Abstract
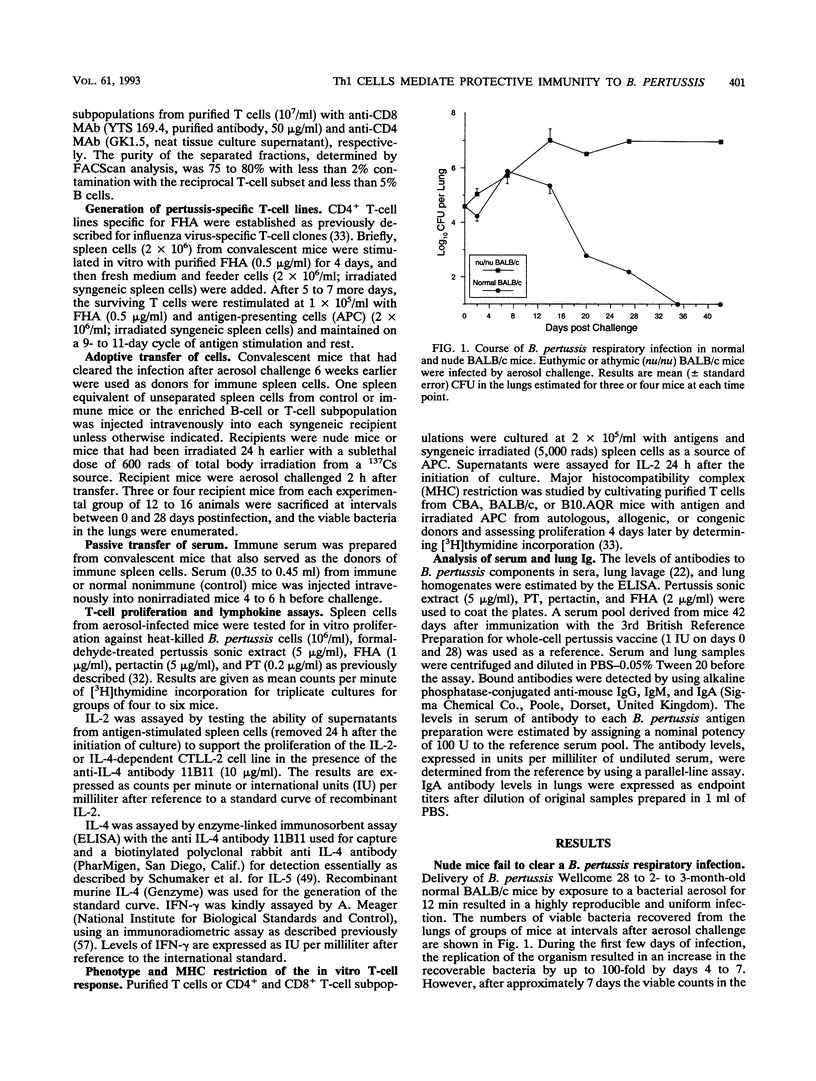
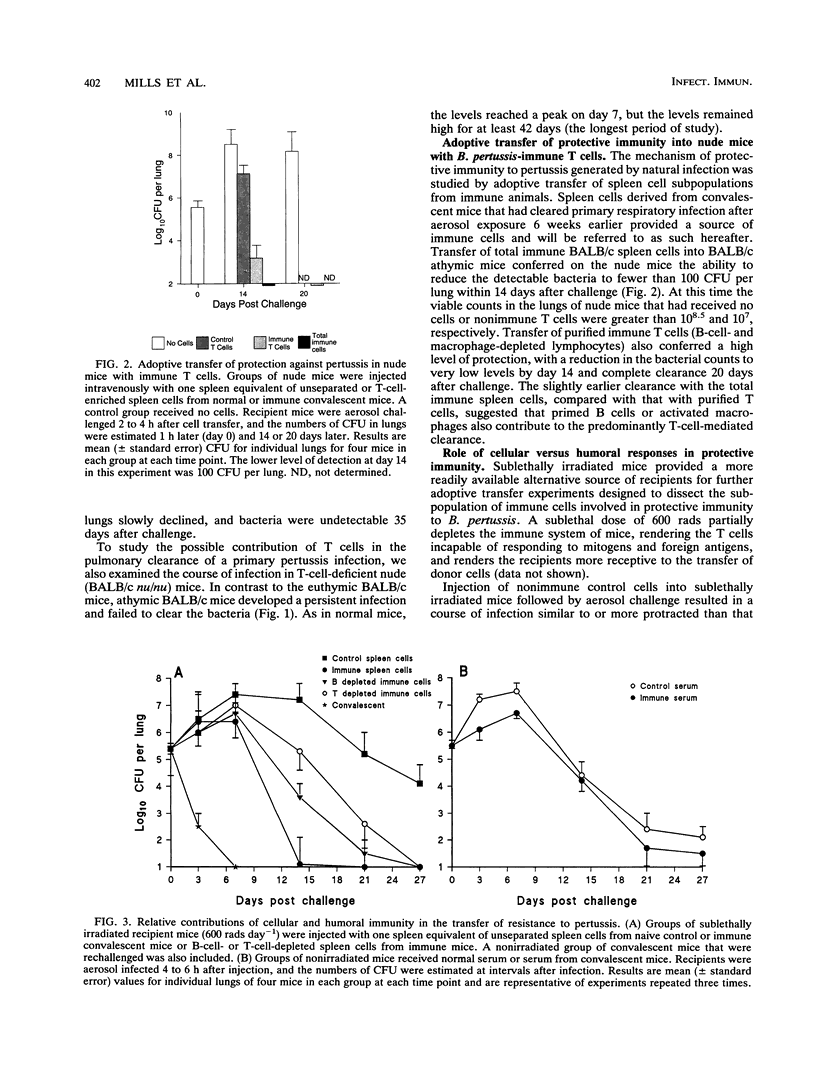
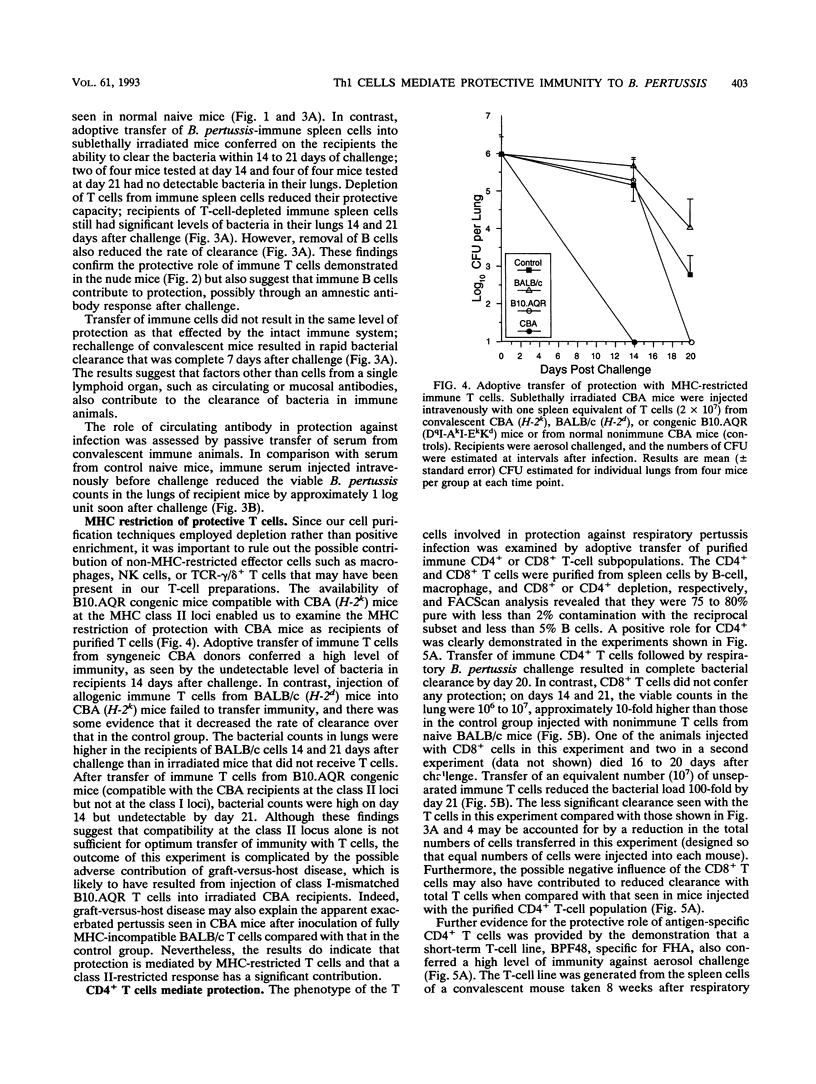
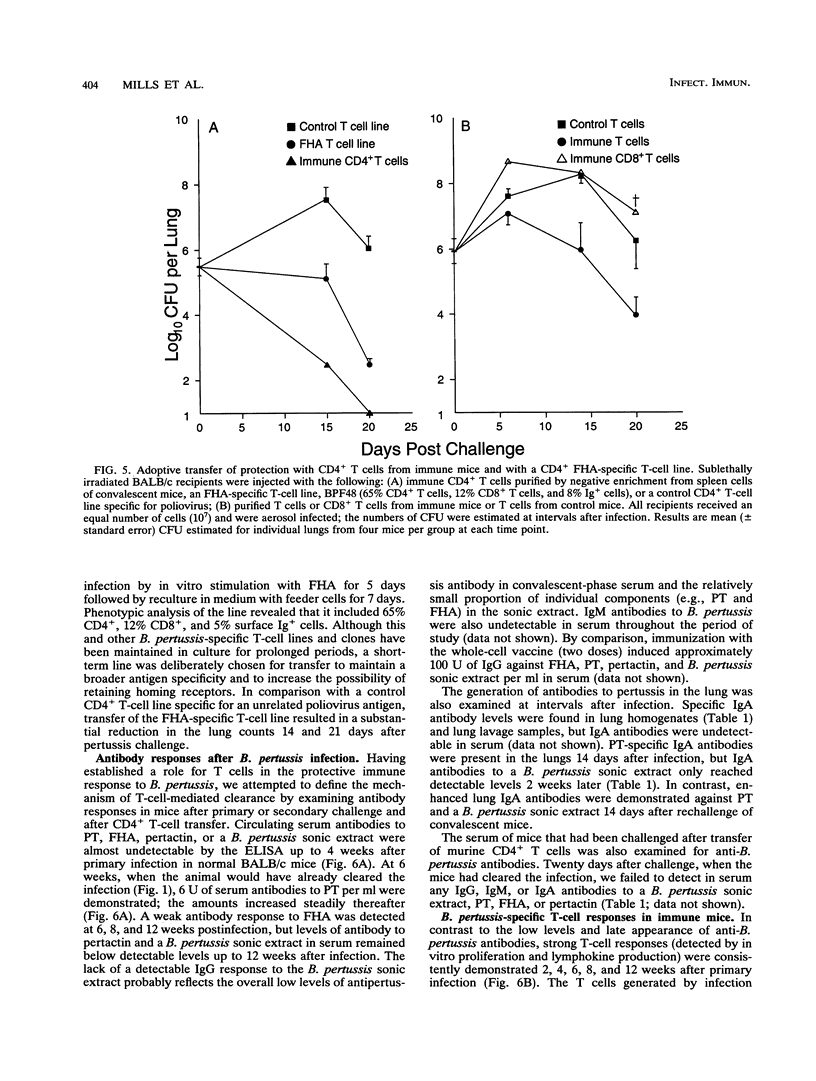
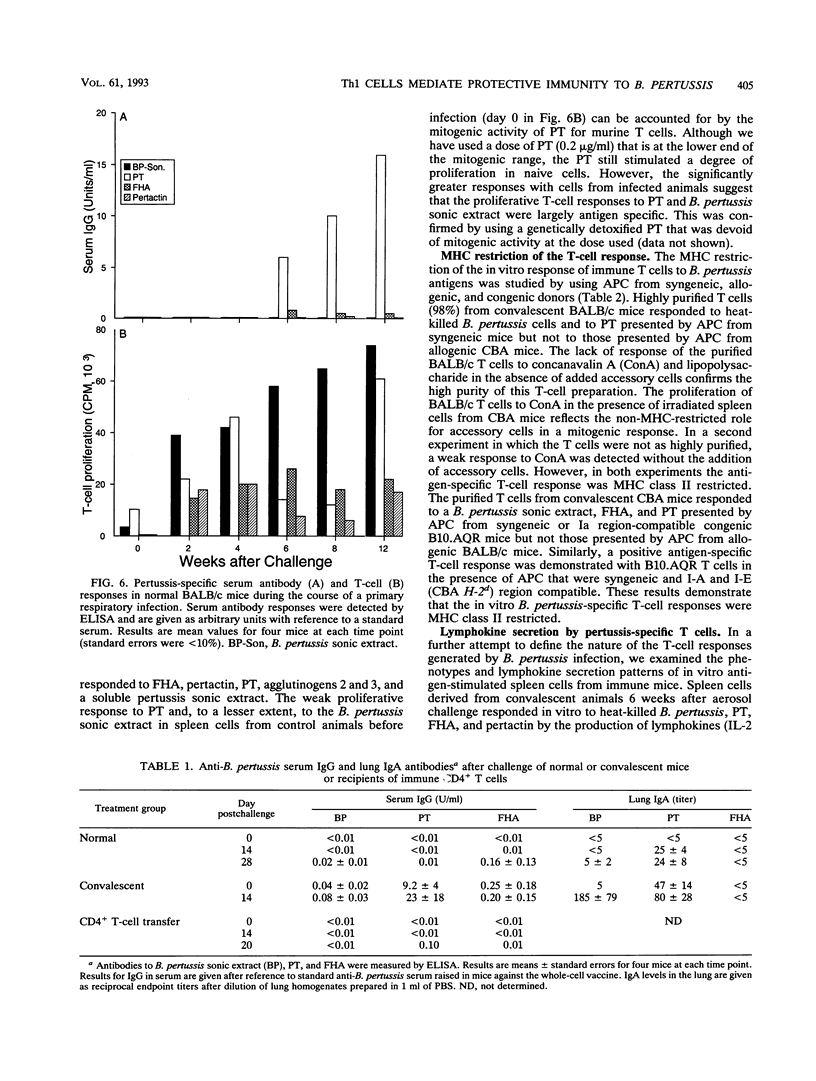
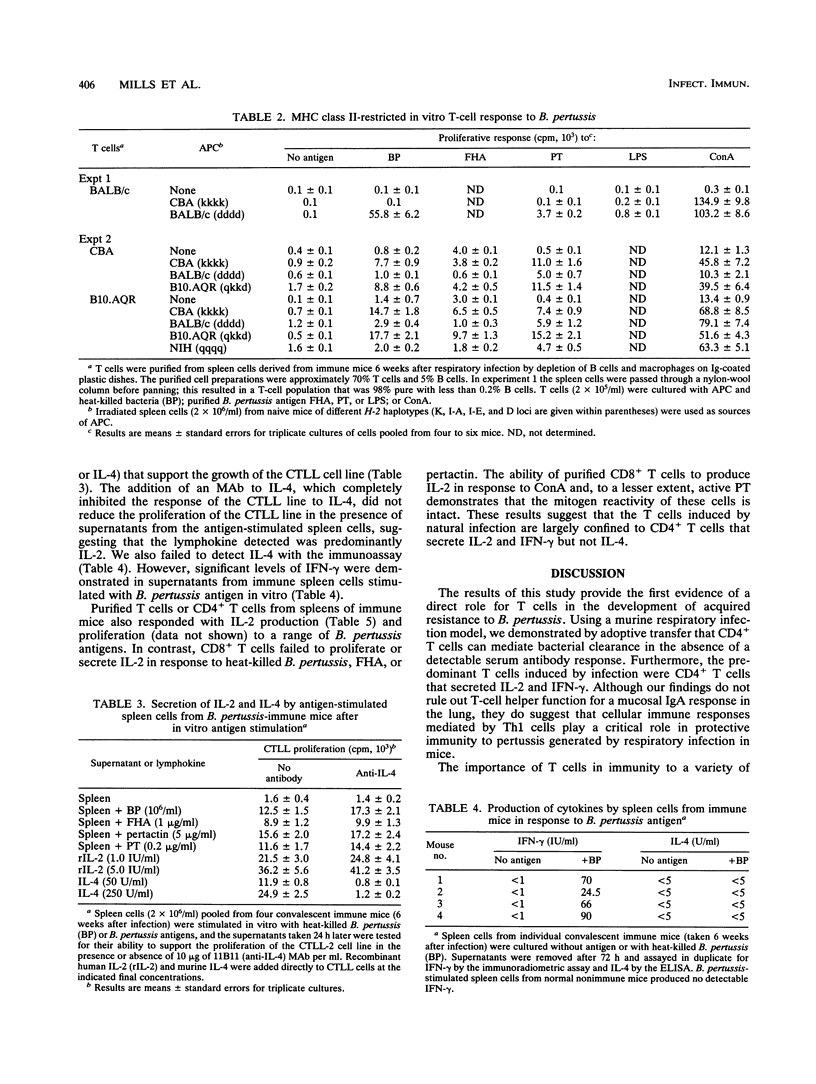
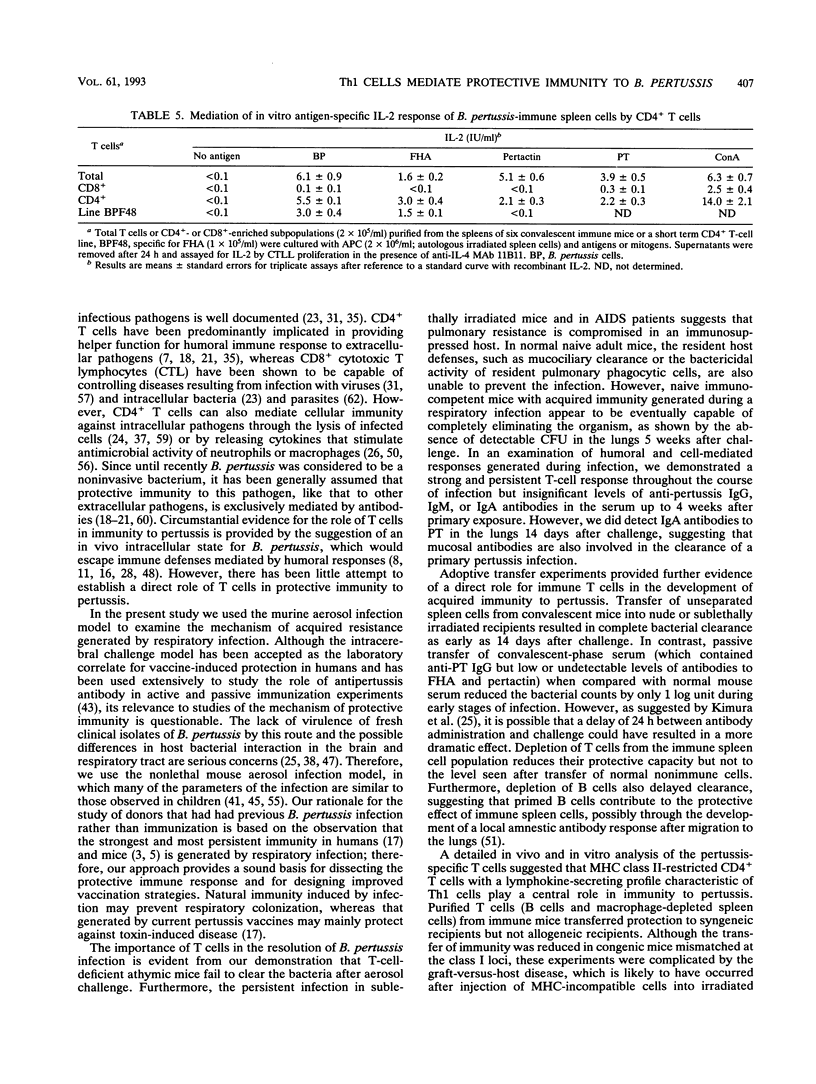
A murine respiratory infection model was used to study the mechanism of protective immunity to Bordetella pertussis. We found that nude mice, which are deficient in T cells, developed a persistent infection and failed to clear the bacteria after aerosol inoculation. In contrast, normal adult nonimmune mice cleared a respiratory infection approximately 35 days after challenge. Before bacterial clearance, antipertussis antibody levels in serum were low or undetectable, whereas consistent antigen-specific T-cell responses were demonstrated throughout the course of infection. The in vitro responses detected in immune spleen cells were mediated by a population of CD4+ major histocompatibility complex class II-restricted Th1-like cells that secreted interleukin-2 and gamma interferon but not interleukin-4. Adoptive transfer of immune spleen cells into nude or sublethally irradiated immunosuppressed mice before challenge resulted in bacterial clearance within 14 to 21 days. In contrast, injection of serum from convalescent mice before challenge only marginally reduced the bacterial load early in the course of infection. Furthermore, transfer of enriched T cells or purified CD4+ T cells but not CD8+ T cells from immune mice conferred a high level of protection. Recipients of CD4+ T cells cleared the bacteria from the lungs within 20 days of challenge, at which time B. pertussis-specific antibodies in the serum were undetectable. Although we do not rule out a contribution of mucosal immunoglobulin A, our findings suggest that cellular responses mediated by CD4+ Th1 cells play an important role in protective immunity to B. pertussis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson P. C., Wu T. C., Meade B. D., Rubin M., Manclark C. R., Pizzo P. A. Pertussis in a previously immunized child with human immunodeficiency virus infection. J Pediatr. 1989 Oct;115(4):589–592. doi: 10.1016/s0022-3476(89)80288-4. [DOI] [PubMed] [Google Scholar]
- Ashworth L. A., Fitzgeorge R. B., Irons L. I., Morgan C. P., Robinson A. Rabbit nasopharyngeal colonization by Bordetella pertussis: the effects of immunization on clearance and on serum and nasal antibody levels. J Hyg (Lond) 1982 Jun;88(3):475–486. doi: 10.1017/s0022172400070339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley K. W., Eldridge J. H., Lee F., Kiyono H., Everson M. P., Koopman W. J., Hirano T., Kishimoto T., McGhee J. R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989 Jun 1;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bromberg K., Tannis G., Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991 Dec;59(12):4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Li J. L., Roberts M., Beesley K., Romanos M., Pickard D. J., Francis M., Campbell D., Dougan G., Brennan M. J. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur J Immunol. 1991 May;21(5):1147–1153. doi: 10.1002/eji.1830210509. [DOI] [PubMed] [Google Scholar]
- Cheers C., Gray D. F. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969 Dec;17(6):875–887. [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Lebman D. A., Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989 Sep 1;170(3):1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Shrader B., Carty J., Mosmann T. R., Bond M. W. A mouse T cell product that preferentially enhances IgA production. I. Biologic characterization. J Immunol. 1987 Dec 1;139(11):3685–3690. [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. The recurrence of whooping cough: possible implications for assessment of vaccine efficacy. Lancet. 1982 Mar 20;1(8273):666–669. doi: 10.1016/s0140-6736(82)92214-0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Granström M., Granström G., Gillenius P., Askelöf P. Neutralizing antibodies to pertussis toxin in whooping cough. J Infect Dis. 1985 Apr;151(4):646–649. doi: 10.1093/infdis/151.4.646. [DOI] [PubMed] [Google Scholar]
- Grob P. R., Crowder M. J., Robbins J. F. Effect of vaccination on severity and dissemination of whooping cough. Br Med J (Clin Res Ed) 1981 Jun 13;282(6280):1925–1928. doi: 10.1136/bmj.282.6280.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J Immunol Methods. 1979;27(2):189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A., Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991 Jan 15;146(2):762–767. [PubMed] [Google Scholar]
- Kunimoto D. Y., Harriman G. R., Strober W. Regulation of IgA differentiation in CH12LX B cells by lymphokines. IL-4 induces membrane IgM-positive CH12LX cells to express membrane IgA and IL-5 induces membrane IgA-positive CH12LX cells to secrete IgA. J Immunol. 1988 Aug 1;141(3):713–720. [PubMed] [Google Scholar]
- Miller D. L., Ross E. M., Alderslade R., Bellman M. H., Rawson N. S. Pertussis immunisation and serious acute neurological illness in children. Br Med J (Clin Res Ed) 1981 May 16;282(6276):1595–1599. doi: 10.1136/bmj.282.6276.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. H., Burt D. S., Skehel J. J., Thomas D. B. Fine specificity of murine class II-restricted T cell clones for synthetic peptides of influenza virus hemagglutinin. Heterogeneity of antigen interaction with the T cell and the Ia molecule. J Immunol. 1988 Jun 15;140(12):4083–4090. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Murray P. D., Swain S. L., Kagnoff M. P. Regulation of the IgM and IgA anti-dextran B1355S response: synergy between IFN-gamma, BCGF II, and IL 2. J Immunol. 1985 Dec;135(6):4015–4020. [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Judd A. K., Ko C., Lim M., Fernandez R., Schoolnik G. K., Steinman L. MHC-restricted recognition of immunogenic T cell epitopes of pertussis toxin reveals determinants in man distinct from the ADP-ribosylase active site. J Exp Med. 1988 Nov 1;168(5):1855–1864. doi: 10.1084/jem.168.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppoloni S., Nencioni L., Di Tommaso A., Tagliabue A., Parronchi P., Romagnani S., Rappuoli R., De Magistris M. T. Lymphokine secretion and cytotoxic activity of human CD4+ T-cell clones against Bordetella pertussis. Infect Immun. 1991 Oct;59(10):3768–3773. doi: 10.1128/iai.59.10.3768-3773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M., Furman B. L., Wardlaw A. C. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis. 1980 Jul;142(1):56–66. doi: 10.1093/infdis/142.1.56. [DOI] [PubMed] [Google Scholar]
- Roberts M., Maskell D., Novotny P., Dougan G. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect Immun. 1990 Mar;58(3):732–739. doi: 10.1128/iai.58.3.732-739.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Irons L. I., Ashworth L. A. Pertussis vaccine: present status and future prospects. Vaccine. 1985 Mar;3(1):11–22. doi: 10.1016/0264-410x(85)90004-0. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O., Noel A., Putto-Laurila A., Pêtre J., Capiau C., Delem A., Vandevoorde D., Simoen E., Teuwen D. E., Bogaerts H. Development of an acellular pertussis vaccine and its administration as a booster in healthy adults. Vaccine. 1991 Feb;9(2):117–121. doi: 10.1016/0264-410x(91)90267-a. [DOI] [PubMed] [Google Scholar]
- Sato H., Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984 Nov;46(2):415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin R. D., Witvliet M. H., Manclark C. R. Mechanism of pertussis toxin B oligomer-mediated protection against Bordetella pertussis respiratory infection. Infect Immun. 1990 Dec;58(12):4063–4068. doi: 10.1128/iai.58.12.4063-4068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989 Feb 1;142(3):760–765. [PubMed] [Google Scholar]
- Taylor P. M., Meager A., Askonas B. A. Influenza virus-specific T cells lead to early interferon gamma in lungs of infected hosts: development of a sensitive radioimmunoassay. J Gen Virol. 1989 Apr;70(Pt 4):975–978. doi: 10.1099/0022-1317-70-4-975. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Romero P., Nussenzweig R. S., Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990 Nov 1;172(5):1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Zapiain L. A., Galvan P., Hewlett E. L. Characterization of antibody inhibiting adherence of Bordetella pertussis to human respiratory epithelial cells. J Clin Microbiol. 1984 Aug;20(2):167–170. doi: 10.1128/jcm.20.2.167-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


