Abstract
This case report describes a case of congenital sideroblastic anaemia, one of the prototype disorders of erythroid haem biosynthesis. In this instance it was not recognised until after the patient had undergone splenectomy and developed refractory thromboembolic disease.
Background
For reasons that are not well known, patients with X linked sideroblastic anaemia (XLSA) develop recurrent thromboembolic disease after splenectomy. This has been reported in a small number (<20) of patients with the disease. The thrombophilia is usually refractory to conventional anticoagulation measures and is fatal in a high percentage of cases.
Case presentation
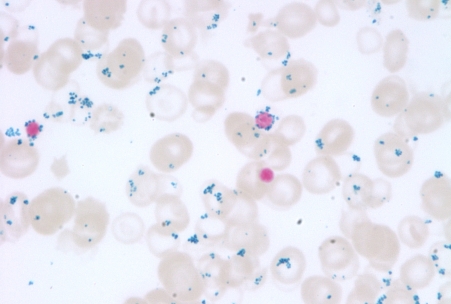
A 24-year-old man was admitted to our institution with refractory thromboembolic disease. His medical history began at 7 months of age when he was found to have a haemoglobin value of 10.0 g/dl during closure of a ventricular septal defect. A peripheral smear showed ‘target cells and siderocytes’. No investigation was performed until age 14 when a bone marrow biopsy was performed. Results showed a normocellular marrow with increased iron stores, ring sideroblasts and erythroid hypoplasia (figure 1). Treatment with vitamin B6 200 mg by mouth daily was initiated but the patient was not compliant with treatment. He was lost to follow-up until the age of 20 when he underwent open splenectomy for splenic rupture in the setting of infectious mononucleosis. Pathology of the spleen described findings consistent with viral infection.
Figure 1.
Insufficient protoporphyrin production decreases use of iron delivered to the erythroblasts. Iron accumulates in the mitochondria leading to the development of characteristic ring sideroblasts (arrows).
The family history was remarkable for his mother dying suddenly at age 47 due to unknown causes; an autopsy was not performed. She had had multiple miscarriages while she was alive. A brother with a history of diabetes and hypertension died at age 39 secondary to a possible drug overdose. He has a paternal aunt diagnosed as having colon cancer at age 57 and a paternal uncle who died at age 50 with lung and throat cancer. There was no family history of anaemia, thrombosis or iron overload.
Approximately 4 months after the splenectomy, the patient developed pain and swelling of the left lower extremity. Duplex ultrasonography revealed acute deep vein thrombosis (DVT). He experienced two further episodes of lower extremity DVT while on warfarin; the prothrombin (PT) and international normalised ratio (INR) (PT/INR) status is not known during these events. Thereafter a Greenfield filter was inserted and warfarin was continued.
Approximately 3 years later, the patient presented with left upper extremity pain. A duplex ultrasound showed evidence of acute DVT in the left basilic vein in the setting of a subtherapeutic PT/INR. At the time of admission, peripheral smear showed target cells with microcytes, nucleated red blood cells, basophilic stippling and Pappenheimer bodies with possible spherocytes. Heparin bridging was administered until the PT/INR was therapeutic on warfarin. While awaiting a therapeutic PT/INR, the patient developed left knee pain. The duplex ultrasound showed findings consistent with a left popliteal vein DVT. He was transferred to our institution for evaluation of thrombophilia.
He was seen in the outpatient's clinic after discharge and was again given pyridoxine 200 mg by mouth daily. He tolerated this well but had no improvement in his anaemia. He was followed by our haematology clinic who sought to keep his PT/INR between a goal of 2.5 and 3.5. At 6 months post discharge from the hospital, he returned to the Emergency Department with superficial vein thrombosis in the setting of a supratherapeutic INR. This was determined to be a failure while on warfarin and twice daily weight-based enoxaparin was given instead.
Approximately 1 year later, the enoxaparin was held for 2 days at the discretion of his dentist and he developed bilateral pulmonary emboli. He was discharged on twice-daily weight-based enoxaparin. A factor Xa level was drawn after discharge and returned 0.26 IU/ml. Enoxaparin was subsequently increased from 90 to 110 mg twice daily at the direction of our haematologists.
Investigations
The patient underwent a bone marrow examination, which revealed normal morphology of the haematopoietic cell lines, numerous ringed sideroblasts and increased iron stores. A formal analysis for the presence of the aminolevulinate synthase 2 (ALAS2) gene mutation was performed which showed a missense mutation (c. 1611 C>T) in exon 10, predicting the substitution of leucine for proline at residue 520 (Pro520Leu), thus establishing the diagnosis of XLSA. The serum transferrin saturation and ferritin values indicated advanced iron overload; the Human hemochromatosis protein (HFE) gene mutations Cyt282Trp and His63Asp were absent.
Differential diagnosis
Large spleen size and an underlying myeloproliferative disorders are considered to be risk factors for postsplenectomy thrombosis with the majority of cases occurring in the portal vein.1 2 In any patient presenting with a first-time or recurrent, non-situational acute DVT, a hypercoagulable condition such as deficiency of protein C, protein S and antithrombin III should be considered as possible causes however several tests used in the screening for inherited causes of thrombophilia must be performed in the absence of anticoagulant treatment. Discontinuation of anticoagulation was felt to pose a significant risk in this patient.
Treatment
Supportive treatments can provide normal survival in most cases of congenital sideroblastic anaemia and are aimed at control of symptoms of the anaemia and prevention of organ damage from the associated iron overload. Only in XLSA, the anaemia may respond to pyridoxine supplements, which is dictated by whether the specific mutation affects the binding of the pyridoxal-5′-phosphate (PLP) cofactor to the ALAS2 enzyme. Accordingly, a partial, a complete or no response may occur. In severely anaemic individuals transfusions are necessary to relieve symptoms but should be kept to a minimum to lessen further iron overload.
Haematopoietic stem cell transplantation provides the only curative treatment of congenital sideroblastic anaemias but with the attendant risks of the procedure. To date, it has been successful in several reported cases and three unreported cases (S Bottomley, personal communication) who were mostly children.
Outcome and follow-up
At 7 months after his enoxaparin was held for a dental extraction, the patient missed two additional doses of enoxaparin. He was readmitted to the hospital with chest pain, dyspnoea and syncope. CT imaging revealed bilateral submassive pulmonary emboli with evidence of right heart strain. Due to persistent hypoxia, he underwent open embolectomy. He was discharged on enoxaparin 110 mg twice daily and has had no further thrombotic disease.
Discussion
To date, close to 50 distinct mutations have been identified among about 80 unrelated kindred or individuals and nearly all involve the catalytic domain of the enzyme.3 The Pro520Lys mutation detected in our patient was previously reported in two unrelated men, one without anaemia and the other with severe sideroblastic anaemia who also had a second ALAS2 mutation (Arg560His).4 Recurrent thromboembolism after splenectomy is a recognised phenomenon in other hereditary anaemias including hereditary non-spherocytic haemolytic anaemia, haemoglobin H disease, hereditary stomatocytosis and thalassaemia intermedia.5–7 Similarly, thrombosis is common in congenital sideroblastic anaemia following splenectomy and venous thromboembolic disease is fatal in a large percentage of these patients. While the pathogenesis is not known with certainty, platelet adhesiveness8 and increased PT generation by exposed procoagulant phospholipids on altered membranes of the most abnormal erythrocytes (which are no longer removed by splenic macrophages) observed in patients who have been splenectomised and have thalassaemia are considered to play a principal role in the thromboembolic events. Similar mechanisms can be expected to be operative in sideroblastic anaemias. Although the thrombocytosis after splenectomy may be greater and more prolonged than in normal individuals, evidence is lacking that it contributes to the thrombotic risk.
Any temptation to perform splenectomy in these disorders must be importantly tempered with the clinical urgency, for example, the presence of traumatic splenic rupture or otherwise untreatable splenic abscess,9 because of the very common complication of subsequent thrombotic events that are often fatal. Whether partial splenectomy may prevent this complication is not known.
Learning points.
The principal manifestations of congenital sideroblastic anaemia are anaemia, life-threatening thromboembolic disease after splenectomy and iron overload.
Following splenectomy, the thrombophilia is often severe and refractory to medical treatment.
In patients with severe anaemia, transfusions are necessary to alleviate symptoms but should be kept to a minimum to lessen further iron overload.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Hassn AM, Al-Fallouji MA, Ouf TI, et al. Portal vein thrombosis following splenectomy. Br J Surg 2000;87:362–73 [DOI] [PubMed] [Google Scholar]
- 2.Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–5; discussion 635–6 [DOI] [PubMed] [Google Scholar]
- 3.Bottomley S. Sideroblastic anemias. In: Greer JP, Foerster J, Lukens J, eds. Wintrobe's Clinical Hematology. Philadelphia, PA: Lippincott Williams & Wilkins, 2008:835–56 [Google Scholar]
- 4.Lee PL, Barton JC, Rao SV, et al. Three kinships with ALAS2 P520L (c. 1559 C –> T) mutation, two in association with severe iron overload, and one with sideroblastic anemia and severe iron overload. Blood Cells Mol Dis 2006;36:292–7 [DOI] [PubMed] [Google Scholar]
- 5.Hirsh J, Dacie JV. Persistent post-splenectomy thrombocytosis and thrombo-embolism: a consequence of continuing anaemia. Br J Haematol 1966;12:44–53 [DOI] [PubMed] [Google Scholar]
- 6.Stewart GW, Amess JA, Eber SW, et al. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br J Haematol 1996;93:303–10 [DOI] [PubMed] [Google Scholar]
- 7.Cappellini MD, Robbiolo L, Bottasso BM, et al. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol 2000;111:467–73 [DOI] [PubMed] [Google Scholar]
- 8.Goldschmidt N, Spectre G, Brill A, et al. Increased platelet adhesion under flow conditions is induced by both thalassemic platelets and red blood cells. Thromb Haemost 2008;100:864–70 [PubMed] [Google Scholar]
- 9.Aleali SH, Castro O, Spencer RP, et al. Sideroblastic anemia with splenic abscess and fatal thromboemboli after splenectomy. Ann Intern Med 1975;83:661–3 [DOI] [PubMed] [Google Scholar]