Abstract
Restoration of autophagy represents a potential therapeutic target for neurodegenerative disorders, but factors that regulate autophagic flux are largely unknown. When deprived of trophic factors, cultured Purkinje neurons die by an autophagy associated cell death mechanism. The accumulation of autophagic vesicles and cell death of Purkinje neurons is inhibited by insulin-like growth factor, by a mechanism that enhances autophagic vesicle turnover. In this report, we identify Rab7 as an IGF-I regulated target during neuronal autophagy. Purkinje neurons transfected with EGFP-Rab7-WT and constitutively active EGFP-Rab7-Q67L contained few RFP-LC3 positive autophagosomes and little co-localization with GFP-Rab7 under control conditions. Upon induction of autophagy, RFP-LC3 positive autophagosomes increased and co-localized with GFP-Rab7. Conversely, expression of the dominant negative mutant EGFP-Rab7-T22N increased the accumulation of autophagosomes under control conditions, which accumulated even further during trophic factor withdrawal. There was no vesicular co-localization between Rab7-T22N and RFP-LC3 under control or trophic factor withdrawal conditions. During prolonged trophic factor withdrawal, a condition that leads to the accumulation of autophagic vesicles and cell death, Rab7 activity decreased significantly. IGF-I, added at the time of trophic factor withdrawal, prevented the deactivation of Rab7 and increased the interaction of Rab7 with its interacting protein (RILP), restoring autophagic flux. These results provide a novel mechanism by which IGF-I regulates autophagic flux during neuronal stress.
Keywords: autophagy, Rab7, IGF-I, neurons
Introduction
Macroautophagy, herein referred to as autophagy, is a cellular housekeeping process that degrades the components of the cell through the lysosomal machinery [19,20]. Autophagy plays a vital role in cellular development, homeostasis, and survival under nutrient deprived or stressful conditions. Conversely, autophagy may contribute to cell death, particularly in the nervous system where conditions such as metabolic stress, aggregated mutant proteins and normal aging impair autophagy signaling leading to decreased autophagic vesicle turnover and subsequent autophagy-associated cell death [8,24]. This imbalance of autophagy signaling referred to as “autophagic stress” is thought to contribute to Parkinson’s [1], Huntington’s [18] and Alzheimer’s disease [7], as well as, to stroke and other neuropathies [4,14]. Thus, there is a growing interest in identifying signaling proteins that control neuronal autophagy.
Rab7 is a small GTPase important in membrane trafficking through the endocytic pathway [22] particularly the fusion of late endosomes with lysosomes [5,21]. Recent studies have indicated a role of Rab7 in the late maturation of autophagosomes [11,13].
Studies from our laboratory demonstrate that the rate of autophagosome to lysosome fusion in cultured Purkinje neurons is regulated by insulin-like growth factor-I (IGF-I), an important neurotrophic factor for these neurons [2,3]. The ability of IGF-I to increase autophagic flux under conditions of trophic factor withdrawal (TFW) prevents autophagic vesicle accumulation and autophagy-associated cell death. To identify a potential mechanism by which IGF-I increases vesicle fusion and turnover, we examined whether IGF-I regulates Rab7 activity during neuronal autophagy.
Materials and methods
Reagents
GFP-Rab7, GFP-Rab7-Q67L and GFP-Rab7-T22N were provided by Bo van Deurs (University of Copenhagen). GST-RILP plasmids were provided by Aimee Edinger (University of California-Irvine). The adenoviral RFP-LC3 was from Aviva Tolkovsky (University of Cambridge, Cambridge, England). The polyclonal rabbit antibodies to LC3, GST, GFP, RILP, and Lamp1 were obtained from Abgent (San Diego, CA), Cell Signaling Technology (Beverly, MA), Clontech Laboratories (Mountainview, CA), Abcam Inc (Cambridge, MA) and Sigma-Aldrich (Saint Louis, MO), respectively. Monoclonal Anti-Rab7 and goat polyclonal RILP were obtained from Sigma-Aldrich (Saint Louis, MO) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Cell Culture
Animal procedures were approved by the Institutional Animal Care and Use Committee of The University of Colorado Denver and were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health. Primary rat cerebellar granule neurons were isolated from 7-day old Sprague Dawley rat pups as described previously [26]. Cells were plated on laminin (Invitrogen; Carlsbad, CA) coated coverslips (Thermo Fisher Scientific Inc., Rock- ford, IL), or Poly-L-lysine coated culture dishes (Becton Dickinson, Franklin Lakes, N.J.) in basal modified Eagle’s (BME) medium containing 10% fetal bovine serum, 25 mM KCl, 2 mM L-glutamine, and penicillin(100units/ml)-streptomycin (100μg/ml) (Invitrogen; Carlsbad, CA). Cytosine arabinoside (10 μM) was added to the culture medium 24 hr after plating to limit the growth of non-neuronal cells. On day 2, neurons were infected with adenoviral vector expressing MAP1-LC3 labeled with red fluorescent protein (RFP-LC3) at a multiplicity of infection of 100 for 24 hr. Autophagy was induced by removing the plating medium and replacing it with TFW medium (serum-free BME containing 5 mM KCl).
Amaxa Nucleofection
Rat cerebellar granule neurons were transfected using the Rat Neuron Nucleofector kit from Lonza Cologne AG (Cologne, Germany) at the time of plating using their optimized protocol. A nucleofection reaction containing 4×106 cells was plated onto three laminin-coated coverslips or a 35-mm culture dish and a nucleofection reaction containing 10×106 cells was plated onto a 60-mm culture dish. Immunofluorescence staining demonstrated that 60–80% of cells were transfected with Rab7 and the transfection efficiency was largely comparable for the different constructs (data not shown).
Immunocytochemistry
Immunofluorescent staining was performed as previously described (2). Cells were incubated overnight at 4°C with LC3 (1:50), RILP (1:250), dynein (1:250) or Rab7 (1:250) diluted in PBS containing 0.2% TX-100 and 2% BSA. Cells were then incubated with the appropriate Cy3 (1:500)- or FITC-conjugated (1:500) secondary antibodies and DAPI (1 ug/ml). Fluorescence imaging was performed on a Zeiss Axioplan 2 microscope equipped with a Cooke sensicam deep-cooled CCD camera and images were analyzed and subjected to digital deconvolution using Slidebook (Intelligent Imaging Innovations Inc.; Denver, CO).
Live-cell imaging of autophagy
After the appropriate treatment times, cells were stained at 37°C with Hoechst (20 ng/ml) for 15 min to visualize cellular nuclei and live-cell imaging was performed as previously described [2]. Images of GFP-Rab7, RFP-LC3 and Hoechst fluorescence were captured on the FITC, Cy3 and DAPI channels, respectively, using a 63X water immersion objective.
Western blotting
Basic Western blotting methods were followed as previously described (2). Primary antibodies were diluted as follows: LC3: 1:500, RILP: 1:1000 and Rab7: 1:1000 in blocking solution (phosphate-buffered saline containing 0.1% Tween 20 and 5% BSA) and incubated with the membranes overnight at 4°C. Autoluminograms shown are representative of at least two independent experiments.
Glutathione Transferase-Rab Interacting Lysosomal Protein (GST-RILP) Pull-Down Assay
Pull-down assays were performed as previously described [23]. Briefly, GST alone or GST-RILP was transformed into Escherichia coli strain BL21 at 37°C overnight. Luria broth was inoculated with the overnight culture and grown at 37°C. Isopropyl β-D-thiogalactoside was added to induce protein production. The bacteria were washed with cold phosphate-buffered saline and resuspended in cold lysis buffer. Proteins were purified by adding pre-equilibrated Glutathione-Sepharose 4B beads (GE Healthcare; Piscataway, NJ) to the lysate and incubated for 30 min at room temperature. Beads were washed with lysis buffer, resuspended as 50% slurry and protein levels were quantified using the BCA assay. Each pull-down was performed in 500 μl with 150 μg of cell lysate and 15 μg of beads pre-equilibrated in pull-down buffer. Beads were rocked overnight at 4°C and washed with cold pull-down buffer. Bound proteins were eluted with sample buffer containing DTT and separated by SDS-PAGE.
Data Analysis
Results shown represent the mean ±SEM from three independent experiments. Statistical differences between the means of unpaired sets of data were evaluated using one-way analysis of variance followed by a post hoc Tukey test. A p value of <0.05 was considered statistically significant.
Results and Discussion
Rab7 regulates autophagic flux in Purkinje neurons
Cerebellar cultures were transfected with GFP alone or Rab7-WT, Rab7-Q67L, or Rab7-T22N mutants tagged to GFP, infected with RFP-tagged LC3, a cellular marker for early-to-late autophagosomes, and then incubated overnight in the absence or presence of trophic factors as previously described [2]. In living Purkinje neurons transfected with EGFP-Rab7-WT (Fig. 1A; g,h) and EGFP-Rab7-Q67L (Fig. 1A; i,j), the EGFP signal was punctate throughout the cytoplasm and perinuclear region in control and TFW conditions. Conversely, expression of the dominant negative mutant EGFP-Rab7-T22N, which remains bound to GDP, resulted in a diffuse cytosolic signal under both control and TFW conditions (Fig. 1A; k,l). In the presence of trophic factors (0 hr control), Rab7-WT and Rab7-Q67L expressing neurons contained few RFP-LC3 positive vacuoles indicative of basal autophagy (Figure 1A; m,o, Fig. 1B). There was little co-localization of GFP-Rab7 with RFP-LC3 (Fig. 1A; s,u, Fig. 1C). At 16 hr of TFW, there was a significant increase in RFP-LC3 positive autophagosomes (Fig. 1A; n,p, Fig. 1B, **p<0.01, *p<0.05 compared to Rab7-WT and Rab7-Q67L control, respectively) and increased co-localization with Rab7 (Fig. 1A; t, v, Fig. 1C, **p<0.01, *p<0.05 compared to Rab7-WT and Rab7-Q67L control, respectively). Rab7 colocalization with RFP-LC3 appeared higher in Rab7-Q67L cells compared to Rab7-WT (Figure 1C). Since autophagosome turnover is rapid in these neurons, the observed increase in LC3-II is modest compared to the increase observed when vesicle degradation is inhibited [3]. Expression of Rab7-T22N resulted in an increased accumulation of autophagosomes under control conditions (Fig. 1A; q, Fig. 1B, #p<0.05 compared to Rab7-WT and Rab7-Q67L control) and an even further accumulation of autophagosomes after TFW (Fig. 1A; r, Fig. 1B). There was no vesicular co-localization between Rab7-T22N and RFP-LC3 under control or TFW conditions (Fig. 1A; w,x, Figure 1C). These data confirm previous findings in CHO and HeLa cells that active Rab7 co-localizes with autophagosomes [11,13]; whereas, an inactive Rab7 fails to co-localize with the vesicular compartment of autophagosomes [11]. Although Gutierrez et al. [11] reported the presence of Rab7-T22N on autophagic vacuole membranes, it is important to note that the EGFP Rab7-T22N signal observed was distinct from Rab7-WT and Rab7-Q67L and was not vesicular, but rather a diffuse cytosolic stain similar to our observations and others using Rab7-T22N ([5,6,13]).
Fig. 1.
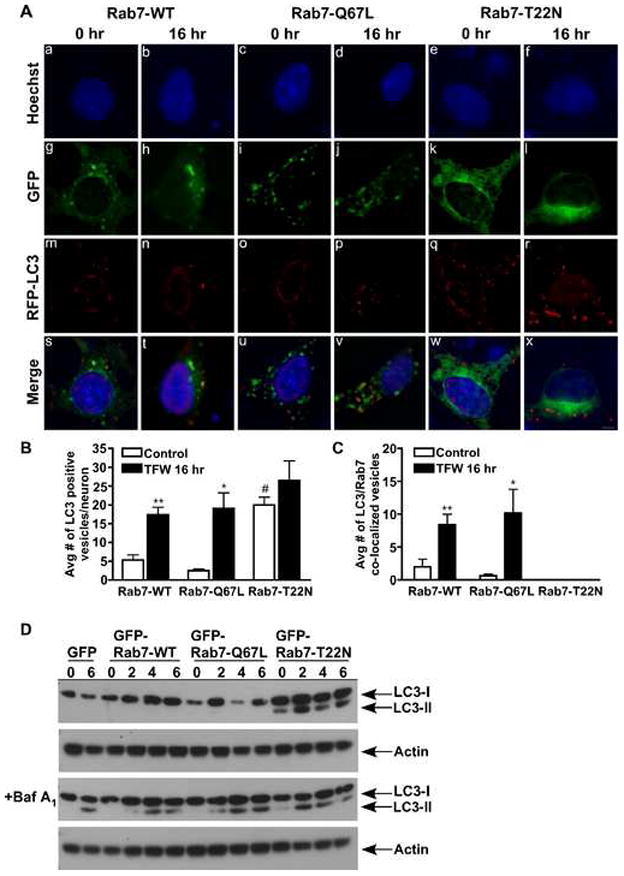
Rab7 regulates autophagic flux in Purkinje neurons. (A) Cerebellar cultures were transfected with GFP or Rab7-WT, Rab7-Q67L, or Rab7-T22N tagged to GFP. Neurons were infected with RFP-LC3, incubated in control or TFW media for 16 hr and subjected to live-cell imaging. Scale bar represents 5 μm. The total number of autophagosomes (B) and Rab7/LC3 co-localized vesicles (C) were counted in 10 Purkinje neurons per treatment condition and averaged for three separate experiments. (D) Rab7 transfected neurons were treated with control or TFW media in the absence or presence of bafilomycin A1 (100 nM) for 0, 2, 4 and 6 hr and an immunoblot analysis was performed against LC3. Actin served as a loading control. Figure 1D is a representative immunoblot of three independent experiments. Data represent mean ± SEM of three independent experiments. (B) **p<0.01 compared to Rab7-WT control, *p<0.05 compared to Rab7-Q67L control, #p<0.05 compared to Rab7-WT and Rab7-Q67L control (C) **p<0.01 compared to Rab7-WT control, *p<0.05 compared to Rab7-Q67L control. One-way ANOVA followed by Tukey’s post hoc test.
During autophagy, cytosolic LC3-I is lipidated to form LC3-II, which binds to the autophagosomal membrane, thus making it a marker of autophagic activity [17]. We analyzed the effects of the Rab7 mutants on LC3-II expression and turnover in Purkinje neurons subjected to TFW. Rab7-WT, Rab7-Q67L or Rab7-T22N transfected cultures were subjected to TFW for 0, 2, 4, and 6 hours in the absence or presence of bafilomycin A1, a vacuolar ATPase inhibitor that inhibits vesicle degradation ensuring accurate measurements of total LC3-II [9]. TFW alone did not result in measurable levels of LC3-II in Rab7-WT and Rab7-Q67L transfected cells confirming previous data [2] that the turnover of autophagic vesicles was rapid in these cultures (Fig. 1D, top blot). However, expression of Rab7-T22N resulted in a dramatic time-dependent increase in LC3-II expression indicative of autophagosome accumulation. As expected, in the presence of bafilomycin A1, TFW resulted in LC3-II induction and accumulation in Rab7-WT and Rab7-Q67L transfected cells, as well as, in Rab7-T22N transfected cells (Fig. 1D bottom blot). Unlike, Rab7-WT and Rab7-Q67L transfected cells, autophagosome accumulation in the presence of Rab7-T22N occurs both in the presence and absence of bafilomycin A1, indicating an impairment of vesicle turnover when Rab7 is inactive.
Rab7 activity during trophic factor withdrawal-induced autophagy
Using a Rab7 effector protein pull-down assay developed by Edinger and colleagues [23], we quantified the interaction of Rab7 with the Rab7-interacting lysosomal protein (RILP), which binds only active GTP-bound Rab7 [6,16] in Rab7-WT and Rab7-T22N transfected neurons in the absence or presence of trophic factors and IGF-I (Fig. 2A). Compared to control, 16 hr of TFW significantly decreased the amount of Rab7-WT precipitated by GST-RILP-coated beads indicating a lower level of GTP-bound Rab7 (Fig. 2A and 2B, *p<0.05 compared to control). IGF-I treatment during TFW maintained Rab7-RILP binding at levels indistinguishable from control levels (Fig. 2A and 2B, #p<0.05 compared to TFW). Under control conditions, IGF-I had a small but insignificant effect on Rab7-RILP binding compared to control (Fig. 2A). As expected, GST-RILP-coated beads did not precipitate constitutively GDP-bound Rab7-T22N confirming the specificity of GST-RILP to GTP-bound Rab7 (Fig. 2A). Since IGF-I is known to signal via the serine/threonine kinase, Akt, we examined the role of Akt in mediating the effect of IGF-I on Rab7 activity. When the specific Akt inhibitor, SH-5 (1.0 μM), was included with IGF-I in TFW conditions, it completely blocked the effect of IGF-I on rescuing Rab7-RILP binding (Fig. 2C and 2D). SH-5 had no additional effect on Rab7-RILP binding in the absence of IGF-I treatment (Fig. 2C).
Fig. 2.
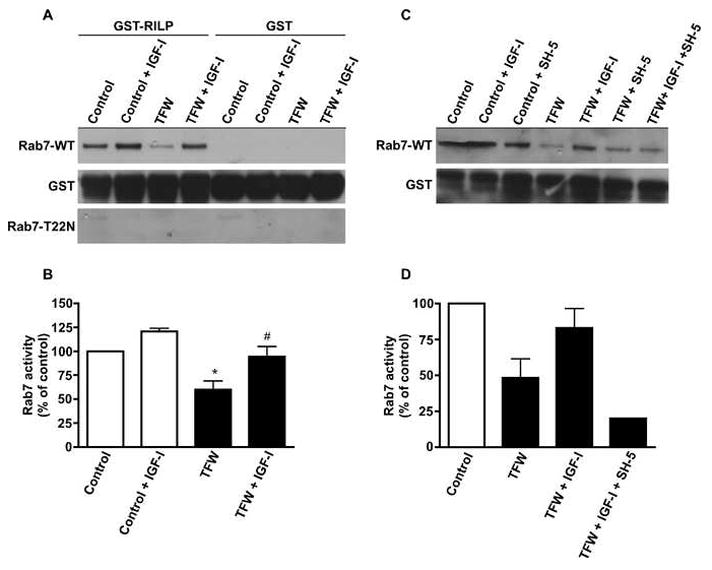
Rab7 activity during TFW-induced autophagy is regulated by IGF-I. (A) and (C) Cerebellar cultures were transfected with GFP or GFP-tagged Rab7-WT or Rab7-T22N and subjected to 16 hr of TFW in the absence or presence of IGF-I (200 ng/ml) or IGF-I plus the Akt inhibitor, SH-5 (1.0 μm). GST or GST-RILP were added to the cell lysates to precipitate active GTP-bound Rab7. Total GST served as a loading control. (B,D) Quantification of results shown in (A and C). The amount of Rab7 detected in the GST-RILP pull-down was normalized to GST and expressed as Rab7 activity, percent of control. Data represent mean ± SEM of three independent experiments. (B) *p<0.05 compared to control, #p<0.05 compared to TFW. One-way ANOVA followed by Tukey’s post hoc test.
These data are the first to indicate that IGF-I regulates Rab7 activity during autophagy. We hypothesize that IGF-I acts similar to constitutively active Rab7 by preventing Rab7 deactivation, thus maintaining autophagic vesicle turnover at a steady rate. As observed in Figure 1A, Rab7-Q67L increased RFP-LC3 colocalization with Rab7 in response to TFW when compared to wild-type. This effect parallels the IGF-I mediated increase in fusion between RFP-LC3 and lysosomes following TFW [2]. The addition of IGF-I to Rab7-Q67L transfected cells subjected to TFW did not further affect autophagic flux when compared to the Rab7 mutant alone (data not shown). Taken together with previous findings that IGF-I increases the rate of fusion between autophagosomes and lysosomes under conditions of TFW, these data provide a new mechanism by which IGF-I regulates autophagic flux in neurons. This action of IGF-I leads to decreased accumulation of autophagosomes during TFW-induced stress and prevents autophagy-associated cell death.
Studies by Yamamoto et al. [29] correlate with our IGF-I data using a serum withdrawal independent model of autophagy. In the presence of serum, IGF-I treatment induced autophagy-mediated degradation of accumulated huntingtin aggregates in HeLa cells expressing mutant exon1htt and occurred under conditions of IRS-2, Akt and mTOR activation [29]. Previously published studies from our lab have also established Nerve Growth Factor as an inhibitor of autophagy-associated Purkinje neuron death in response to serum withdrawal, which was dependent on the p75 neurotrophin receptor [10]. We are confident that the observed regulation of autophagy by IGF-I is not simply a starvation reversal or specific to IGF-I, but a novel neuroprotective signaling response of neurotrophic factors.
The mechanism by which IGF-I regulates Rab7-GTP binding appears to involve protein phosphorylation downstream of the protein kinase Akt. Rab7 oscillates between an inactive, soluble, GDP-bound form and an active, membrane, GTP-bound form at a frequency determined by the rate of GDP-GTP exchange, which is controlled by guanine-nucleotide exchange factors (GEFs) and GTP activating factors (GAPs). In addition, guanine-nucleotide dissociation inhibitors (GDIs) play a role in the recycling Rab7. GDI binds Rab7 in the GDP-bound form through the geranyl-geranyl group covalently linked to residues in the C-terminus of Rab7 [25]. Through this activity, GDI can retrieve Rab7 from the membrane following bilayer fusion. Further identification and characterization of GEFs, GAPs, and GDIs specific for Rab7 will allow investigation of their potential role in the IGF-I signaling pathway regulating Rab7.
The role of RILP in neuronal autophagy
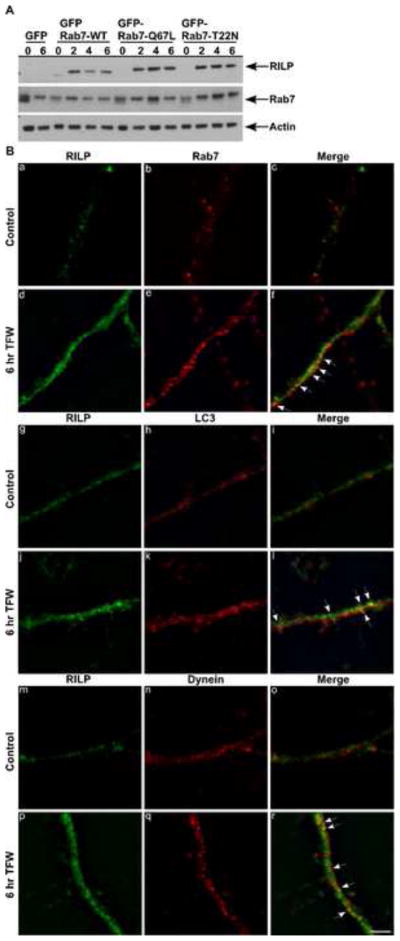
We next examined endogenous RILP expression and its potential role in autophagy. Western blots indicated that the levels of RILP in Purkinje neurons were low under control conditions. However, within two hours of TFW, levels of RILP dramatically increased and remained high for at least 6 hours after inducing autophagy (Fig. 3A). Endogenous Rab7 levels remained unchanged during these time points (Fig. 3A). Immunocytochemical staining demonstrated that RILP expression increased along neuronal processes during TFW (Fig. 3B) where it co-localized with a fraction of Rab7 and LC3 stained vesicles (Fig. 3B; f, l arrows). RILP staining also partially overlapped with staining for the microtubule motor protein dynein (Fig. 3B; r arrows).
Fig. 3.
RILP expression levels and localization during TFW-induced autophagy. (A) Cerebellar cultures were transfected with GFP or Rab7-WT, Rab7-Q67L or Rab7-T22N tagged to GFP. Neurons were treated in the absence or presence of trophic factors for 0, 2, 4 and 6 hr. Immunoblot analysis was performed using a polyclonal antibody to RILP and Rab7. Actin served as a loading control. (B) Cerebellar cultures were maintained in control or TFW medium for 6 hr, fixed and stained simultaneously with antibodies against RILP (green) and Rab7 (red), RILP (green) and LC3 (red) or RILP (green) and dynein (red). Shown are representative images of the immunofluorescence findings. Scale bar represents 5 μm.
Previous studies in nonneuronal cells have shown that a Rab7-RILP complex recruits the dynein-dynactin motor complex for the minus-end transport of late endocytic vesicles and phagosomes along microtubules [12,15,16]. The observed dramatic increase in RILP expression during TFW implicates RILP in the process of neuronal autophagy. However, a paradox lies between the decreased association of Rab7 with RILP and increased expression of RILP during TFW. Increased RILP expression upon TFW was observed by Western blot in all three Rab7 mutants and by immunocytochemistry in nontransfected cells. It remains possible that the RILP effect is independent of the GDP/GTP exchange status of Rab7. Overexpression of RILP alone is sufficient to redistribute lysosomes even in the face of inactive GDP-bound Rab7 [6]. This suggests that RILP may also recruit the dynein-dynactin complex independent of Rab7 or interact with a separate protein involved in vesicle transport. One such protein, Rab34, a golgi/cytosolic small GTPases, has recently been identified as a second interacting partner to RILP in the regulation of lysosomal morphology and distribution and may act in parallel with Rab7 [27,28].
Our findings of dramatic induction of RILP expression during autophagy and its co-localization with dynein, Rab7 and LC3 suggests that a similar Rab7-RILP-mediated recruitment of the dynein-dynactin motor complex occurs on mature autophagosomes in neurons, possibly facilitating the transport of autophagosomes in neuronal processes to perinuclear lysosomes for fusion. The observed deactivation and disruption of Rab7 interaction with RILP during TFW represents a severe consequence of prolonged stress as it likely disrupts autophagic vesicle trafficking resulting in vesicle accumulation and neuronal death. The ability of an IGF-I/Akt pathway to restore the levels of GTP-bound Rab7-RILP demonstrates a novel target of IGF-I for neuronal survival.
Research Highlights.
LC3 colocalizes with Rab7 during trophic factor withdrawal-induced autophagy.
Expression of dominant negative Rab7-T22N results in autophagosome accumulation during trophic factor withdrawal-induced autophagy.
The interaction of Rab7 with its interacting protein, RILP decreases during trophic factor withdrawal.
IGF-I restores the interaction of Rab7 and RILP during trophic factor withdrawal-induced autophagy.
Acknowledgments
We are grateful to Dr. Bo van Deurs for the Rab7 plasmids, Dr. Aimee Edinger for the GST-RILP plasmid and Dr. Aviva M Tolkovsky for the adeno-RFP-LC3. This work was supported by Department of Veterans Affairs Merit Award, NIH grant NS045560 and NIH/NINDS grant 1F32NS062534-01A1. Molecular Biology Core Services were supported by the NIH DERC grant P30-DK57516.
Abbreviations
- IGF-I
Insulin-like growth factor I
- RFP-LC3
MAP1-LC3 labeled with red fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 2.Bains M, Florez-McClure ML, Heidenreich KA. Insulin-like growth factor-I prevents the accumulation of autophagic vesicles and cell death in Purkinje neurons by increasing the rate of autophagosome-to-lysosome fusion and degradation. J Biol Chem. 2009;284:20398–20407. doi: 10.1074/jbc.M109.011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bains M, Heidenreich KA. Live-cell imaging of autophagy induction and autophagosome-lysosome fusion in primary cultured neurons. Methods Enzymol. 2009;453:145–158. doi: 10.1016/S0076-6879(08)04007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boellaard JW, Kao M, Schlote W, Diringer H. Neuronal autophagy in experimental scrapie. Acta Neuropathol. 1991;82:225–228. doi: 10.1007/BF00294449. [DOI] [PubMed] [Google Scholar]
- 5.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease. J Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 9.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–36316. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 10.Florez-McClure ML, Linseman DA, Chu CT, Barker PA, Bouchard RJ, Le SS, Laessig TA, Heidenreich KA. The p75 neurotrophin receptor can induce autophagy and death of cerebellar Purkinje neurons. J Neurosci. 2004;24:4498–4509. doi: 10.1523/JNEUROSCI.5744-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 12.Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 14.Jeffrey M, Scott JR, Williams A, Fraser H. Ultrastructural features of spongiform encephalopathy transmitted to mice from three species of bovidae. Acta Neuropathol. 1992;84:559–569. doi: 10.1007/BF00304476. [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 17.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 21.Meresse S, Gorvel JP, Chavrier P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci. 1995;108( Pt 11):3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- 22.Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero Rosales K, Peralta ER, Guenther GG, Wong SY, Edinger AL. Rab7 activation by growth factor withdrawal contributes to the induction of apoptosis. Mol Biol Cell. 2009;20:2831–2840. doi: 10.1091/mbc.E08-09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadasivan S, Waghray A, Larner SF, Dunn WA, Jr, Hayes RL, Wang KK. Amino acid starvation induced autophagic cell death in PC-12 cells: evidence for activation of caspase-3 but not calpain-1. Apoptosis. 2006;11:1573–1582. doi: 10.1007/s10495-006-7690-6. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- 26.Selimi F, Lohof AM, Heitz S, Lalouette A, Jarvis CI, Bailly Y, Mariani J. Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells. Neuron. 2003;37:813–819. doi: 10.1016/s0896-6273(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Hong W. Assay and functional properties of Rab34 interaction with RILP in lysosome morphogenesis. Methods Enzymol. 2005;403:675–687. doi: 10.1016/S0076-6879(05)03058-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Hong W. Interorganellar regulation of lysosome positioning by the Golgi apparatus through Rab34 interaction with Rab-interacting lysosomal protein. Mol Biol Cell. 2002;13:4317–4332. doi: 10.1091/mbc.E02-05-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]