Abstract
Tetrapod vertebrates possess multiple α- and β-like globin genes that are ontogenetically regulated, such that functionally distinct hemoglobin (Hb) isoforms are synthesized during different stages of development. The α- and β-like globin genes of amphibians, birds and mammals are differentially expressed during embryonic development and postnatal life, but little is known about the developmental regulation of globin gene expression in non-avian reptiles. Here we report an investigation into the developmental regulation of Hb synthesis in the green anole lizard Anolis carolinensis. We tested two hypotheses derived from comparative genomic studies of the globin gene clusters in tetrapod vertebrates. First, we tested whether the product of the Anolis αD-globin gene is incorporated into embryonic Hb, thereby performing the role that would normally be performed by the embyronic αE-globin gene (which has been deleted from the green anole genome). Second, we tested whether two ‘lizard-specific’ β-globin paralogs have independently evolved a division of labor between an early-expressed embryonic gene and a later-expressed adult gene. Results of a proteomic analysis revealed that α- and β-like globin genes of the anole are differentially expressed during embryonic development. However, the same repertoire of α- and β-chain Hb isoforms was expressed during all stages of development and postnatal life, and the ontogenetic shifts in isoform composition were relatively subtle. In contrast to the pattern that has been documented in other tetrapod vertebrates, it appears that the developmental regulation of Hb synthesis in the green anole lizard does not involve discrete, stage-specific switches in gene activation and gene silencing.
Keywords: Anolis, gene duplication, globin gene family, hemoglobin, reptile genomics
INTRODUCTION
In vertebrates, the multimeric hemoglobin (Hb) protein binds and transports oxygen and other gaseous ligands in support of cellular aerobic metabolism. The protein carries out these functions under the diverse range of physiological conditions that are encountered during the various stages of prenatal development and postnatal life (Brittain, 2002). The Hb of jawed vertebrates is a heterotetramer composed of two α-chain subunits and two β-chain subunits that are encoded by members of two paralogous gene families. During the course of vertebrate evolution, multiple rounds of gene duplication and divergence have given rise to families of α- and β-like globin genes that are ontogenetically regulated, such that functionally distinct Hb isoforms (isoHbs) are synthesized in embryonic and adult erythroid cells (Collins and Weissman, 1984; Hardison, 1998; Hardison, 2001). For example, the α- and β-like globin genes of Xenopus are differentially expressed in the larval and adult stages (Banville and Williams, 1985a; Banville and Williams, 1985b; Fuchs et al., 2006), and the α- and β-like globin genes of birds and mammals are also differentially expressed during embryonic development and postnatal life (Hardison, 2001; Alev et al., 2008; Alev et al., 2009). However, very little is known about the developmental regulation of Hb synthesis in non-avian reptiles.
In the α-globin gene family, the physiological division of labor between early- and late-expressed genes was established in the common ancestor of tetrapod vertebrates and it appears to have been retained in nearly all descendant lineages. The ancestral arrangement of the tetrapod α-globin gene cluster consists of three linked genes, 5′-αE, αD, αA-3′ (Hoffmann and Storz, 2007; Hoffmann et al., 2010). The αE- and αA-globin genes originated via tandem duplication of an ancestral proto α-globin gene after the stem lineage of teleost fishes split from the stem lineage of tetrapods, and the αD-globin gene originated subsequently via tandem duplication of the proto αE-globin gene in the common ancestor of tetrapods (Fig. 1A) (Hoffmann and Storz, 2007). The embryonic αE-globin gene (known as αL-globin in amphibians, π-globin in birds and ζ-globin in mammals) is exclusively expressed in primitive erythroid cells derived from the yolk sac, and the adult αA-globin gene is expressed in definitive erythroid cells during later stages of prenatal development and postnatal life (Proudfoot et al., 1982; Higgs et al., 1989; Hardison, 2001). Among tetrapod vertebrates, the green anole lizard (Anolis carolinensis) represents the one documented exception to this highly conserved pattern of developmental regulation due to the apparent absence of the αE-globin gene in this species (Hoffmann et al., 2010). It remains to be seen whether this gene is absent from the genomes of other squamate reptiles as well. As the αD-globin gene originated via duplication of an α-like globin gene that had an ancestral larval/embryonic function (Hoffmann and Storz, 2007), it may be that αD-chain isoHbs perform the necessary oxygen-binding and oxygen-transport functions during early stages of embryonic development in lizards and other reptiles that do not possess an ortholog of αE-globin. In some species of birds, for example, the αD-globin gene is only expressed during embryonic development (Ikehara et al., 1997) whereas other species express αD-globin in both primitive and definitive erythroid cells (Alev et al., 2008; Alev et al., 2009).
Fig. 1.
Schematic cladograms depicting the inferred phylogenetic relationships among members of the α- and β-globin gene families in tetrapod vertebrates [based on data from Hoffmann and Storz (Hoffmann and Storz, 2007), Opazo et al. (Opazo et al., 2008a) and Hoffmann et al. (Hoffmann et al., 2010)]. In each tree, filled symbols denote nodes that represent gene duplication events. (A) Phylogeny of α-like globin genes in tetrapods. Note that the three paralogs (αE-, αD- and αA-globin) are reciprocally monophyletic relative to one another, and that the αE-globin gene (which is exclusively expressed in primitive erythroid cells during embryonic development) and the αD-globin gene are products of a duplication event that occurred in the stem lineage of tetrapods. (B) Phylogeny of β-like globin genes in tetrapods. Note that birds, non-avian reptiles, eutherian mammals, monotremes and amphibians each inherited an ortholog of the same proto β-globin gene, which then underwent one or more rounds of duplication and divergence to produce distinct repertoires of β-like globin genes in each descendant lineage.
In contrast to the ancient functional diversification of the α-globin gene cluster, the physiological division of labor between early- and late-expressed genes in the β-globin gene cluster appears to have evolved independently in several different tetrapod lineages (Hoffmann et al., 2010). Each of the main lineages of tetrapods inherited an ortholog of the same proto β-globin gene, which then underwent one or more rounds of duplication and divergence to produce distinct repertoires of β-like globin genes in each descendant lineage (Fig. 1B) (Opazo et al., 2008a; Opazo et al., 2008b; Patel et al., 2008; Hoffmann et al., 2010). For example, an inventory of globin genes in the green anole genome revealed that this species possesses a pair of highly distinct β-like globin genes, βI and βII, that are distinguished from one another by 25% sequence divergence at the amino acid level (Hoffmann et al., 2010). Phylogenetic analysis of reptile β-like globin genes revealed that orthologs of the Anolis βI- and βII-globin genes are shared by other lizards, but it is not yet clear whether they are shared more widely among other lepidosaurs, and nothing is known about the developmental expression profiles of these genes. Given that the developmental regulation of β-like globin genes has evolved independently in amphibians, birds and mammals (Hoffmann et al., 2010), it is possible that the βI- and βII-globin paralogs of lizards have evolved a functionally similar physiological division of labor between an early-expressed embryonic gene and a later-expressed adult gene.
Here we report the results of a developmental study of Hb synthesis in the green anole lizard A. carolinensis. We tested two main predictions that were derived from our current understanding of globin gene family evolution in tetrapod vertebrates. The first prediction is that the product of the Anolis αD-globin gene is incorporated into embryonic Hb, thereby performing the role that would normally be performed by the embryonic αE-globin gene (which is missing from the green anole genome). The second prediction is that the two β-globin paralogs of Anolis (which are products of a lineage-specific duplication event) are developmentally regulated such that one of the two paralogs is expressed exclusively in primitive erythroid cells of early-stage embryos, while the other paralog is expressed in definitive erythrocytes during later stages of prenatal development and postnatal life.
MATERIALS AND METHODS
Specimen collection
To test our predictions regarding the developmental regulation of Hb synthesis in Anolis we collected tissues from embryonic specimens and blood samples from adult specimens of Anolis carolinensis Voigt 1832. We sampled early, mid- and late-stage embryos corresponding to stages 5, 5/6, 11 and 17 of Anolis development (Fig. 2) (Sanger et al., 2008a). Relative to most avian eggs, A. carolinensis eggs are laid at a relatively advanced embryonic stage, represented by stage 5. At this stage, limb buds, hind-, mid- and forebrain segments, heart and many other structures are already present in the embryo (Fig. 2A). Blood is visible in the embryo several stages prior to hatching, but peripheral blood vessels are not yet visible in the extremities. By stage 11 the embryonic liver is yellow-brown in color, and blood is easily visible in the heart and in the extremities. After approximately 30 days the embryo is fully developed and capable of living outside of the egg. We sampled just before this time at stage 17.
Fig. 2.
Representative developmental stages of Anolis carolinensis. Pictures in panels A, B and C correspond to stages 5, 11 and 17, respectively. Scale bars=2 mm.
Lizard husbandry and embryo collection were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee of Harvard University (IACUC# 28-14 and 26-11), and details are provided by Sanger et al. (Sanger et al., 2008a; Sanger et al., 2008b). Briefly, sexually mature A. carolinensis lizards were collected from the wild and purchased from Candy's Quality Reptiles (La Place, LA, USA). To collect embryonic tissues, we housed 3-5 females in a standard rat cage with one male and several sticks to act as perches. A potted plant within each cage was checked every one to three days for new eggs. We incubated eggs in coarse-grained vermiculite at 27°C and approximately 75% humidity. We collected embryos of the appropriate stage by submerging the egg in phosphate-buffered saline (PBS) in a Petri dish. To remove the embryo from the egg, a shallow incision was made over the embryo using #5 watchmaker's forceps allowing the eggshell to be folded away. The embryo was then cleared from the yolk and rinsed in fresh PBS. Early embryos (stages 5 and 5/6) were immediately flash frozen. The liver was dissected from later stage embryos (stages 11 and 17) by opening the abdomen and chest cavity using 3 mm spring scissors while the embryo was submerged in fresh PBS. After being flash frozen, all tissues were stored at –80°C until the time of processing. Blood samples from four adult lizards were collected by filling a capillary tube from a small incision along the ventrum of the tail of a partially anesthetized lizard (Sellers et al., 1980).
Characterization of isoHb diversity
In the case of the adult A. carolinensis specimens, the isoHb composition of mature erythrocytes was characterized by means of isoelectric focusing (IEF; PhastSystem, GE Healthcare Bio-Sciences, Piscataway, NJ, USA). After separating native Hbs by means of IEF, gel bands were excised and digested with trypsin. The resultant peptides were then identified by means of tandem mass spectrometry (MS/MS) (cf. Nakachi et al., 2008; Campbell et al., 2010; Storz et al., 2010). The peak lists of the MS/MS data were generated by Distiller (Matrix Science, London, UK) using the charge state recognition and de-isotoping with default parameters for quadrupole time-of-flight data. Database searches of the resultant MS/MS spectra were performed using Mascot (Matrix Science, v1.9.0, London, UK). Specifically, peptide mass fingerprints derived from the MS/MS analysis were used to query a custom database of Anolis α- and β-globin sequences. These amino acid sequences were derived from conceptual translations of all annotated α- and β-globin genes from the current assembly of the green anole genome (release 54 of the Ensembl database).
A previous examination of the green anole genome (Hoffmann et al., 2010) revealed two putatively functional α-like globin genes (located in scaffolds 2790:1-15038 and 1188:1-163036). Phylogenetic reconstructions based on coding sequence indicated that one of the genes is orthologous to the αA-globin gene of other tetrapods, and the other gene is orthologous to the αD-globin gene of other amniotes (see Fig. 1A). However, there was no trace of the embryonic αE-globin gene in the current green anole genome assembly or in independent EST databases. Hoffmann et al. also annotated two putatively functional β-like globin genes (βI- and βII-globin, located in scaffolds 7008:1-4809 and 3777:1-10310) (Hoffmann et al., 2010). Phylogenetic reconstructions indicated that the βI-globin genes of the green anole and common iguana are 1:1 orthologs, as are the βII-globin genes from the same species pair (Hoffmann et al., 2010). The βI- and βII-globin genes of the green anole and iguana appear to be products of a lizard-specific or squamate-specific duplication event. In the reference database for the MS/MS analysis, we also included the α- and β-globin sequences from one amphibian (Xenopus tropicalis Daudin 1802), two additional squamate reptiles (common iguana, Iguana iguana Linnaeus 1758, and Indian python, Python molurus Linnaeus 1758), one sphenodont reptile (tuatara, Sphenodon punctatus Gray 1842), two crocodilians (Nile crocodile, Crocodylus niloticus Laurenti 1768, and American alligator, Alligator mississippiensis Daudin 1802), two testudines (loggerhead turtle, Caretta caretta Linnaeus 1758, and Galapagos tortoise, Geochelone nigra Quoy and Gaimard 1824), two birds (chicken, Gallus gallus Linnaeus 1758, and zebra finch, Taeniopygia guttata Vieillot 1817) and two mammals (human, Homo sapiens Linnaeus 1758, and platypus, Ornithorhynchus anatinus Shaw 1799). The following search parameters were used for the MS/MS analysis: no restriction on protein molecular weight or isoelectric point, and methionine oxidation allowed as a variable peptide modification. Mass accuracy settings were 0.15 Da for peptide mass and 0.12 Da for fragment ion masses. We identified all significant protein hits that matched more than one peptide with P<0.05.
Quantitative proteomic analysis of Hb expression
In the case of Hb components separated by IEF, the relative concentrations of the different isoHbs were quantified densitometrically using Image J (Abramoff et al., 2004). In the case of the A. carolinensis embryos, we pooled whole embryos (stages 5 and 5/6) or dissected liver tissues (stages 11 and 17) for trypsin digests and subsequent MS/MS analyses. Tissues from 5-10 embryos were pooled for each of the four developmental stages. To estimate the relative abundance of different isoHbs in each sample, we measured the exponentially modified protein abundance index, emPAI, using the number of identified peptides per protein (Ishihama et al., 2005).
RESULTS
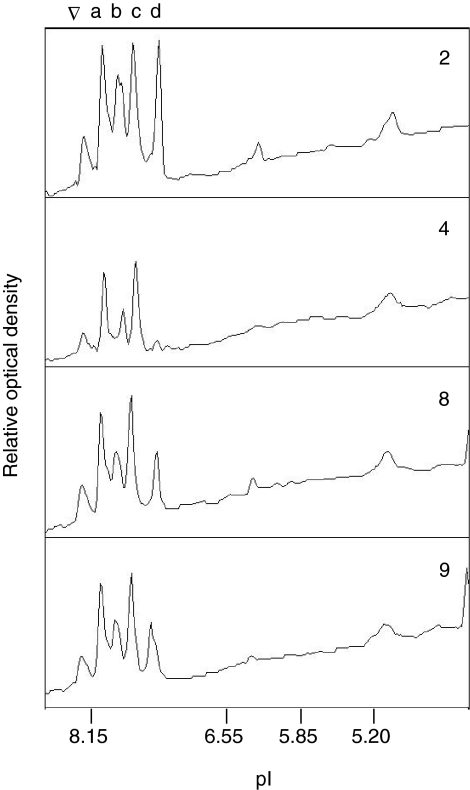
The IEF analysis revealed that adult lizards express four distinct isoHb components (Fig. 3), and the MS/MS analysis revealed that each of the subunit components represent products of the previously annotated α- and β-like globin genes in the Anolis genome assembly (Fig. 4). There were no peptide matches corresponding to the products of genes other than the αA-, αD-, βI- and βII-globin genes of Anolis. The MS/MS analysis revealed that adult lizards express each of the four possible tetrameric α2β2 subunit isoHb combinations, which were present in the following rank order of protein abundance: αA2βI2>αD2βII2≥αD2βI2>αA2βII2. In the mature erythrocytes of adult lizards, the mean ratio of αD/αA-chain isoHbs was 1.13 (range=1.09-1.17), and the mean ratio of βI/βII-chain isoHbs was 1.38 (range=1.30-1.52; N=4 individuals)
Fig. 3.
Densitometric scans of isoelectric focusing (IEF) gels showing hemoglobin isoform (isoHb) diversity in the red blood cells of adult Anolis carolinensis (N=4; specimens 2, 4, 8 and 9). The four major peaks in each trace (labeled a, b, c and d) represent CO-derivatives of structurally distinct isoHb tetramers. The ∇ symbol denotes the position of the loading well in the IEF gel.
Fig. 4.
Alignment of amino acid sequences representing the complete repertoire of α- and β-like globin genes from Anolis carolinensis and two bird species, chicken and zebra finch. Sequences of α- and β-like globin genes are shown in panels A and B, respectively. The highlighted portions of each Anolis sequence denote the coverage of matched peptides in the mass spectrometry analysis (see text for details).
In the case of the developmental study, results of the MS/MS analysis revealed that the α- and β-globin genes of the green anole are differentially expressed during the course of embryonic development (Fig. 5). However, the same subunit isoHbs that were identified in the mature erythrocytes of adult lizards were also expressed throughout the entire course of prenatal development. Thus, although expression levels undergo subtle changes during the course of development, the MS/MS data demonstrate that the αA-, αD-, βI- and βII-globin genes were expressed in both primitive and definitive erythroid cells.
Fig. 5.
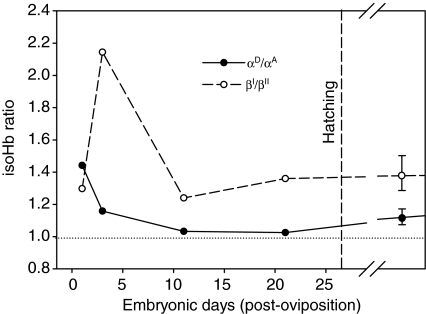
Changes in the relative abundance of α- and β-chain hemoglobin isoforms (isoHbs) during embryonic development and postnatal life in Anolis carolinensis. Mean isoHb ratios from red blood cells of four adult lizards are shown to the right of the axis break, and the bars denote the minimum and maximum values.
With regard to the α-like globin genes, the αD-chain isoHbs were most highly expressed during the earliest stages of embryogenesis, and the relative abundance of αD-chain isoHbs consistently exceeded that of αA-chain isoHbs over the course of development (Fig. 5). The ratio of αD/αA-chain isoHbs decreased from 1.44 at day 1 post-oviposition (stage 5) to 1.03 at day 21 (stage 17). Compared with the embryos at stage 17, the ratio of the two α-chain isoHbs remained remarkably similar to the ratio measured in the mature erythrocytes of adult lizards.
With regard to the β-like globin genes, the βI-chain isoHbs were most highly expressed during the earliest stages of embryonic development, exhibiting a twofold increase in relative abundance at day 4 post-oviposition (stage 5/6), followed by a gradual decline up to the pre-hatching stage (stage 17). Aside from the early spike in the relative abundance of the βI-chain isoHb, the ratios of βI/βII-chain isoHbs during the remaining stages of embryonic development were quite similar to the ratio measured in the mature erythrocytes of adult lizards.
DISCUSSION
By characterizing the developmental regulation of Hb synthesis in the green anole lizard, we were able to test two hypotheses derived from comparative genomic studies of the globin gene clusters in tetrapod vertebrates. First, we tested whether the product of the Anolis αD-globin gene is incorporated into embryonic Hb, thereby performing the role that would normally be performed by αE-globin. Second, we tested whether the two ‘lizard-specific’ β-globin paralogs are developmentally regulated such that one of the two paralogs is expressed exclusively in primitive erythroid cells of early-stage embryos, while the other paralog is expressed in definitive erythrocytes during later stages of embryonic development and postnatal life. Below we describe tests of both predictions in turn.
Expression of α-chain isoHbs
Results of the MS/MS analysis revealed that the highest relative expression of αD-chain isoHbs occurred during the earliest post-oviposition stage of embryogenesis (Fig. 5). This suggests that the product of αD-globin may play a key role in oxygen uptake and/or oxygen scavenging in early-stage lizard embryos. However, in contrast to the αE-globin gene of other tetrapods, the αD-globin gene in the green anole is also expressed at a high level throughout the course of embryonic development, and αD-chain isoHbs are present at a slightly higher concentration than αA-chain isoHbs in the mature erythrocytes of adult lizards. The high-level expression of αD-globin during postnatal life is quite different from the typical pattern observed in birds. In the definitive erythroid cells of adult birds, the αD-chain isoform typically constitutes the minor fraction of adult Hb and the αA-chain isoform typically constitutes the major fraction, although the relative abundance of the two isoforms is quite variable among species (Borgese and Bertles, 1965; Brown and Ingram, 1974; Hiebl et al., 1987; Ikehara et al., 1997).
The function of αD-Hb has always been something of an enigma, and its evolutionary origin and phylogenetic affinities have only recently been illuminated (Hoffmann and Storz, 2007). In mammals, the αE- and αA-globin genes are often present in multiple copies, but the αD-globin gene has been deleted independently in multiple lineages (Hughes et al., 2005; Cooper et al., 2006; Hoffmann et al., 2008). The αD-globin gene has also been secondarily lost from Xenopus (Fuchs et al., 2006; Hoffmann and Storz, 2007), and it remains to be seen whether this gene is absent from the genomes of all amphibians. Products of the αD-globin gene have not been recovered from the erythrocytes of adult crocodilians (reviewed by Gorr et al., 1998), but in lieu of a complete genome sequence it remains possible that crocodilians possess a transcriptionally active αD-globin gene that has thus far evaded detection because it is exclusively expressed during embryonic development. In summary, our experimental results suggest that αD-chain isoHbs may play an especially important role during the earliest stages of embryonic development in the green anole, as this is the stage where it is most highly expressed. However, unlike the embryonic αE-globin gene of other tetrapods, the αD-globin gene of the green anole does not appear to be performing a specialized function that is specific to early embryogenesis, as the same gene is highly expressed during all stages of development and postnatal life.
Expression of β-chain isoHbs
As the two β-globin paralogs of Anolis are products of a lizard- or squamate-specific duplication event and are therefore not orthologous to embryonic β-like globin genes of other tetrapods (Hoffmann et al., 2010), we tested whether the two genes independently evolved different stage-specific expression patterns. The MS/MS analysis revealed ontogenetic shifts in the relative abundance of βI- and βII-chain isoHbs. However, both isoHbs were also co-expressed in the mature erythrocytes of adult lizards. Thus, although the high level of amino acid sequence divergence between the two lizard-specific β-globin paralogs suggests that they may have evolved some form of physiological division of labor (cf. Weber et al., 2000; Storz et al., 2008; Runck et al., 2009), the isoHb differentiation is not associated with any discrete differences in the developmental timing of expression. The two lizard-specific β-globin paralogs exhibit subtle changes in expression during the course of prenatal development, but the pattern appears to be qualitatively different from the more discrete gene switching that characterizes the ontogenetic regulation of Hb synthesis in other tetrapod vertebrates (Banville and Williams, 1985a; Banville and Williams, 1985b; Hardison, 2001; Nagel and Steinberg, 2001; Fuchs et al., 2006; Nakazawa et al., 2006; Alev et al., 2008; McIntyre et al., 2008; Nagai and Sheng, 2008; Alev et al., 2009).
Conclusions and future directions
Results of the MS/MS analysis revealed that α- and β-like globin genes of the green anole lizard are differentially expressed during the course of embryonic development. However, the same repertoire of α- and β-chain isoHbs was expressed during all stages of embryonic development and postnatal life, and the ontogenetic shifts in isoHb composition were relatively subtle. In birds and mammals, by contrast, the embryonic α- and β-like globin genes are exclusively expressed in primitive erythroid cells derived from the yolk sac, and they are not re-activated in definitive erythroid cells during postnatal life. Contrary to the pattern that has been documented in other tetrapod vertebrates, it appears that the developmental regulation of Hb synthesis in the green anole does not involve discrete, stage-specific switches in gene activation and gene silencing.
Developmental changes in blood-gas transport have been documented in almost all tetrapod species that have been examined in sufficient detail. In some taxa, developmental changes in blood-oxygen affinity are at least partly attributable to the stage-specific expression of functionally distinct isoHbs. This Hb switching has been documented in a number of birds (Borgese and Nagel, 1977; Baumann et al., 1982), crocodilians (Grigg et al., 1993), snakes (Pough, 1977; Birchard et al., 1984; Ragsdale and Ingermann, 1991) and turtles (Wells and Baldwin, 1994). In some taxa, such as the viviparous lizard, Sphenomorphus quoyii (Grigg and Harlow, 1981), developmental changes in blood-oxygen affinity are primarily attributable to changes in the red cell concentrations of organic phosphates or other allosteric cofactors that modulate Hb-oxygen affinity. It remains to be seen whether oviparous lizards like Anolis conform to this same pattern. In the future, it will be important to characterize functional properties of the various α- and β-chain isoHbs that are differentially expressed during prenatal development and adulthood in the anole lizard. In A. carolinensis, products of the αA- and αD-globins are distinguished by 75 amino acid substitutions (53% amino acid sequence divergence), and products of the βI- and βII-globins are distinguished by 36 amino acid substitutions (25% divergence; Fig. 4). Given the high levels of amino acid divergence between paralogous genes that encode the same subunit types, there would seem to be ample scope for functional isoHb differentiation in these lizards. It will also be important to assess whether developmental changes in blood-gas transport are attributable to changes in red cell pH and/or changes in the intracellular concentration of organic phosphates such as ATP and GTP that are known to modulate Hb-oxygen affinity in other non-avian reptiles.
Acknowledgments
We thank T. A. Gorr and two anonymous reviewers for helpful comments and suggestions.
Footnotes
This work was funded by grants from the National Science Foundation and the National Institutes of Health/NHLBI. We thank K. Williams for valuable laboratory assistance. Deposited in PMC for release after 12 months.
REFERENCES
- Abramoff M. D., Magelhaes P. J., Ram S. J. (2004). Image processing with Image J. Biophotonics Int. 11, 36-42 [Google Scholar]
- Alev C., McIntyre B. A. S., Nagai H., Shin M., Shinmyozu K., Jakt L. M., Sheng G. (2008). BetaA, the major beta globin in definitive red blood cells, is present from the onset of primitive erythropoiesis in chicken. Dev. Dyn. 237, 1193-1197 [DOI] [PubMed] [Google Scholar]
- Alev C., Shinmyozu K., McIntyre B. A. S., Sheng G. (2009). Genomic organization of zebra finch alpha and beta globin genes and their expression in primitive and definitive blood in comparison with globins in chicken. Dev. Genes Evol. 219, 353-360 [DOI] [PubMed] [Google Scholar]
- Banville D., Williams J. G. (1985a). Developmental changes in the pattern of larval beta-globin gene expression in Xenopus laevis. Identification of two early larval beta-globin mRNA sequences. J. Mol. Biol. 184, 611-620 [DOI] [PubMed] [Google Scholar]
- Banville D., Williams J. G. (1985b). The pattern of expression of the Xenopus laevis tadpole α-globin genes and the amino acid sequence of the three major tadpole α-globin polypeptides. Nucleic Acids Res. 13, 5407-5421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R., Padeken S., Haller E. A. (1982). Functional properties of embryonic chicken hemoglobins. J. Appl. Physiol. 53, 1439-1448 [DOI] [PubMed] [Google Scholar]
- Birchard G. F., Black C. P., Schuett G. W., Black V. (1984). Foetal-maternal blood respiratory properties of an ovoviparous snake, the cottonmouth, Agkistrodon piscivorus. J. Exp. Biol. 108, 247-255 [Google Scholar]
- Borgese T. A., Bertles J. F. (1965). Hemoglobin heterogeneity: embryonic hemoglobin in the duckling and its disappearance in the adult. Science 148, 509-511 [DOI] [PubMed] [Google Scholar]
- Borgese T. A., Nagel R. L. (1977). Differential effects of 2,3-DPG, ATP and inositol pentaphosphate (IP5) on the oxygen equilibrium of duck embryonic, fetal and adult hemoglobins. Comp. Biochem. Physiol. 56A, 539-543 [Google Scholar]
- Brittain T. (2002). Molecular aspects of embryonic hemoglobin function. Mol. Aspects Med. 23, 293-342 [DOI] [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. (1974). Structural studies on chick embryonic hemoglobins. J. Biol. Chem. 249, 3960-3972 [PubMed] [Google Scholar]
- Campbell K. L., Storz J. F., Signore A. V., Moriyama H., Catania K. C., Payson A. P., Bonaventura J., Stetefeld J., Weber R. E. (2010). Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol. Biol. 10, 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. (1984). The molecular genetics of human hemoglobin. Prog. Nucleic Acid Res. Mol. Biol. 31, 315-462 [DOI] [PubMed] [Google Scholar]
- Cooper S. J. B., Wheeler D., De Leo A., Cheng J., Holland R. A. B., Marshall Graves J. A., Hope R. M. (2006). The mammalian alphaD-globin gene lineage and a new model for the molecular evolution of alpha-globin gene clusters at the stem of the mammalian radiation. Mol. Phylogenet. Evol. 38, 439-448 [DOI] [PubMed] [Google Scholar]
- Fuchs C., Burmester T., Hankeln T. (2006). The amphibian globin gene repertoire as revealed by the Xenopus genome. Cytogenet. Genome Res. 112, 296-306 [DOI] [PubMed] [Google Scholar]
- Gorr T. A., Mable B. K., Kleinschmidt T. (1998). Phylogenetic analysis of reptilian hemoglobins: trees rates and divergences. J. Mol. Evol. 47, 471-485 [DOI] [PubMed] [Google Scholar]
- Grigg G. C., Harlow P. (1981). A fetal-maternal shift of blood oxygen affinity in an Australian viviparous lizard, Sphenomorphus quoyii (Reptilia: Scincidae). J. Comp. Physiol. 142, 495-499 [Google Scholar]
- Grigg G. C., Wells R. M. G., Beard L. A. (1993). Allosteric control of oxygen binding by haemoglobin during development in the crocodile Crocodylus porosus: the role of red cell organic phosphates and carbon dioxide. J. Exp. Biol. 175, 15-32 [Google Scholar]
- Hardison R. (1998). Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J. Exp. Biol. 201, 1099-1117 [DOI] [PubMed] [Google Scholar]
- Hardison R. (2001). Organization, evolution, and regulation of the globin genes. In Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (eds Steinberg M. H., Forget B. G., Higgs D. R., Nagel R. L.), pp. 95-115 Cambridge: Cambridge University Press; [Google Scholar]
- Hiebl I., Braunitzer G., Schneeganss D. (1987). The primary structures of the major and minor hemoglobin-components of adult Andean goose (Chloephaga melanoptera Anatidae): the mutation Leu→Ser in position 55 of the β-chains. Biol. Chem. Hoppe-Seyler 368, 1559-1569 [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Vickers M. A., Wilkie A. O., Pretorius I. M., Jarman A. P., Weatherall D. J. (1989). A review of the molecular genetics of the human α-globin gene cluster. Blood 73, 1081-1104 [PubMed] [Google Scholar]
- Hoffmann F. G., Storz J. F. (2007). The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol. Biol. Evol. 24, 1982-1990 [DOI] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C., Storz J. F. (2008). Rapid rates of lineage-specific gene duplication and deletion in the α-globin gene family. Mol. Biol. Evol. 25, 591-602 [DOI] [PubMed] [Google Scholar]
- Hoffmann F. G., Storz J. F., Gorr T. A., Opazo J. C. (2010). Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol. Biol. Evol. 27, 1126-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R., Cheng J., Ventress N., Prabhakar S., Clark K., Anguita E., De Gobbi M., de Jong P., Rubin E., Higgs D. R. (2005). Annotation of cis-regulatory elements by identification, subclassification, and functional assessment of multispecies conserved sequences. Proc. Natl. Acad. Sci. USA 102, 9830-9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara T., Eguchi Y., Kayo S., Takei H. (1997). Isolation and sequencing of two alpha-globin genes alpha(A) and alpha(D) in pigeon and evidence for embryo-specific expression of the alpha(D)-globin gene. Biochem. Biophys. Res. Commun. 234, 450-453 [DOI] [PubMed] [Google Scholar]
- Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. (2005). Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4, 1265-1272 [DOI] [PubMed] [Google Scholar]
- McIntyre B. A. S., Alev C., Tarui H., Jakt L. M., Sheng G. (2008). Expression profiling of circulating non-red blood cells in embryonic blood. BMC Dev. Biol. 8, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Sheng G. (2008). Definitive erythropoiesis in chicken yolk sac. Dev. Dyn. 237, 3332-3341 [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Steinberg M. H. (2001). Hemoglobins of the embryo and fetus and minor hemoglobins of adults. In Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (eds Steinberg M. H., Forget B. G., Higgs D. R., Nagel R. L.), pp. 197-230 Cambridge: Cambridge University Press; [Google Scholar]
- Nakachi M., Hoshi M., Matsumoto M., Moriyama H. (2008). Conserved sequences of sperm-activating peptide and its receptor throughout evolution, despite speciation in the sea star Asterias amurensis and closely related species. Zygote 16, 229-237 [DOI] [PubMed] [Google Scholar]
- Nakazawa F., Nagai H., Shin M., Sheng G. (2006). Negative regulation of primitive hematopoeisis by the FGF signaling pathway. Blood 108, 3335-3343 [DOI] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G., Storz J. F. (2008a). Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proc. Natl. Acad. Sci. USA 105, 1590-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G., Storz J. F. (2008b). Differential loss of embryonic globin genes during the radiation of placental mammals. Proc. Natl. Acad. Sci. USA 105, 12950-12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. S., Cooper S. J. B., Deakin J. E., Fulton B., Graves T., Warren W. C., Wilson R. K., Graves J. A. M. (2008). Platypus globin genes and flanking loci suggest a new insertional model for β-globin evolution in birds and mammals. BMC Biol. 6, 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pough F. H. (1977). Ontogenetic change in molecular and functional properties of blood of garter snakes. J. Exp. Zool. 201, 47-56 [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. (1982). The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell 31, 553-563 [DOI] [PubMed] [Google Scholar]
- Ragsdale F. R., Ingermann R. L. (1991). Influence of pregnancy on the oxygen affinity of red cells from the Northern Pacific rattlesnake Crotalis viridis oreganus. J. Exp. Biol. 159, 501-505 [DOI] [PubMed] [Google Scholar]
- Runck A. M., Moriyama H., Storz J. F. (2009). Evolution of duplicated β-globin genes and the structural basis of hemoglobin isoform differentiation in Mus. Mol. Biol. Evol. 26, 2521-2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger T. J., Losos J. B., Gibson-Brown J. J. (2008a). A developmental staging series for the lizard genus Anolis: a new system for the integration of evolution, development, and ecology. J. Morphol. 269, 129-137 [DOI] [PubMed] [Google Scholar]
- Sanger T. J., Hime P. M., Johnson M. A., Diani J. (2008b). Protocols for husbandry and embryo collection of Anolis lizards. Herpetol. Rev. 39, 58-63 [Google Scholar]
- Sellers J. C., Trauth S. E., Wit L. C. (1980). A method of caudal blood collection. J. Herpetol. 14, 185-187 [Google Scholar]
- Storz J. F., Hoffmann F. G., Opazo J. C., Moriyama H. (2008). Adaptive functional divergence among triplicated α-globin genes in rodents. Genetics 178, 1623-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E., Fago A. (2010). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R. E., Fago A., Val A. L., Bang A., Van Hauwaert M. L., Dewilde S., Zal F., Moens L. (2000). Isohemoglobin differentiation in the bimodal-breathing amazon catfish Hoplosternum littorale. J. Biol. Chem. 275, 17297-17305 [DOI] [PubMed] [Google Scholar]
- Wells R. M. G., Baldwin J. (1994). Oxygen transport in marine green turtle (Chelonia mydas) hatchlings: blood viscosity and control of hemoglobin-oxygen affinity. J. Exp. Biol. 188, 103-114 [DOI] [PubMed] [Google Scholar]