, bioluminescence
1 Introduction
The concept of bioluminescence to study biochemistry has been around for many years, for example, as the basis for quantifying ATP in snap frozen histological specimens or tissue extracts (1, 2). However, in vivo application has been spearheaded by Contag et al. (3) and promoted by Xenogen (now Caliper Lifesciences). In less than a decade, BLI has become a routine modality for use in cancer biology, particularly suited for assessing tumor burden and metastatic spread. In vivo BLI has been reviewed many times (3–6) and readers are directed to these papers and other chapters of this book for further insight.
In its most popular format, the bioluminescent reaction requires luciferase enzyme derived from the American firefly (Photinus pyralis) and D-luciferin substrate. Luciferase is generated by cells following transfection. It is important to select clones with high stable expression, usually based on lentiviral transfection, which tends to be more stable than plasmid transfection. It is important to recognize that clones isolated for high expression may not behave identically to parallel lines or the parental system (e.g., differential growth rates). Thus, tumor models can be highly effective in terms of assessing tumor development and response to therapy, but they may not perfectly replicate parental cell lines.
Pharmacokinetics of the luciferin substrate are important. Remarkably, luciferin appears to readily permeate every tissue including crossing the blood-brain and -placental barriers (4). However, the kinetics of light-emission can differ with tumor location, and thus, it is critical to establish reproducibility of light-emission curves prior to embarking on large scale studies. The most popular route of administration of luciferin is IP (intra peritoneal) (7), but while this is apparently facile, we find a significant failure rate (8), where no light-emission is observed following substrate administration, yet repeat one hour later gives expected bioluminescence. We attribute this to poor injection, possibly into the intestines. Intravenous (IV) administration can give much higher light-emission (9), but more transiently so that any variation in the timing of image capture and/or integration time can generate poorer reproducibility (8). Intravenous injection is also technically more challenging. Direct intra tumor (IT) injection generates the most intense bioluminescence, but is obviously invasive and only feasible for easily accessible tumors (7, 10). We favor subcutaneous (SC) administration of luciferin in the back neck region. The technique is facile with overwhelming success in observing expected signal and the kinetics provide intense light over several minutes (8, 11).
Light detection is strongest from subcutaneous tumor sites although in this case caliper measurements may be just as effective and cheaper for simple tumor volume assessment. However, BLI is particularly effective for low tumor burdens, and indeed, sub-palpable volumes can be detected and quantified. For large tumors, self absorption and scatter of light can bias apparent relative tumor volume. Planar BLI appears to accurately reflect the volume of small tumors, but becomes less linear for larger tumors, although continuing to increase monotonically (12, 13). Light is subject to significant absorption and scattering from deep tumors, and thus, equivalent tumors located at depth are expected to provide much less detectable light. Thus, for longitudinal studies it is crucial to view an animal from the same direction on successive occasions to ensure a reproducible solid viewing angle and consistent absorption by any intervening tissues. Nude mice are preferred, though light may also be detected from white or black mice with hair: some investigators prefer to shave the animals or apply depilating agents.
Bioluminescent imaging systems can be constructed quite easily and cheaply based on several recipes in the literature, primarily from the amateur astronomy field, where there is a similar need to detect weak signals against a low background based on long-term signal integration (14). To date, our BLI service uses a home built system, which has been described elsewhere (7, 8, 15). The primary protocol below describes the procedures with this system (Cyclops). However, the instrument is technically complex requiring a BLI technician and engineering support. Sophisticated commercial systems are available, which are user friendly (Caliper Xenogen1 and Berthtold2), and we have recently acquired both IVIS® Lumina and Spectrum systems for use by multiple research teams. These provide both bioluminescence and fluorescence imaging including depth resolved capabilities for the Spectrum. D-luciferin can cost $100 per 100 mg, but bulk purchases should allow better than $400 per gram, which is important for high-throughput screening.
Although BLI is simple, several properties require consideration. The light-emission can by characterized by parameters including area under the curve (AUC), maximum signal intensity, time to maximum intensity or light integration over a specified period. We routinely use a dose of 450 mg/kg administered subcutaneously into an anaesthetized nude mouse with imaging for a period of five minutes starting ten minutes after luciferin administration. Weak signals may require longer integration to achieve useful signal to noise, but many investigators prefer a constant acquisition method even though small tumors than provide essentially zero signal.
2 Materials
2.1 Preparation of Luciferin
D-Luciferin Firefly, sodium salt monohydrate (synthetic)-(Biosynth, CH-9422 Staad, Switzerland)3 stored in dark at −20 °C.
Sorensen’s phosphate buffer, 0.2M, pH 7.2 (Phosphate Mixed Solution Salts) stored at 2–8 °C (Electron Microscopy Sciences, Hatfield, PA 19440)
2.2 Administration of Luciferin
Lo-dose B-D 1/2 cc 28G1/2, U-100 Insulin Syringes, Latex free syringe, micro-fine IV (Becton-Dickinson and Company, Franklin Lakes, NJ, USA)
Veterinary Isoflurane, USP- (Webster, Sterling, MA)
USP Medical Grade oxygen compressed USP, 99% Pure, CGA connection 540, 281 CU FT cylinder tank.
2.3 Imaging Equipment
A CCD (SITe SI-032AB) non-color, back-illuminated, full frame image sensor with 512×512 pixels, (Scientific Imaging Technologies Inc., Tigard, OR) 4.
1400 Duo-Seal Vacuum Pump- (Welch, Niles, IL 60714)
FP88-HL Ultra-low Refrigerated Circulator (Julabo, Allentown, PA 18109)
Dark box to accommodate imaging system
Dehumidifier (Kenmore)
NIST-traceable research radiometer (IL 1700, International Light, Inc. Newburyport, MA) for camera calibration
Deltaphase isothermal warming pad (Model 39 DP; Braintree Scientific, Inc., Braintree, MA)
Anesthesia system Matrix Medical Inc. (VMC model 100F, Orchard Park, NY).
-
Data acquisition and processing computer running IGOR Pro (Wavemetrics, Seattle, WA, USA), and custom image analysis routines
or
Caliper Xenogen IVIS® Lumina with the LivingImage software (Hopkinton, MA)
3 Methods
3.1 Luciferin preparation
Luciferin is used at 40 mg/ml of D-Luciferin sodium salt monohydrate for both mouse and rat studies.
The substrate is mixed with 2M Sorensen’s Phosphate buffer solution.
After making the solution the vial must be covered (i.e. foil), since the luciferin is light sensitive and stored in a laboratory refrigerator.
3.2 Injection of Luciferin
Mouse is anesthetized with induction dose of isoflurane- 2.5% in oxygen 5.
A dose of 450 mg/kg (280 μl for 25 g mouse) is injected subcutaneously in the back neck region using a single use Lo-dose insulin syringe 10 minutes prior to imaging6. It is important that the tine to start imaging is kept constant between individuals and for repeat measurements, since the light emitted is strongly time dependant.
Depending on the region of interest, the animal is placed on a secure netted bed and the snout is placed in an anesthesia nose cone and maintained with 1.5% isoflurane and 1 l/min oxygen in the imaging box.
3.3 Imaging with Cyclops (Advanced Radiological Sciences Imaging System)
The vacuum pump must be turned on followed by the coolant pump of the refrigerated circulation system.
After the refrigerant has cooled to −1.0 °C, the charge-coupled cameras (CCD) are turned on and cooled to 230 K (−43 °C) using internal thermoelectric device.
The light box is closed. Prior to imaging the animal, a dark image is acquired to allow subtraction of dark current signal and interference noise from auxiliary equipment. The dark image integration time should be the same as the bioluminescent imaging time, which depends on how intense is the BLI signal. During dark image acquisition a mechanical shutter shields the camera sensor and therefore an image is taken without any light.
Diffuse light sources are applied and a 700 ms light image is acquired for image overlay to show the body of the animal for orientation and anatomical co-registration.
A 5 minute BLI image is taken immediately after the light image 7. In some cases multiple sequential images are acquired to reveal light emission dynamics. In high throughput mode usually a single image is captured for each animal.
At the end of the imaging series a second dark image is acquired.
Mice are returned to cage and monitored until fully recovered from anesthetic, usually within 5 mins.
Between groups of mice, the animal bed and support structure are sprayed with Quatricide disinfectant (Pharmacal Research Laboratories Inc., Waterbury, CT) to reduce risk of pathogen spread.
3.3.1 Processing Images
The images are saved on a personal computer and processed using IGOR Pro software with a set of custom image analysis routines 8.
Upload the Dark Image 1, then the Dark Image 2 and average them to allow subtraction of background/instrument noise.
Upload the BLI image and subtract averaged dark image.
The signal is measured by creating a region of interest (ROI) and integrating the signal. Signals are either measured in relative light units (RLUs) per second, radiance units photons/s/cm2/sr, or total light emission photons/s.
Upload the Light Image and overlay the bioluminescence image after the background is rendered transparent.
Save images as JPG or TIFF files.
3.3.2 Measuring the signal
Using Igor-Pro access ROI under the Image Tools.
Draw a ROI around the signal. The signal is measured by creating a box around the signal and integrating the region of interest.
Save the data in an Excel file and the picture in your computer drive as JPEG or TIFF files.
3.4 Xenogen Lumina IVIS System 9
The Lumina system includes anesthesia unit, heated platform and can image up to three mice at a time.
To begin, the user must Initiate IVIS System to begin the cooling down process. The optimal time between injection and imaging depends on the route of luciferin injection and tumor site.
When ready to image, place the mouse in the anesthesia induction chamber. After turning on the oxygen, the gas valve on the anesthesia is opened.
Turn on the evacuation pump before opening the induction chamber.
To image the mouse, place the mouse on the platform and open the anesthesia flow for the IVIS box. The door must be closed in order to begin imaging. For multiple mice a black shield is placed between animals to reduce cross-illumination.
There are 4 fields of view ranging from A (the closest in length to the cameras) and D (the farthest).
The user must select Bioluminescence since the Lumina has both fluorescent and bioluminescent capabilities. Exposure time is set in minutes and the pixel binning is usually set at medium.
After acquiring the image, the mouse must be taken out of the box and the oxygen tank, anesthesia unit and gas valve must be turned off.
The system remains on overnight to allow the dark images to be taken and saved. This is done automatically.
The images are saved on a personal computer and can be analyzed using the Living Image Data Software provided by Caliper.
The Lumina system automatically corrects light intensity for camera-subject distance and calibrates for dark images daily.
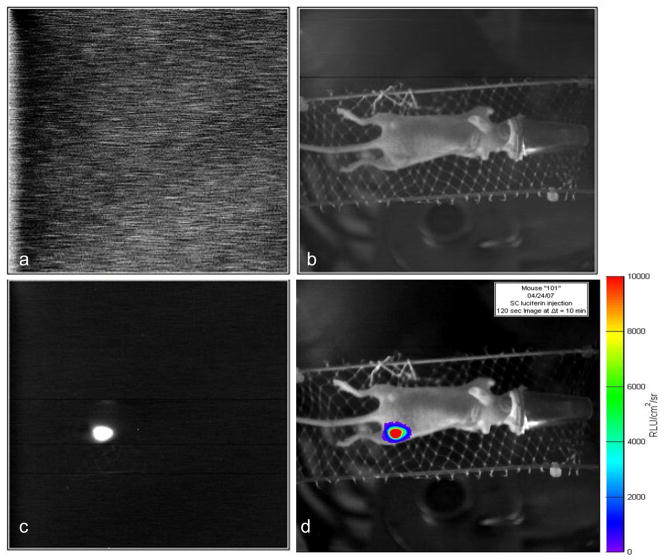
Figure 1. Images associated with BLI.
Images acquired with home-built Cyclops BLI system. A) Dark image to allow subtraction of background noise; b) Light image based on surface external illumination for anatomical registration; c) Bioluminescent image; c) Overlay of bioluminescent signal intensity on anatomical image.
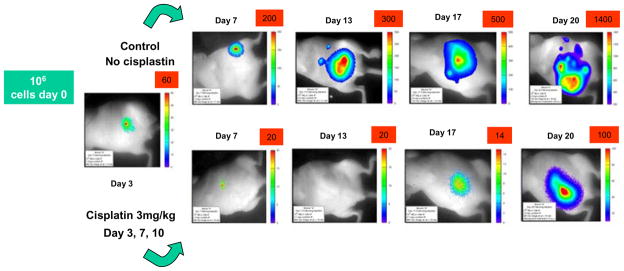
Figure 2. Assessing chemotherapy by BLI.
Series of images of two nude mice imaged at various times after injecting HeLa-luc cells into the peritoneum. On each occasion mice were anesthetized and 150 mg/kg luciferin was administered followed by a 10 min. image. The upper mouse served as control, while the lower series of images show a mouse which received cis-platinum chemotherapy at 3 mg/kg on days 3, 7 and 10 following introduction of tumor cells. Note images have been scaled differently to accommodate the massive changes in dynamic range.
Acknowledgments
Supported in part by grants from the DOD Breast Cancer Initiative (IDEA award DAMD17-03-1-0343), the NIH Cancer Imaging Program (P20 CA86354 and U24 CA126608) and the Simmons Cancer Center. We are grateful to Drs. Li Liu, Robert Bollinger, Jerry Shay, and Peter Antich for bringing the vision of BLI to UT Southwestern.
Footnotes
D-luciferin may be obtained from many sources as either synthetic or natural material. Sodium or potassium salts may be used. We have no evidence for differential quality. Other sources include D-Luciferin sodium salt (Catalog #10102; Biotium, Hayward, CA, USA); D-Luciferin Potassium Salt (P/N 122769) isolated from firefly (Caliper Life Sciences: http://www.caliperls.com/products/dluciferin-potassium-salt.htm)
In earlier work we had used a TC245 Charge-coupled device camera-(Texas Instruments, Dallas TX) (7). and a system based on the French Audine astronomical camera with a high performance Kodak KAF-0402ME CCD (14).
Other forms of anesthesia such as ketamine can also be used.
Other doses of luciferin may be used. Caliper recommends a dose of 150 mg/kg for mice with its Lumina Imaging system. Our experience favors the higher dose. Caliper Lifesciences recommends 10 to 15 minutes between injection and imaging.
The exposure time may be altered to avoid over-exposing intense signals or to detect weak signals. In practice, we may use anywhere from 1 to 30 mins. Many investigators like to maintain a constant imaging time, where 5 mins is typical.
The macros are really outside the scope of this chapter and interested readers are referred to (8)
Instructions derived from the instrument user manual.
References
- 1.Schaefer C, Mayer WK, Krüger W, Vaupel P. Microregional distributions of glucose, lactate, ATP and tissue pH in experimental tumours upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol. 1993;119:599–608. doi: 10.1007/BF01372723. [DOI] [PubMed] [Google Scholar]
- 2.Lundin A. Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Bioluminescence and Chemiluminescence. 2000;305(Pt C):346–370. doi: 10.1016/s0076-6879(00)05499-9. [DOI] [PubMed] [Google Scholar]
- 3.Contag CH, Ross BD. It’s not just about anatomy: In vivo bioluminescence imaging as an eyepiece into biology. JMRI. 2002;16:378–387. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SH, Contag CH. Using in vivo bioluminescence imaging to shed light on cancer biology. Proc IEEE. 2005;93:750–762. [Google Scholar]
- 5.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 6.Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci (USA) 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paroo Z, Bollinger RA, Braasch DA, Richer E, Corey DR, Antich PP, Mason RP. Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Molecular Imaging. 2004;3:117–124. doi: 10.1162/15353500200403172. [DOI] [PubMed] [Google Scholar]
- 8.Bollinger RA. PhD. Dallas; UT Southwestern: 2006. Evaluation of the Light Emission Kinetics in Luciferin/Luciferase-Based In Vivo Bioluminescence Imaging for Guidance in the Development of Small Animal Imaging Study Design Vol. [Google Scholar]
- 9.Wang W, El-Deiry WS. Bioluminescent Molecular Imaging of Endogenous and Exogenous p53-Mediated Transcription In Vitro and In Vivo Using an HCT116 Human Colon Carcinoma Xenograft Model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Cecic I, Chan DA, Sutphin P, Ray P, Gambhir SS, Giaccia AJ, EGE Oxygen Sensitivity of Reporter Genes: Implications for Preclinical Imaging of Tumor Hypoxia. Molecular Imaging and Biology. 2007;6:219–228. [PubMed] [Google Scholar]
- 11.Karam JA, Fan J, Stanfield J, Richer E, Benaim EA, Frenkel E, Antich P, Sagalowsky AI, Mason RP, Hsieh JT. The use of histone deacetylase inhibitor FK228 and DNA hypomethylation agent 5-Azacytidine in human bladder cancer therapy. Int J Cancer. 2007;120:1795–1802. doi: 10.1002/ijc.22405. [DOI] [PubMed] [Google Scholar]
- 12.Klerk CP, Overmeer RM, Niers TM, Versteeg HH, Richel DJ, Buckle T, Van Noorden CJ, van Tellingen O. Validity of bioluminescence measurements for noninvasive in vivo imaging of tumor load in small animals. Biotechniques. 2007;43:7–13. doi: 10.2144/000112515. [DOI] [PubMed] [Google Scholar]
- 13.Sarraf-Yazdi S, Mi J, Dewhirst MW, Clary BM. Use of in vivo bioluminescence imaging to predict hepatic tumor burden in mice. J Surg Res. 2004;120:249–255. doi: 10.1016/j.jss.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Kanto V, Munger J, Berry R. The CCD Camera Cookbook. Richmond, VA: Willman-Bell, Inc; 1994. [Google Scholar]
- 15.Dikmen ZG, Gellert G, Dogan P, Mason R, Antich P, Richer E, Wright WE, Shay JE. A New Diagnostic System in Cancer Research: Bioluminescent Imaging (BLI) Turk J Med Sci. 2005;35:65–70. [Google Scholar]