Abstract
Endothelin-2 (EDN2) was recently proposed as a granulosa cell-derived contractile signal that facilitates ovulation (Ko et al., Endocrinology 147(4):1770-1779, 2006). Spatially, Edn2 mRNA expression is restricted to granulosa cells of periovulatory follicles. Temporally, mRNA for this contractile peptide is expressed immediately prior to follicle rupture. The primary objective of this study was to test the hypothesis that hypoxia mediates END2 expression in granulosa cells at ovulation and if so, to determine the region within the EDN2 promoter responsible for this effect. To determine the effect of hypoxia on Edn2 mRNA expression, immature mice were treated with 5 IU PMSG followed 48 h later by 5 IU hCG. Granulosa cells were isolated at 9 h after hCG, cultured under normal or hypoxic conditions and the expression level of mRNA for Edn2 was compared. Edn2 mRNA expression was increased when granulosa cells were cultured in a hypoxic environment (p < 0.05). Subsequent promoter analysis found that the 5′upstream region of the EDN2 promoter (between -1894 and -1407 bp) was responsible for hypoxia-mediated changes in EDN2 expression. This promoter region contains multiple sites for potential transcriptional regulation including that by hypoxia-inducible factor 1 (HIF-1; ACGTG) at -1297 bp. The second objective of this study was to determine whether the progesterone receptor (PR) or cyclooxygenase-2 (COX-2), two key regulators of periovulatory events, controlled EDN2 expression. To accomplish this, gonadotropin-primed mice were treated with RU-486 or indomethacin and expression of mRNA for Edn2 was determined in ovaries collected at 12 h after hCG. Treatment with RU-486 or indomethacin did not affect expression of mRNA for Edn2 (p > 0.05). Taken together, it is believed that hypoxia, but not the PR or COX-2, regulate gonadotropin-induced EDN2 expression in the periovulatory follicle.
Keywords: endothelin-2, ovulation, ovary, hypoxia
Introduction
Follicular development and ovulation are dynamic processes. Our understanding of the regulatory mechanisms controlling the growth and final differentiation of a mammalian follicle has advanced exponentially since the introduction of modern molecular biology techniques. However, our understanding of even the most fundamental pathways is still not complete. The regulatory mechanism controlling production of endothelin-2 (EDN2) is one of these pathways. Endothelin-2 (EDN2) is a small, 21 amino acid peptide that is produced by granulosa cells at the time of ovulation (Ko et al., 2006). Expression of mRNA for Edn2, but not Edn1 or Edn3, is dramatically increased in the periovulatory follicle for only a very brief period of time (1-2 h) around ovulation and blockade of endothelin receptor binding will delay/inhibit the process of follicular rupture (Ko et al., 2006). This rather unique pattern of mRNA expression suggests the involvement of direct signaling pathways to accurately coordinate controlled production of this peptide and we propose hypoxia as one such regulatory signal.
Intra-follicular changes in oxygen concentration are expected when the structure of the growing follicle is taken into consideration. Granulosa cell differentiation, proliferation and the accumulation of follicular fluid are occurring in an avascular compartment, adjacent to a richly vascularized theca externa which is acting under the coordinated action of an array of angiogenic factors (reviewed in (Berkholtz et al., 2006; Fraser, 2006)). Indeed, oxygen partial pressure is reported to decrease with increasing follicular size in women (Fischer et al., 1992) and swine (Basini et al., 2004). Furthermore, hCG regulates expression of hypoxia inducible factor-2α in human granulosa-lutein cells (Herr et al., 2004) and we have observed increased expression of hypoxia-inducible factor-1-responsive gene in granulosa cells during the periovulatory period in rats (unpublished microarray data). Overall, several lines of evidence are consistent with the induction of hypoxic stress within the periovulatory follicle prior to ovulation.
Hence, we hypothesized that hypoxia is regulating expression of EDN2 in granulosa cells at the time of ovulation. In this manuscript experiments are described that first evaluated whether a standard gonadotropin-primed granulosa cell culture system (Jo and Curry, 2006) was a suitable model to study EDN2 gene regulation in vitro. We then modified the protocol for cell collection to more thoroughly test the effect of hypoxia on periovulatory EDN2 gene expression. Following this, we proceeded to evaluate the role of the two most common mediators of periovulatory gene expression (the progesterone receptor and cyclooxygenase-2 (reviewed in (Richards et al., 2002; Sirois et al., 2004)) as potential mediators of EDN2 production. Finally, we used a dual-luciferase reporter assay to identify regions of the EDN2 promoter that may contain important hypoxia-mediated transcriptional elements for future study.
Materials and Methods
Reagents
Pregnant mare's serum gonadotropin (PMSG), human chorionic gonadotropin (hCG), RU-486, indomethacin and all other reagents used for cell culture were purchased from Sigma (St. Louis, MO). Reagents used for real-time PCR were purchased from Applied Biosystems (Foster City, CA). All other specific reagents are listed below.
Animals
Immature female CD-1 mice were purchased from Harlan Inc. (Harlan, IN). Animals were maintained in a 14 h light, 10 h dark cycle and given a continuous supply of chow and water. Follicular development and ovulation were induced by treatment of 23-25 day old mice with 5 IU of PMSG followed 48 hours later by 5 IU hCG. Animals were sacrificed for tissue collection, at the times described for each experiment, by CO2 inhalation followed by cervical luxation. The University of Kentucky Animal Care and Use Committee approved all animal procedures.
Granulosa cell culture
Ovaries were collected from gonadotropin-primed mice and granulosa cells isolated by follicular puncture, as described previously (Ko et al., 1999). Briefly, granulosa cells were collected in cold serum-free 4F medium consisting of 15 mM HEPES (pH 7.4), 50% DMEM and 50% Ham's F12 with bovine transferrin (5 μg/ml), human insulin (2 mg/ml), hydrocortisone (40 ng/ml) and antibiotics. Cells were washed three times in 4F medium then plated on serum-coated six-well plates at a density of approximately 1 ×106 cells per well and incubated at 37°C in a humidified atmosphere using modular incubation chambers under either normal (20% O2, 5% CO2, 75% N2) or hypoxic (0.1% O2, 5% CO2 with N2 base) conditions. To examine the induction of mRNA for Edn2 by treatment with an ovulatory dose of hCG in vivo versus in vitro, granulosa cells were collected from ovaries of gonadotropin-primed mice sacrificed at 48 h after PMSG and cultured with or without 5 IU hCG under normal oxygen conditions for 0, 6 or 12 h. Granulosa cells collected from ovaries obtained at 12 h after hCG served as in vivo controls. To examine the effect of hypoxia on expression of mRNA for Edn2, granulosa cells were collected from gonadotropin-primed mice sacrificed at 9 h after treatment with hCG and cultured under normal or hypoxic condition for 0, 3, 6 or 9 h. All cultures were performed in duplicate and replicated 3 times on different days.
In vivo experiment
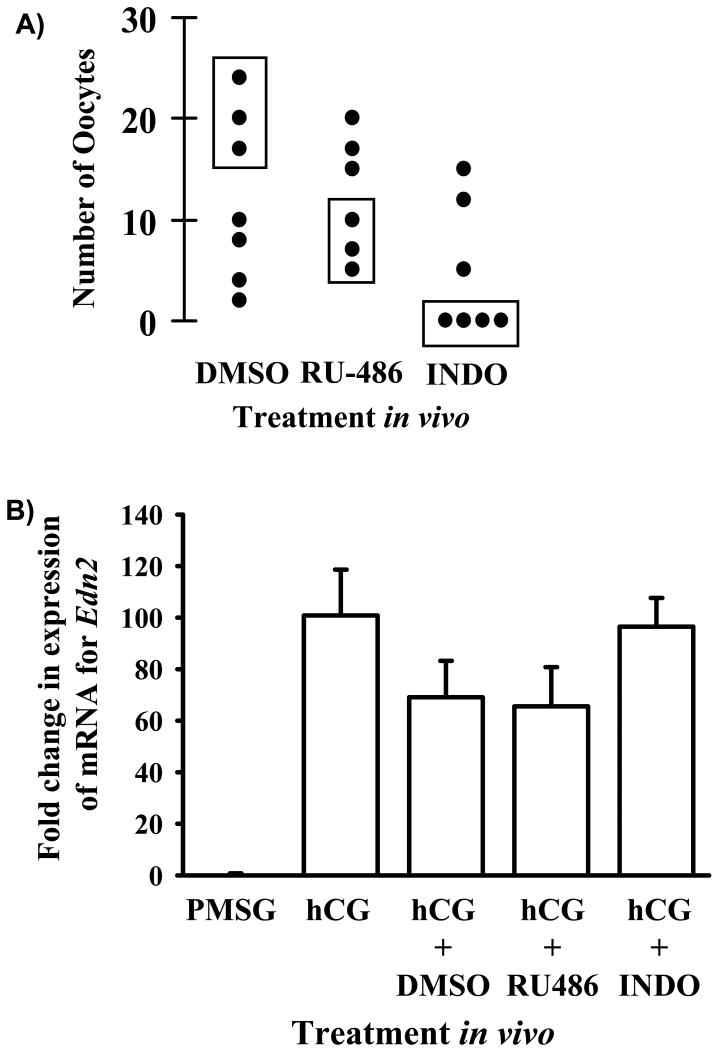
The progesterone receptor (PR) and cyclooxygenase-2 (COX-2), two well defined mediators of periovulatory events within the ovary, are required for ovulation (Dinchuk et al., 1995; Lydon et al., 1996; Lim et al., 1997). Recently, it was proposed that expression of EDN2 in granulosa cells was regulated by the progesterone receptor (Palanisamy et al., 2006). To test this hypothesis and further explore the regulatory pathway of EDN2 gene expression, expression of mRNA for Edn2 was determined in ovaries collected from mice treated with the PR antagonist RU-486 or the cyclooxygenase inhibitor, indomethacin. Gonadotropin-primed mice (n = 6-8 per treatment) were left untreated (as a control for the vehicle), or treated with vehicle alone (DMSO), with 10 mg/kg body weight RU-486 i.p. at 47 h after PMSG or with 10 mg/kg body weight indomethacin at 49 h after PMSG (1 h after hCG). Ovulation was induced in all mice by the administration of hCG at 48 h after PMSG. At 12 h after hCG, mice were anaesthetized and one ovary / mouse collected and snap frozen on dry ice for later RNA extraction and measurement of Edn2 mRNA by real-time PCR. Mice were sacrificed at 20 h after hCG and ovulation rate of the contralateral ovary determined, as described previously (Ko et al., 2006; Al-Alem et al., 2007). Due to the inconsistent effect of treatment with either DMSO, RU-486 or indomethacin on ovulation rate (Fig. 4A), it was determined that the best approach to test the hypothesis that EDN2 gene expression was regulated by the PR and/or COX-2 was to measure mRNA for Edn2 in a selected group of these ovaries (discussed later). Thus, mRNA for Edn2 was measured in ovaries collected at 12 h after hCG when the contralateral ovary was shown to ovulate normally (DMSO) or when ovulation rate was low (RU-486 and indomethacin).
Figure 4.
Effect of PR or COX-2 on Edn2 expression. A) Ovulation rate in response to treatment with vehicle, RU-486 or indomethacin. Immature mice were treated with PMSG and 48 h later with hCG. Vehicle (DMSO), RU-486 (10 mg/kg body weight) or indomethacin (10 mg/kg body weight) were administered at PMSG + 48 h, 47 h and 49 h, respectively. One ovary per mouse was collected at 12 h after hCG for RNA extraction. The rate of ovulation for the contralateral ovary was determined at 20 h after hCG. Filled circles represent individual mice. Ovaries collected at 12 h after hCG from the mice represented by filled circles enclosed in boxes were utilized for the determination of Edn2 mRNA expression. B) Real-time PCR analysis of Edn2 mRNA expression, relative to GAPDH. No effect of treatment with vehicle, RU-486 or indomethacin on the expression of mRNA for Edn2 was detected (p > 0.05).
Real-time PCR analysis
To determine the level of expression of mRNA for Edn2 in mice during follicular development, ovulation and luteal development, real-time PCR analysis was performed using ovaries collected at 48 h after PMSG administration and at 6 h, 12 h or 24 h after hCG injection (n = 3 animals/time point). To determine the effect of hypoxia or hCG on EDN2 gene expression in vitro, real-time PCR was performed using granulosa cells collected at the completion of the aforementioned cultures. In each case, total RNA was extracted from ovaries or granulosa cells with TRIzol (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's directions and the integrity of RNA verified by visualization of distinct 18S and 28S rRNA bands after ethidium bromide staining in an agarose gel. cDNA was reverse transcribed from each sample of total RNA as described previously (Ko et al., 2006) and real-time PCR performed using the Applied Biosystems 7300 detection system (Applied Biosystems, Foster City, CA). Expression levels of mRNA for Edn2 were determined relative to GAPDH as an endogenous control. cDNA for EDN2 and GAPDH was amplified using TaqMan® Universal PCR Master Mix (Applied Biosystems) and the following EDN2 gene-specific primers and probes: Edn2-F 5′-CTC CTG GCT TGA CAA GGA ATG-3; Edn2-R 5′-GCT GTC TGT CCC GCA GTG TT-3′; Edn2-Probe 5′-/56-FAM/TCT GCC ACC TGG ACA TCA TCT GGG T/36-TAMSp/-3′ and GAPDH primer and probe were used from TaqMan® Rodent GAPDH Control Reagents (Applied Biosystems). cDNA generated from the appropriate cells at each treatment or time point was included in each PCR reaction and the analysis was replicated 2-3 times on different days. DEPC-treated water was used to replace cDNA and act as a negative control for each analysis. The relative amount of transcript was calculated by the ΔΔCT method (Livak and Schmittgen, 2001) and normalized to GAPDH.
Northern blot analysis
Northern blot analyses were carried out as described previously (Gieske et al., 2005). Briefly, total RNA was extracted from ovaries using TRIzol reagent according to the manufacturer's protocol and quantified by spectrophotometry. Ten micrograms of total RNA were separated by electrophoresis, capillary transferred to a nylon membrane (0.2-μm pore size, Nytran N; Schleicher & Schuell Inc., Keene, NH) and fixed to the membrane by UV cross-linking (UV crosslinker, UVP CL-1000; VWR, West Chester, PA). Plasmids containing partial cDNA for the gene of interest were digested from an appropriate restriction enzyme and purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). A [32P]-dCTP-labeled cDNA probe was synthesized using the Prime-It II random-primer labeling kit (Stratagene, La Jolla, CA). Random oligonucleotide primers were annealed to 25 ng of template DNA, followed by incubation with [α-32P]-dCTP (10 mCi/ml) and unlabeled deoxynucleotides in the presence of Exo(2) Klenow enzyme. Unincorporated nucleotides were removed with mini Quick Spin RNA columns (Roche, IN, USA). The membrane was prehybridized in UltraHyb hybridization buffer (Ambion Inc., Austin, TX) for 30 min at 68°C, and probe was added to a concentration of 106 cpm/ml and then hybridized at 68°C for 16 h. Blots were washed twice (5 min each) with shaking in 2× SSC, 0.1% SDS at room temperature, then washed at high stringency (0.1× SSC, 0.1% SDS) twice for 30min each at 68°C. The membrane was exposed to film (Kodak Biomax XAR) for 48 h at -80°C.
5′ RACE PCR
To determine the transcription initiation site of the 5′-end of the EDN2 gene, GeneRacer (Invitrogen, Carlsbad, CA) was used according to the manufacturer's protocol. In this procedure, total RNA (1 μg) is treated with calf intestinal phosphatase to remove 5′ phosphates. The sample is then treated with tobacco acid pyrophosphatase to remove the 5′ cap from the full-length mRNA while leaving a 5′ phosphate required for ligation. An RNA oligonucleotide of known sequence is ligated to the 5′ ends of decapped mRNA with T4 RNA ligase, which provides a known priming site for the GeneRacer PCR primers. A reverse transcription reaction is then performed, using the 5′ oligomer-ligated mRNA as template for the GeneRacer oligo(dT) primer and avian myeloblastosis virus reverse transcriptase. A touchdown PCR amplification of the resulting reverse transcription is then performed with proofreading Platinum Pfx DNA polymerase, the GeneRacer 5′ primer (specific to the GeneRacer RNA oligomer derived sequences), and a gene specific primer (EDN2sp1; CGC AGT GTT CAC CCA GAT GAT G). After successful amplification is confirmed by agarose gel electrophoresis, a second round of nested PCR amplification is performed as above, except that the GeneRacer 5′ nested primer is used in place of the GeneRacer 5′ primer and a gene specific primer (EDN2sp2; CAG AAG TAC ACA CAT TCC TTG TCA AG). The amplified PCR products are then isolated from an agarose gel by excising the band, loaded into a SNAP column and microfuged. They are then cloned into plasmid vector pCR4-TOPO and transformed into competent One Shot TOP10 cells with a TOPO TA Cloning kit (part of the GeneRacer kit package). The clones were sequenced commercially (UK-AGTC, Lexington, KY) and sequences were analyzed to determine the transcription initiation site and promoter region of the endothelin gene. The nucleotide sequence of the endothelin promoter region was obtained from GenBank and used to find putative transcription factor binding sites using TESS (Transcription Element Search System; http://www.cbil.upenn.edu/tess).
Plasmid constructs
The mouse genomic 5′-flanking region of Edn2 mRNA was characterized by 5′-RACE PCR as described above. Promoter regions of different sizes (+117 bp to -413 bp, -945 bp, -1407 bp and -1894 bp) were amplified from PCR using specific primers (Table 1). The PCR was conducted at 94°C for 5 min followed by 40 cycles at 94°C for 1 min, 58°C for 1 min and 72°C for 5 min. The amplified PCR fragments were then subcloned into a pCRII-TOPO vector (Invitrogen) and cloned PCR sequences were verified by nucleotide sequencing. Each promoter fragment was excised with Mlu I and Nco I digestion (Promega, Madison WI, USA) and ligated into the equivalent site of the pGL3-basic vector (Promega) to form the END2 promoter-luciferase reporter constructs. The resulting constructs were confirmed by restriction enzyme digestion and nucleotide sequencing.
Table1. Sequences of oligonucleotides used to create EDN2 promoter constructs.
| Gene Name | Primer Sequence | Length (bp) |
|---|---|---|
| EDN2 P1 | 5′-CGCGT(Mlu1)CATATGCCAGCTATGTGGCTGA-3′ | 1979 |
| EDN2 P2 | 5′-ACGCGT(Mlu1)CCTTCTACTTTGCCTTCCTCCT-3′ | 1494 |
| EDN2 P3 | 5′-ACGCGT(Mlu1)CAGCCTCTGGCCTCTCCA-3′ | 1032 |
| EDN2 P4 | 5′-ACGCGT(Mlu1)CTTGCTTCACCGTGGGCAG-3′ | 500 |
| EDN2 PR | 5′-AGCAGCAGCGGCAGAGTGCCATGG(Nco1)-3′ |
Reporter gene assays
Granulosa cells were isolated from ovaries collected from gonadotropin-primed mice sacrificed at 9 h after hCG and incubated under normal oxygen conditions at 37°C in 1.5 ml F12-DMEM medium without serum and ampicillin for preparation of the constructs to transfect. The cells were then transfected with lipofectimine 2000 (Invitrogen) in 6-well plates. Each well contained 1 × 106 cells, 5 μg pGL3-EDN2 promoter, 1 μg of the internal control vector phRL-TK (Promega), 10 μl lipofectimine 2000, and 500 ml Opti-MEM I (Invitrogen) medium without serum and antibiotics. The cells were then incubated for 24 h under normal or hypoxic conditions, as described above. At the completion of the transfection, the dual-luciferase reporter assay was performed. The activity of firefly luciferase in pGL3 and Renilla luciferase in pRL-TK were determined following the dual-luciferase reporter assay protocol (Promega). The cells were rinsed with PBS and cell lysates were prepared by manually scraping the cells from the culture plates in the presence of 1 × passive lysis buffer (PLB). Cell lysate (20 μl) was transferred into luminometer tubes containing 100 μl Luciferase Assay Reagent II (LAR II). Firefly luciferase activity (M1) was determined first, and following the addition of 100 μl of Stop&Glo Reagent, Renilla luciferase activity (M2) was recorded. All transfections were performed in duplicate and each experiment was conducted on at least three separate occasions. Data are presented as relative luciferase activity of firefly (M1)/Renilla luciferase (M2).
Statistical analysis
Data are presented as means ± SEM. Data sets were first tested for homogeneity of variance. If heterogeneity was detected, data were log-(base 10) transformed before statistical analysis. Statistical analysis was performed using commercially available software from SPSS (Version 12.0, SPSS Inc.) and statistical significance was defined as a P-value of less than 0.05. If differences were detected, Tukey's test was used to determine which means differed. Non-transformed data are depicted in all the figures.
Results
Expression of mRNA for Edn2 in the mouse ovary
Real-time PCR was utilized to determine the relative level of expression of mRNA for Edn2 in the mouse ovary during the ovulatory cascade (Fig. 1A). Expression of mRNA for Edn2 in ovaries obtained at 48 h after treatment with PMSG and at 6 h after subsequent treatment with hCG was low. A dramatic increase in expression of mRNA for Edn2 was observed at 12 h after hCG (p < 0.05), with expression of mRNA returning to basal levels by the final collection point, 24 h after hCG. This temporal expression pattern was confirmed by Northern blot analysis, which showed a dramatic increase in Edn2 mRNA expression at 12 h after hCG (Fig. 1B). Overall, the pattern of expression of mRNA for Edn2 in the mouse ovary during the ovulatory cascade is consistent with our microarray analysis (data not shown) and with results previously described for the rat (Ko et al., 2006; Al-Alem et al., 2007).
Figure 1.
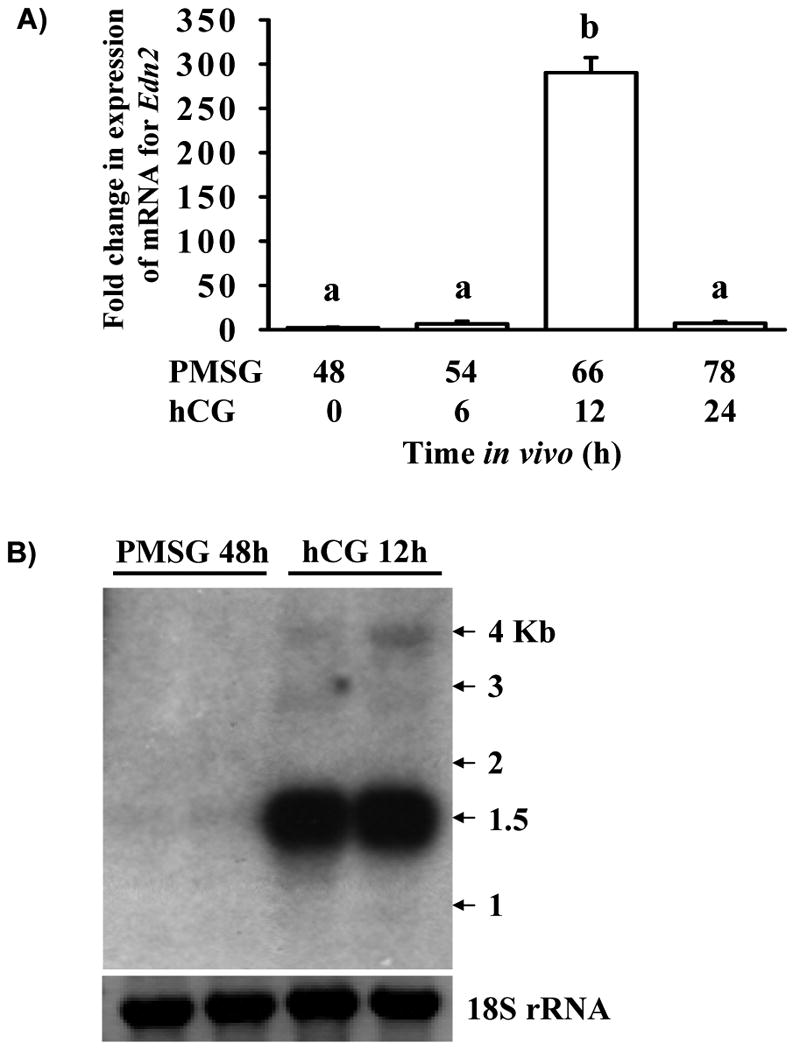
Expression of mRNA for Edn2 in the mouse ovary during gonadotropin-primed follicular development and ovulation. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. A) Animals were sacrificed for tissue collection at 48 h after PMSG and at 6, 12 and 24 h after hCG (n = 3 per time point). Real-time PCR was performed to determine expression of mRNA for Edn2. Expression of mRNA for GAPDH was used to normalize data. Expression of mRNA for Edn2 was increased at 12 h after hCG in comparison to all other time points examined (p < 0.05). B) Animals were sacrificed for tissue collection at 48 h after PMSG and at 12 h after hCG (n = 2 per time point). Northern analysis was used to determine expression and transcript size of mRNA for Edn2. Expression of 18S rRNA was used as a loading control.
Induction of mRNA for Edn2 by hCG: in vivo vs. in vitro
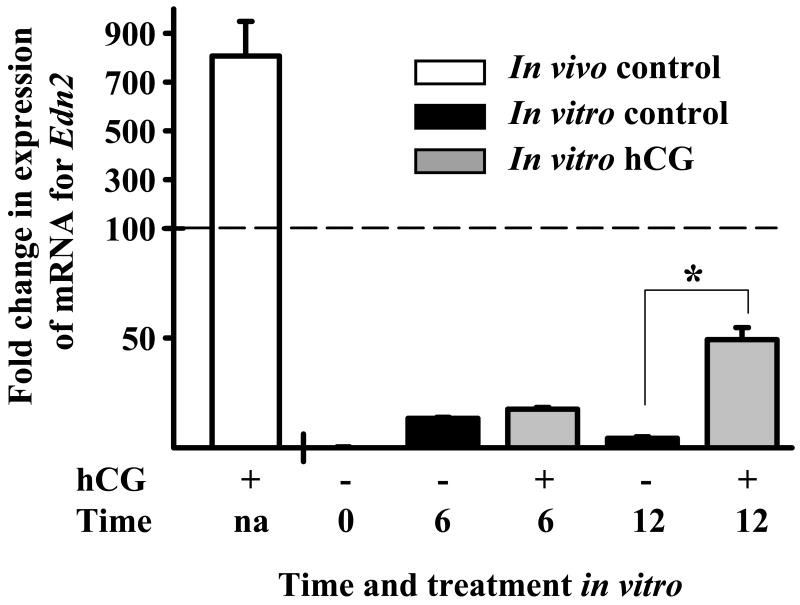
To determine whether the dramatic induction of mRNA for Edn2 observed in ovaries collected at 12 h after hCG in vivo could be mimicked by the addition of hCG in vitro, granulosa cells were isolated from ovaries collected from mice at 48 h after treatment with PMSG and incubated for 0, 6 and 12 h with or without the addition of hCG (5 IU/ml). Total RNA isolated from granulosa cells collected at the end of each culture was assessed relative to RNA extracted from granulosa cells obtained at 12 h after hCG in vivo. Real-time PCR analysis revealed that expression of mRNA for Edn2 was increased by hCG at 12 h in vitro (p < 0.05, Fig. 2), however the level of induction of mRNA was low when compared to the in vivo control, bringing into question on the validity of using hCG in vitro to examine the regulatory mechanism of EDN2 expression.
Figure 2.
Induction of mRNA for Edn2 by hCG in vivo vs. in vitro. Real-time PCR analysis of the induction of mRNA for Edn2 in ovaries of PMSG/hCG-primed mice sacrificed at 12 h after treatment with hCG (in vivo control) and in granulosa cells collected from PMSG-primed mice sacrificed at 48 h after treatment with PMSG and cultured for 0, 6 and 12 h with 0 or 5 IU hCG. Expression of mRNA for Edn2 was increased in hCG-treated granulosa cells after 12 h in culture (p < 0.05). However expression of mRNA for Edn2 after treatment of granulosa cells with hCG for 12 h in vitro was low compared to the in vivo control.
Effect of hypoxia on expression of mRNA for Edn2
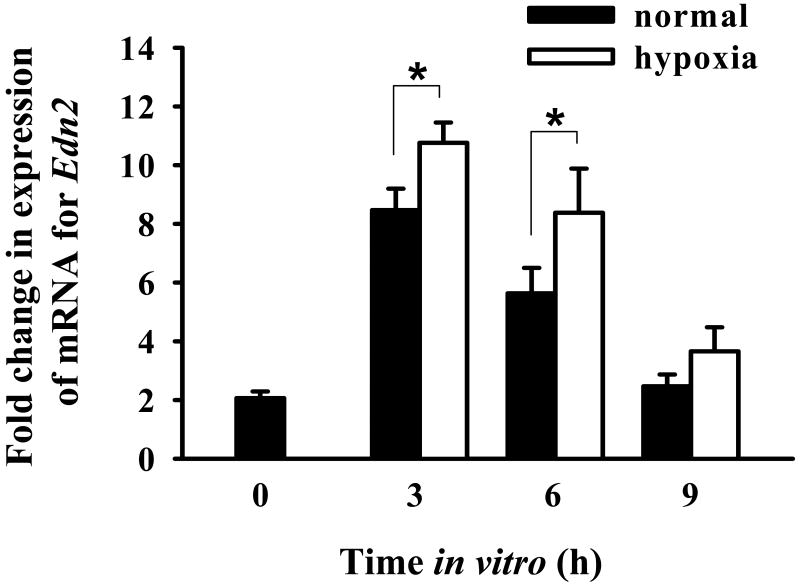
To determine whether expression of mRNA for Edn2 by granulosa cells was affected by hypoxia, modular incubation chambers were utilized to control O2 levels within each culture. Granulosa cells were isolated from ovaries collected from gonadotropin-primed mice sacrificed at 9 h after hCG and cultured under normal (20% O2, 5% CO2, 75% N2) or hypoxic (0.1% O2, 5% CO2 with a N2 base) conditions for 0, 3, 6 and 9 h. Maximal expression of Edn2 was observed after 3 h in culture, under both normal and hypoxic conditions (Fig. 3). At both 3 and 6 h of culture, expression of mRNA for Edn2 was higher in the granulosa cells that were incubated under hypoxic conditions (p < 0.05), suggesting modulation of EDN2 expression by O2 concentration.
Figure 3.
Effect of hypoxia on expression of mRNA for Edn2. Immature mice were treated with PMSG and 48 h later with hCG. Granulosa cells were obtained from ovaries at 9 h after hCG and cultured under normal or hypoxic condition for 0, 3, 6 or 9 h. Real-time PCR was performed to determine expression of mRNA for Edn2 in granulosa cells collected at the end of each culture. Expression of mRNA for GAPDH was used to normalize data. Expression of mRNA for Edn2 was increased when granulosa cells were cultured under a hypoxic environment for 3 and 6 h (p < 0.05).
Effect of PR or COX-2 on expression of mRNA for Edn2
To determine whether the PR and/or COX-2 play a role in the regulation of EDN2 gene expression in the ovary, we proposed to measure gene expression after treatment of mice with RU-486 (a PR antagonist) or indomethacin (a COX inhibitor) in vivo. As a control for the effectiveness of the treatments, one ovary was collected at 12 h after hCG for RNA extraction, and the other left in situ for determination of ovulation rate. Inconsistency was observed in the rate of ovulation after treatment with either the vehicle or antagonists (Fig. 4A). Therefore, mRNA for Edn2 was measured in a subset of the mice, those with the expected antagonist-induced reduction in ovulation rate. Real-time PCR analysis revealed no effect of treatment with RU-486 or indomethacin on the expression of mRNA for Edn2 (p > 0.05, Fig. 4B). Considering the conclusions drawn by others (that PR regulates EDN2 expression, (Palanisamy et al., 2006)) with the results reported herein, it is suggested that the potential regulation of EDN2 expression by either the PR or COX-2 is investigated further.
Promoter analysis
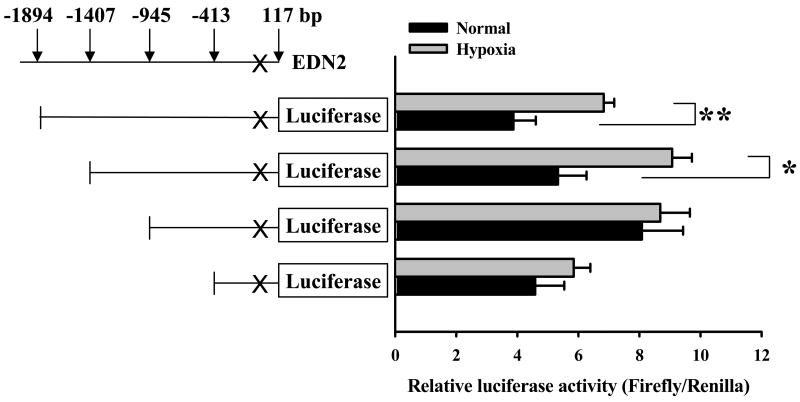
To identify the promoter region of the EDN2 gene, the transcription initiation site was identified by 5′ RACE PCR using total RNA isolated from ovaries collected 12 h after hCG. The 5′ RACE PCR amplified a major band and some minor secondary bands (Fig. 5A). Subsequent cloning and sequencing of the bands identified a single transcription initiation site at the location of 87 bp upstream of the translation initiation codon of the END2 gene (Fig. 5B). To determine the proximal region of EDN2 promoter that is responsible for the response to hypoxia, a promoter-reporter assay was performed using constructs in which four different sizes of the END2 promoter fragments were flanked by firefly luciferase (Fig. 6). Granulosa cells collected at 9 h after hCG were transfected with the constructs, cultured under normal or hypoxic condition for 24 h, and the promoter activity was measured by the dual luciferase assay. In the granulosa cells that were transfected with either -1894 or -1407 bp construct, hypoxia increased EDN2 promoter activity (p < 0.05). No significant difference, however, was induced by hypoxia when granulosa cells were transfected with either the -945 or -413 bp constructs. The 1894 bp of EDN2 promoter was analyzed using a filter-string based search supported by TESS program to identify any potential regulatory elements. A putative hypoxia-inducible factor 1 (HIF-1) binding site at -1297 bp (Fig. 5B) was found that is identical with the hypoxia response element (HRE) sequence (ACGTG) described in the zfIGFBP-1 gene promoter of zebrafish (Kajimura et al., 2006) and in the vascular endothelial growth factor (VEGF) gene promoter of human (Liu et al., 1995). Whether this element is functional bears further investigation.
Figure 5.
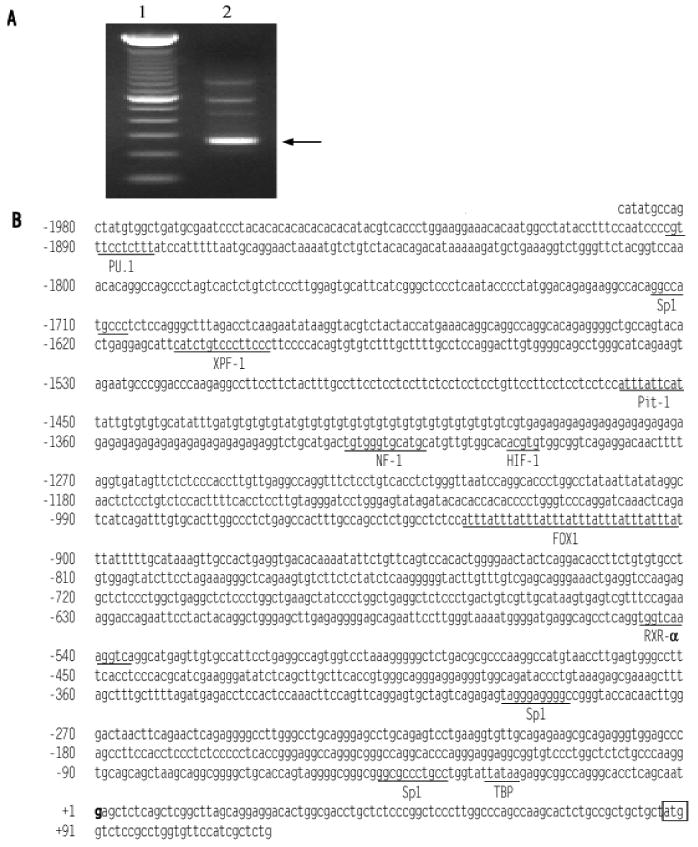
Analysis of Edn2 mRNA by RACE PCR and the nucleotide sequence of the EDN2 proximal promoter region. To determine the transcription initiation site (TIS), 5′ RACE PCR was carried out from total RNA extracted from an ovary at 12 after hCG. The PCR products were separated on agarose gel. A) The main PCR product is indicated by an arrow (Lane 1, 100 bp marker; lane 2, RACE PCR products). B) The nucleotide sequence of the EDN2 promoter region and part of exon 1. The TIS is bolded and assigned by the number +1. The translational initiation codon is boxed. Possible regulatory elements and binding factor were searched on TESS program. Elements with a likelihood value of 20 or greater are underlined with the names of their possible interacting factors.
Figure 6.
Dual luciferase assay of the EDN2 promoter. Promoter constructs of different sizes (+117 to -413, -945, -1407 and -1894 bp) that were flanked by firefly luciferase were transfected using granulosa cells collected at 9 h after hCG and cultured under normal or hypoxic conditions for 24 h. Hypoxia increased EDN2 promoter activity in the granulosa cells that were transfected with either the -1894 or -1407 bp constructs (p < 0.05), but not the -945 or -413 bp constructs (p > 0.05).
Discussion
The primary goal of the experiments described herein was to test the hypothesis that hypoxia mediates the induction of EDN2 by periovulatory granulosa cells. Promoter analysis could then be utilized to identify regions on the EDN2 promoter bearing important transcription sites for future investigation. However, experiments were also conducted to determine EDN2 gene expression profiles in the mouse (our previous studies utilized rats (Ko et al., 2006; Al-Alem et al., 2007)), to evaluate gonadotropin-treatment of granulosa cells as an in vitro model for the examination of the regulatory mechanism of EDN2 production and to test whether the PR and/or COX-2 played a role in the regulation of EDN2 expression. Overall, a new model for the production of EDN2 by granulosa cells is presented, with hypoxia but not PR or COX-2 regulating this final signal of the ovulatory cascade.
Expression of mRNA for Edn2 is increased in a very transient manner, immediately prior to the time of follicular rupture (Ko et al., 2006). EDN2 is a contractile peptide and we previously hypothesized that production of EDN2 by granulosa cells of the periovulatory follicle was inducing contraction of the smooth muscle layers within the ovary, facilitating expulsion of the cumulus-oocyte complex through an already weakened follicular structure (Ko et al., 2006). The regulatory pathway for EDN2 production and ovulation has not been closely examined and the results generated herein suggest that care must be taken when examining the mechanism of EDN2 production within the ovary. Although treatment of granulosa cells collected from PMSG-primed mice with hCG did increase expression of mRNA for Edn2, suggesting potential use as an in vitro model, the magnitude of the induction observed in vitro was not comparable to that observed when the granulosa cells were exposed to hCG in vivo. Perhaps similar in context to the preference of using hCG versus forskolin as an “ovulatory” treatment of granulosa cells, arguments could be made for or against whether the difference in the magnitude of gene expression when hCG is used in vivo versus in vitro should dictate exclusion of this commonly used culture model (treatment of granulosa cells with hCG in vitro) from future studies examining the regulation of periovulatory EDN2 expression. As such, the results are presented for the interpretation of others pursuing this area of reproductive biology.
The results generated suggest that EDN2 is not under the regulation of PR or COX-2. Levels of mRNA for Edn2 at 12 h after hCG did not differ when mice were previously treated with a PR antagonist or a COX inhibitor. However, this result must also be interpreted with an open mind. Palanisamy et al. (2006) reported that PR was driving EDN2 gene expression in mice. We used an alternate approach to test their results, however inconsistency in the effect of treatment with both the vehicle and the inhibitors led us to evaluate Edn2 expression in only a subset of the mice we injected and our results should be examined with this in mind. Interestingly, and similar to our observations using mice, when injecting these compounds into immature gonadotropin-primed rats, RU-486 appears not as effective as indomethacin in the expected reduction of ovulation (Gaytan et al., 2004). Perhaps pertinent to hypoxia and ovulation, DMSO (the vehicle used to administer both RU-486 and indomethacin) is a known hydroxyl radical scavenger (Panganamala et al., 1976; Del Maestro et al., 1980). Whether this may have influenced our results, or the anesthesia of mice at the time of ovulation, warrants further investigation. Due to the inconsistency we observed in ovulation, we measured the level of expression of mRNA in mice with normal ovulation in response to treatment with DMSO and low ovulation after treatment with RU486 and indomethacin. Levels of mRNA for Edn2 between these treatment groups did not differ, indicating that these treatments (which effectively reduced ovulation) did not affect Edn2 mRNA expression. Overall this would suggest that PR and/or COX-2 are not involved in the regulation of EDN2 gene expression in mice.
It appears that hypoxia is a regulator of EDN2 expression at ovulation in the mouse. When granulosa cells were collected at 9 h after hCG and cultured under normal or hypoxic conditions, expression of mRNA for Edn2 was increased in the hypoxic treatment group. Coupled with reports of decreasing oxygen tension with advancing follicular development (Fischer et al., 1992; Basini et al., 2004), and hCG regulation of hypoxia-related genes in granulosa cells in vitro (Herr et al., 2004) and in vivo (unpublished microarray data), it appears that hypoxia may be a physiological regulator of this final contractile mediator of ovulation. Results of the dual luciferase assay suggest that a relatively complex regulatory mechanism is in effect. As expected, analysis of the longest construct (-1894 bp) revealed increased luciferase (EDN2 promoter) activity under hypoxic conditions. While the next largest construct (-1407 bp) also revealed hypoxia-induced promoter activity, analysis of third construct (-945 bp) revealed no difference. Interestingly, under normal conditions EDN2 promoter activity appeared to increase with decreasing promoter length (-1894 to -945 bp) suggesting that inhibitory transcriptional elements are present between -1894 to -1407 and -1407 to -945 bp. Among the regulatory elements searched by the TESS program, a PU.1 binding site at -1982 bp is a potential inhibitory element. PU.1 functions as a negative regulator in the expression of the flt3 gene (Inomata M et al., 2006) and c-myc gene by forming a complex with mSin3A and HDAC1 in vivo (Kihara-Negishi et al., 2001). In contrast, when the -945 and -413 bp constructs are compared, no effect of hypoxia on promoter expression is observed. However, promoter activity appears to be increased in the larger of these two constructs, suggesting the presence of a stimulatory transcriptional element in this section of the EDN2 gene promoter. Overall, the results suggest that both inhibitory and stimulatory transcriptional elements are present on the END2 promoter with hypoxia-induced elements located from -1894 to -945 bp upstream of the transcription initiation site.
In summary, the experiments described herein identified hypoxia as a novel mediator of periovulatory EDN2 production, with promoter analysis identifying likely sites of transcriptional regulation for future investigation. Results are also presented that indicate care should be taken with both in vivo and in vitro models when studying the regulatory pathway of this final contractile signal of ovulation.
Acknowledgments
Supported by grant RO1HD052694 (C.K.) and P20 RR15592 (P.B. and C.K.) from the National Institutes of Health.
Literature Cited
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Basini G, Bianco F, Grasselli F, Tirelli M, Bussolati S, Tamanini C. The effects of reduced oxygen tension on swine granulosa cell. Regul Pept. 2004;120:69–75. doi: 10.1016/j.regpep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24:262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R, Thaw HH, Bjork J, Planker M, Arfors KE. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Dunkern TR, Fritz G, Kaina B. Ultraviolet light-induced DNA damage triggers apoptosis in nucleotide excision repair-deficient cells via Bcl-2 decline and caspase-3/-8 activation. Oncogene. 2001;20:6026–6038. doi: 10.1038/sj.onc.1204754. [DOI] [PubMed] [Google Scholar]
- Fischer B, Kunzel W, Kleinstein J, Gips H. Oxygen tension in follicular fluid falls with follicle maturation. Eur J Obstet Gynecol Reprod Biol. 1992;43:39–43. doi: 10.1016/0028-2243(92)90241-p. [DOI] [PubMed] [Google Scholar]
- Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan M, Bellido C, Morales C, Gonzalez-Padilla M, Sanchez-Criado JE, Gaytan F. Immature rats show ovulatory defects similar to those in adult rats lacking prostaglandin and progesterone actions. Reprod Biol Endocrinol. 2004;2:63. doi: 10.1186/1477-7827-2-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieske MC, Na GY, Koo Y, Jo M, Curry TE, Jr, Ko C. Decay-accelerating factor in the periovulatory rat ovary. J Endocrinol. 2005;186:303–313. doi: 10.1677/joe.1.06218. [DOI] [PubMed] [Google Scholar]
- Herr D, Keck C, Tempfer C, Pietrowski D. Chorionic gonadotropin regulates the transcript level of VHL, p53, and HIF-2alpha in human granulosa lutein cells. Mol Reprod Dev. 2004;69:397–401. doi: 10.1002/mrd.20137. [DOI] [PubMed] [Google Scholar]
- Inomata M, Takahashi S, Harigae H, Kameoka J, Kaku M, Sasaki T. Inverse correlation between Flt3 and PU.1 expression in acute myeloblastic leukemias. Leuk Res. 2006;30:659–64. doi: 10.1016/j.leukres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jo M, Curry TE., Jr Luteinizing Hormone-Induced RUNX1 Regulates the Expression of Genes in Granulosa Cells of Rat Periovulatory Follicles. Mol Endocrinol. 2006;20:2156–2172. doi: 10.1210/me.2005-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Aida K, Duan C. Understanding Hypoxia-Induced Gene Expression in Early Development: In Vitro and In Vivo Analysis of Hypoxia-Inducible Factor 1-Regulated Zebra Fish Insulin-Like Growth Factor Binding Protein 1 Gene Expression. Mol Cell Biol. 2006;26:1142–1155. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara-Negishi F, Yamamoto H, Suzuki M, Yamada T, Sakurai T, Tamura T, Oikawa T. In vivo cpmplex formation of PU.1 with HDAC1 associated with PU.1-mediated transcriptional repression. Oncogene. 2001;20:6039–6047. doi: 10.1038/sj.onc.1204756. [DOI] [PubMed] [Google Scholar]
- Ko C, In YH, Park-Sarge OK. Role of Progesterone Receptor Activation in Pituitary Adenylate Cyclase Activating Polypeptide Gene Expression in Rat Ovary. Endocrinology. 1999;140:5185–5194. doi: 10.1210/endo.140.11.7149. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia Regulates Vascular Endothelial Growth Factor Gene Expression in Endothelial Cells. Identification of a 5′ Enhancer. Circulation Research. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A Novel Pathway Involving Progesterone Receptor, Endothelin-2, and Endothelin Receptor B Controls Ovulation in Mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Panganamala RV, Sharma HM, Heikkila RE, Geer JC, Cornwell DG. Role of hydroxyl radical scavengers dimethyl sulfoxide, alcohols and methional in the inhibition of prostaglandin biosynthesis. Prostaglandins. 1976;11:599–607. doi: 10.1016/0090-6980(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel Signaling Pathways That Control Ovarian Follicular Development, Ovulation, and Luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]