Abstract
In order to successfully feed and transmit disease agents, ticks are thought to inject serine protease inhibitors (serpins) into the host to modulate host defense responses to tick feeding, such as inflammation, the complement activation pathway and blood coagulation. In this study, we show that Amblyomma americanum (Aam) serpin (S) 6 is putatively injected into the host during tick feeding, in that the antibody to recombinant (r) AamS6 specifically reacted with the expected ∼43/45 kDa AamS6 protein band on western blots of pilocarpine-induced tick saliva. Additionally, antibodies to tick saliva proteins that were generated by repeated 48 h infestations of rabbits with adult A. americanum specifically reacted with rAamS6. We speculate that AamS6 is associated with regulating events at the start of the tick feeding process, as temporal and spatial RT-PCR and western blot analyses revealed that both AamS6 mRNA and protein are strongly expressed during the first 24–72 h of feeding time before starting to fade from 96 h. The AamS6 protein has an apparently slow turnover rate in that, although the injection of AamS6 dsRNA into unfed ticks triggered complete disruption of the AamS6 mRNA by the 48 h feeding time point, western blot analysis of protein extracts of the same animals showed that the AamS6 protein that may have been expressed prior to disruption of the AamS6 mRNA was not depleted. We speculate that the presence of the AamS6 protein in ticks despite the complete disruption of the AamS6 mRNA explains the observation that RNAi-mediated silencing of the AamS6 mRNA did not affect the ability of A. americanum ticks to attach onto host skin, successfully feed and lay eggs. These findings are discussed in regards to advances in the molecular biology of ticks.
Keywords: Amblyomma americanum, tick saliva serine protease inhibitor, tick feeding regulation
INTRODUCTION
Ticks surpass any other known arthropods in terms of the diversity of the disease-causing pathogens they transmit to humans, companion animals and livestock (Sonenshine, 1993). In livestock production, ticks and tick-borne diseases (TBD) are among the biggest source of economic loss (Uilenberg et al., 2004; Gratz, 2006; Nicholson et al., 2009). In the late 1990s, tick control and treatment of TBDs was estimated at 13 to 18 US dollars per animal, which translates to billions of dollars in global expenditures annually (de Castro, 1997). For many years, ticks and TBDs were by and large considered to be primarily a veterinary problem. However, since the identification of Borrelia burgdorferi as the causative agent of Lyme disease in the 1980s (Burgdorfer et al., 1982; Burgdorfer, 1984), there has been a dramatic rise in the identification of human TBD incidence. Between 1982 and 2004 there have been 15 new tick-borne bacterial agents discovered or recognized as human pathogens (Parola and Roult, 2006).
With all life stages readily feeding on both humans and livestock, one of the biggest tick pests of medical and veterinary importance is Amblyomma americanum (Lone Star tick) (James et al., 2001). This species is the most prevalent tick throughout the Southeastern and South-Central United States but it is also distributed along the Atlantic Coast up to New York and Maine (James et al., 2001; Nicholson et al., 2009). Additionally, A. americanum serves as the vector of several important disease-causing pathogens, including Francisella tularenis, Theileria cervi, Ehrlichia chaffensis, E. ewingii and the suspected agent for southern tick-associated rash illness (STARI) (Childs and Paddock, 2003; Goddard and Varela-Stokes, 2009; Nicholson et al., 2009).
Protection of animals against tick-borne diseases relies on the use of vaccines targeting a particular pathogen and/or the killing of ticks using acaricides. Meeusen et al. (Meeusen et al., 2007) recently reviewed the status of veterinary vaccines. From this review, it is apparent that most commercially available vaccines, some with limited success, are tailored for local geographical regions (Meeussen et al., 2007). Conversely, the killing of ticks using acaricides has a global appeal and it is currently the most widely adopted method to control ticks and tick borne diseases. Although this is the most successful tick control method to date, there are many drawbacks associated with acaricides, including resistance to the active ingredient, contamination of the environment and of food products, detrimental effect to non-target organisms and inefficiency regarding application (Graf et al., 2004; George et al., 2004; Ghosh et al., 2007). These negative aspects have encouraged the search for innovative methods of protection against ticks, the most promising of which is immunological control through an anti-tick vaccine (Sonenshine et al., 2006; Willadsen, 2004; de la Fuente and Kocan, 2006). The limiting step towards the development of animal vaccines against ticks is the discovery and target validation of effective antigens that, when disrupted, will compromise tick feeding success and which, in turn, will limit or completely inhibit pathogen transmission. Our laboratory is interested in tick-encoded serine protease inhibitors (serpins) as target vaccine antigens.
Ticks initiate attachment and feeding by lacerating host tissue and small blood vessels to create a feeding site and then imbibe the blood from the resulting hematoma (Sonenshine, 1993). This injury stimulates the host's tissue repair responses, such as inflammation and blood coagulation, to stop further blood loss. To complete feeding, ticks secrete enzymes that block inflammation and blood clotting, thus ensuring continued blood flow to the feeding site (Nuttall et al., 2006). Given that the host's primary lines of defense to tick feeding activity, inflammation and blood coagulation, are serine protease-mediated pathways that are regulated by serpins (Huntington, 2006; Gettins, 2002), it has been hypothesized that ticks may use serpins to evade the host's immune response (Mulenga et al., 2001a). An increasing number of serpin-encoding cDNAs have been identified in several tick species of medical and veterinary importance, including A. americanum (Mulenga et al., 2007), Haemaphysalis longicornis (Mulenga et al., 2001b; Imamura et al., 2005; Sugino et al., 2003), Ixodes ricinus (Prevot et al., 2006), I. scapularis (Mulenga et al., 2009) and Rhiphicephalus appendiculatus (Mulenga et al., 2003; Imamura et al., 2006; Imamura et al., 2008).
Although sequence information on tick-encoded serpins is accumulating, the biological role(s) of these molecules in tick feeding regulation remains speculative. In this study, the objective was to elucidate the biology of A. americanum serpin 6 (AamS6) in A. americanum tick feeding regulation. AamS6 was previously discovered among 17 A. americanum (Aam) presumptive serpins (formerly referred to as ‘Lospins’, the acronym for Lone Star tick serpins) that are expressed during the first 5 days of feeding (Mulenga et al., 2007). We provide evidence to show that AamS6 is potentially secreted into the host during tick feeding, as it is present in pilocarpine-induced tick saliva.
MATERIALS AND METHODS
Tick dissections, total RNA extraction and cDNA synthesis
Ticks used in these experiments were obtained from an A. americanum L. colony from the laboratory of Dr Pete Teel from the Entomology Department of Texas A&M University, College Station, TX, USA. This colony is maintained by feeding on chickens at the larval and nymphal stage and on cattle at the adult stage. To feed ticks, adult females were placed in cells attached onto the back of a calf with no prior exposure to tick infestation. After 24 h, all unattached ticks were removed from cells. For extraction of total RNA and protein extractions from whole animals, three adult female ticks were collected at the unfed, 24, 48, 72, 96 and 120 h tick feeding time points. These ticks were placed on a glass slide, chopped with a sterile razor blade, and then individually stored in the total RNA extraction reagent Trizol (Invitrogen, Carlsbad, CA, USA). Samples were stored at –80°C until used for RNA extractions. The Trizol reagent allows for the extraction of both total RNA and proteins from the same animal as it separates samples into the aqueous phase containing total RNA and the organic phase containing proteins and genomic DNA. Here, the aqueous phase was processed for total RNA extraction and the organic phase was retained and used for total protein extraction, as detailed below.
For tissue dissections, eight ticks were collected at each time point (24, 48, 72, 96 and 120 h post-attachment). Dissections were routinely done as described by Mulenga et al. (Mulenga et al., 2001b). Ticks were placed under a dissecting scope on a hanging drop glass slide filled with molecular grade water treated with the RNAse inhibitor diethylpyrocarbonate (DEPC). Using a sterile razor blade and soft tissue forceps, the dorsal shield was removed. Tick organs, salivary glands (SG), midgut (MG), ovary (OV), and the carcass remnant after removal of SG, MG and OV (CA), were dissected using forceps and 18 gauge needles. All dissected tissues were pooled in groups of eight in Trizol (Novagen).
To extract total RNA, whole tick and tissue samples were thawed at room temperature and homogenized using a Sonic Dismembrator Model 100 (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was extracted and re-suspended in RNAase-free water according to the manufacturer's protocol provided with the Trizol total RNA extraction reagent. Total RNA was quantified using a nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Total RNA (1 μg) was used to synthesize cDNA using the Verso cDNA synthesis kit according to the manufacturer's instructions (Thermo Fisher Scientific). The resulting cDNA was then quantified as described above and stored at –20°C.
Expression and affinity purification of recombinant AamS6
To construct the expression plasmid, the AamS6 open reading frame without the signal peptide coding region was unidirectionally subcloned into the pRSETA vector (Invitrogen) using primers (For, 5′-GGATCCGACGATGCACTGCTGGCCAAAGCTC-3′; Rev, 5′-AAGCTTGACCTTACCATTTAGTCTTATTCTGCGTG-3′) with added restriction enzyme sites, BamHI and HindIII (shown in bold). The AamS6 ORF was subcloned in frame with an amino-terminal 3 kDa fusion protein that contained a 6-His-tag that was subsequently used for affinity purification below. The recombinant pRSETA-AamS6 expression plasmid was transformed into Escherichia coli BL21 (DE) pLysS cells using routine heat-shock methods. Recombinant (r) AamS6 expression was induced by adding isopropyl β-d-thiogalactoside (IPTG) at a 1 mmol l–1 final concentration to a culture with an OD600 of 0.6. The culture was then incubated for 6 h at 37°C. Recombinant AamS6 was purified by affinity chromatography using the 1 mol l–1 HiTrap Chelating HP column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) that was charged with NiCl2 under denaturing conditions. The expression of recombinant AamS6 was verified by routine sodium dodecyl sulfate (SDS)-PAGE under reducing conditions with Coomassie Blue staining.
Production of rabbit polyclonal antibodies to rAamS6
The production of rabbit polyclonal antibodies to the rAamS6 protein fused with a 6-His tag at the amino-terminal region was subcontracted to a fee-for-service company (Pacific Immunology, Ramona, CA, USA). Approximately 4 mg of rAamS6 quantified by Bradford Protein Assay (Thermo Fisher Scientific) was electrophoresed on two single-well 12.5% SDS-PAGE gels and stained with Coomassie Brilliant Blue. The stained rAamS6 protein band was excised using a razor blade and the gel strip was sent out for rabbit immunization. Pre-immune and rabbit immune sera were shipped to our laboratory at the end of the immunization protocol.
Transcription expression analyses by titration semi-quantitative RT-PCR
To determine temporal and spatial expression patterns, ∼200 ng of cDNA from whole tick and dissected tick organs were used in a PCR reaction containing GoTaq Green PCR Master Mix (Promega, Madison, WI, USA), AamS6 primers (For, 5′-CTGCTATCAGCGAGAGCACGCA-3′; Rev, 5′-TCTGCGTGAAATTTCTGTCATTCTGGA-3′) at 0.1 μmol l–1 final concentration in a 20 μl reaction. Prior to conducting RT-PCR transcription analyses, we conducted preliminary titration RT-PCR and determined that the saturation points for tick actin (load control samples) and AamS6 were 28 and 30 PCR cycles, respectively. On this basis, the cycling conditions were an initial denaturation of 94°C for 2 min, followed by 29 amplification cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 1 min, and a final extension of 72°C for 5 min. For tick actin (sample load control, a similar reaction was repeated at 0.1 μmol l–1 final primer (For, 5′-GGACAGCTACGTGGGCGACGAGG-3′; Rev, 5′-CGATTTCACGCTCAGCCGTGGTGG-3′), concentrations in a 20 μl reaction. The cycling conditions were an initial denaturation of 95°C for 2 min, 27 amplification cycles of 95°C for 45 s, 58°C for 30 s and 72°C for 1 min, and a final extension of 72°C for 5 min. PCR reaction products (10 μl of each) were electrophoresed along with a 1 Kb DNA ladder (Promega) on a 2% agarose gel containing 1 μg ethidium bromide in Tris-acetate-EDTA (TAE) buffer. To determine mRNA abundance, densitograms of amplified PCR bands were determined using the web based ImageJ software (http://rsb.info.nih.gov/ij/). The variations between template concentrations were normalized according to the following formula:
where Y is the normalized mRNA density, V is the observed AamS6 PCR band density in individual samples, H is the highest tick actin PCR band density among tested samples, and X is the tick actin PCR band for the test tissue (Mulenga et al., 2008).
Correlating AamS6 transcription with protein expression production
In order to correlate AamS6 mRNA expression profiles with protein production, total tick proteins that were extracted from the same animals from which total RNA was extracted were subjected to western blot analysis using antibodies to rAamS6. Total tick proteins were extracted from the organic phase of the Trizol reagent that was retained from the total RNA extraction step (see above), according to the manufacturer's instructions. Genomic DNA was first precipitated out using 0.3% ethanol and centrifugation at maximum speed. Subsequently, total proteins were precipitated from the supernatant using isopropanol. The resulting protein pellet was washed four times in 0.3 mol l–1 guanidine hydrochloride in 95% ethanol solution. The pellet was then dried in a Savant DNA 120 SpeedVac Concentrator (Thermo Fisher Scientific) for 20–30 min. The dried pellet was then reconstituted in 1% SDS incubated at 50°C for 10 min. These protein extracts were used in the western blot analysis described below.
To determine temporal and spatial expression profiles of the AamS6 protein, total protein extracts of three individual whole ticks per time point (unfed and partially fed for 24–120 h), and pools of eight dissected organs of ticks that were partially fed for 24–120 h were subjected to western blot analyses using polyclonal antibodies to rAamS6. Tick protein samples with rAamS6 serving as a positive control were resolved on a 12.5% polyacrylamide gel under reducing conditions. Due to host blood contamination of samples, it was technically difficult to quantify tick-derived proteins that were loaded per lane. Thus, we loaded equivalent volumes, rather than equivalent quantities.
The resolved proteins were electroblotted onto an Immobilon PVDF membrane (Millipore, Billerica, MA, USA) using the Xcell SureLock Mini-Cell XCell II Blot Module (Invitrogen). The membranes were washed in 20 ml of PBS-Tween 20 (PBST; 0.05%) at room temperature (RT) and then blocked overnight at 4°C by incubation in 5% blocking solution (1 g dried skim milk added to 20 ml of PBST). The blocked membranes were washed at RT six times for 5 min each with 20 ml PBST. Following washing, the membranes were incubated for 1 h at RT in rabbit pre-immune or immune serum to rAamS6 (1:660, V/V) in blocking solution. After appropriate washing, membranes were subsequently incubated for 1 h at RT with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Millipore; 1:1000, V/V) in blocking solution. After six washes in PBST, western blots were processed for antibody binding detection using a metal-enhanced DAB chromogenic substrate (Thermo Fischer Scientific) in the case of the total tick protein blots or the super signal chemiluminescent substrate (Thermo Fischer Scientific) in the case of the tick saliva blots.
Validating the secretion of AamS6 into tick saliva as evidence for injection into the host during feeding
To validate whether AamS6 was secreted into tick saliva, which is evidence for being injected into the host during tick feeding, antibodies to tick saliva proteins and rAamS6 were, respectively, used to screen western blots of pilocarpine-induced tick saliva and affinity-purified rAamS6. Tick saliva was collected from 30 female ticks at 24, 48/72 and 96/120 h using the published protocol from Ribeiro et al. (Ribeiro et al., 2004). Ticks were placed dorsal-side up on tape, and sterile glass micropipettes were place over the tick's hypostome. To induce salivation, 5 μl of pilocarpine hydrochloride (50 mg ml–1 in 95% ethanol; Sigma-Aldrich, St Louis, MO, USA) was applied to the scutum. The ticks were incubated in a humidified chamber at 35°C for 3 h. Tick saliva was collected from the micropipettes by washing in PBS and stored at –80°C until used for western blot analysis.
To generate rabbit antibodies to tick saliva proteins, two New Zealand white rabbits were repeatedly infested for 48 h periods with adult A. americanum ticks. Ticks were placed on and allowed to attach onto rabbit ears for 48 h and then manually detached. This was repeated up to four times until tick resistance manifested in ticks struggling to attach onto host skin, because of intense exudation from the host and sera specifically reacting with tick protein extracts. Rabbits were exsanguinated under humane conditions and sera collected.
RNA interference (RNAi)-mediated gene silencing
To validate the significance of AamS6 in tick feeding regulation, RNAi-mediated gene silencing was performed as previously published (Mulenga and Khumthong, 2010), with slight modifications. Double-stranded RNA (dsRNA) was generated using the Megascript kit according to the manufacturer's instructions (Ambion, Austin, TX, USA). When compared with other A. americanum serpin sequences the amino-terminal region of AamS6 showed a high similarity to the AamS17 amino-terminal region (Mulenga et al., 2007). Thus, two AamS6 regions were targeted for dsRNA synthesis, the 3′ region unique to AamS6 to silence AamS6 alone, and the 5′ region conserved between AamS6 and AamS17 (AamS6/S17) to silence both AamS6 and AamS17. The forward and reverse primers with an added T7 promoter sequence (5′-AATACGACTCACTATAGGG-3′) that were used for dsRNA template amplification were: 5′-CTGCTATCAGCGAGAGCACGCA-3′ and 5′-CTGCGTGAAATTTCTGTCATTCTGGAA-3′ for the C terminus; and 5′-CCACTTCGCCGTGAAGCTCCTC-3′ and 5′-GCACCTGCGTGGAGTGCGTCTAG-3′ for the amino-terminal domains. After purification, dsRNA was quantified using a nanodrop ND-1000 spectrophotometer and adjusted to a final concentration of 2 μg μl–1. Subsequently four cohorts of 50 unfed female ticks were injected with 1 μl (2 μg μl–1) of AamS6/17 or AamS6, or with GFP (control) dsRNA or TE buffer (pH 8.0) diluent, using 33-gauge half-inch needles attached to a 10 μl gastight syringe (Hamilton, Reno, NV, USA) on the ventral side between the right fourth coax and the anal opening. The ticks were kept at 22°C overnight to observe any mortality resulting from the injection. After the overnight incubation, viable ticks alongside the non-injected tick controls were fed on a calf with no prior tick infestation. Ticks were confined to a feeding area in cells that were secured on the back of the calf using livestock identification cement (Nasco, Ft Atkinson, WI, USA). Ticks were allowed to feed until they naturally detached from the host. Prior to placing female ticks in cells, male ticks (50 per cell) were pre-fed for 3–4 days to stimulate the release of aggregation pheromones. Unattached female ticks were counted and removed from the cells 48 h later. The attached ticks were allowed to feed until spontaneous detachment. Detached females were collected every 24 h, weighed, placed in separate containers at 22°C, and allowed to oviposit.
To validate whether tick injections with dsRNA triggered the disruption of AamS6 mRNA and blocked the expression or caused the depletion of AamS6 protein levels, three ticks per treatment were collected 48 h post-attachment. Ticks were sampled at the 48 h time point to ensure that the ticks were alive and had had a chance to attempt feeding. These ticks were individually processed for total RNA and protein extraction using the Trizol reagent, as detailed above. The extracted total RNA was subjected to a two-step semi-quantitative RT-PCR using specific PCR primers targeting the AamS6 specific and AamS6/S17 domains (shown above). Tick actin PCR primers (Mulenga et al., 2009) were used for sample load controls. Samples from the PCR reactions (10 μl) were analyzed by electrophoresis along with a 1 Kb DNA ladder on a 2% agarose gel containing 1 mg ml–1 ethidium bromide to qualitatively corroborate silencing. To determine whether disruption of AamS6 mRNA blocked expression or caused depletion of AamS6 protein levels, total protein extracts (from the same animals from which total RNA used to validate gene silencing by RT-PCR) were subjected to western analysis using antibodies to rAamS6.
To assess the effects of AamS6 mRNA silencing on tick feeding success, tick-feeding parameters were recorded, including attachment rates (the number of attached ticks was determined by subtracting the number of unattached ticks from the total number of ticks that were placed on the animal), mortality (number of ticks dead after 48 h subtracted from number of ticks placed on animal) and engorgement mass (EM) (mass of the engorged tick after spontaneous detachment). To assess the effect of silencing on fecundity, engorged ticks were incubated at 25°C for 4 weeks to lay eggs. Subsequently, the egg mass conversion ratio (EMCR; the mass of the egg clutch divided by EM) was calculated to assay the effects of silencing on the tick's ability to convert its blood meal to eggs. To analyze the statistical significance of the differences observed between the silenced and control groups, a one-way ANOVA and post-ANOVA pair-wise comparisons using Tukey's HSD test were conducted. The web based Grubbs test (http://www.graphpad.com) was used to identify outlier samples, which were subsequently removed from the analysis.
RESULTS
AamS6 mRNA is expressed in unfed and partially fed ticks during 5 days of feeding
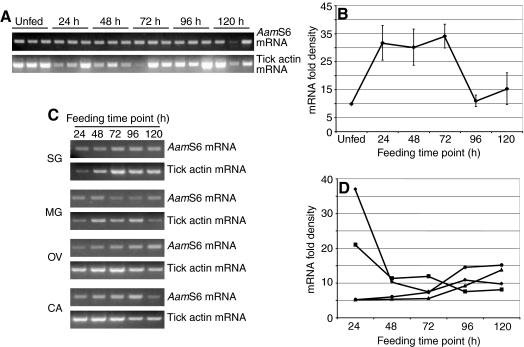
The temporal and spatial AamS6 transcription analyses summarized in Fig. 1 showed that AamS6 mRNA was constitutively expressed (Fig. 1A) and that, during feeding, it was predominantly expressed in the salivary gland and midgut, followed by the ovaries, during the first 24–72 h of feeding, before starting to wane from the 96 h feeding time point (Fig. 1B). Normalization of PCR band densities in whole ticks (Fig. 1A) revealed that AamS6 transcript abundance had increased by ∼3-fold by 24 h post attachment before dropping by a similar margin from the 96 h feeding time points (Fig. 1B). At the organ level, AamS6 transcript abundance did not appear to vary in ovary and the carcass [the tick remnant after removal of the salivary glands (SG) and midguts (MG)]. However in the SG and MG, AamS6 transcript abundance was respectively downregulated by ∼2- and 5-fold between the 24 h and the 96 h feeding time points (Fig. 1D), which was consistent with the observation in whole ticks (Fig. 1B).
Fig. 1.
Temporal and spatial RT-PCR expression analyses. Total RNA extracted from (A) three whole ticks per feeding time point (unfed, 24, 48, 72, 96 and 120 h), and from (C) dissected tick organs [salivary glands (SG), midgut (MG), ovary (OV) and carcass (CA, tick remnant after removal of SG, MG and OV)] pooled from eight ticks were subjected to titration two-step semi-quantitative RT-PCR to amplify the AamS6 fragment and tick actin (sample load control). Densities of AamS6 PCR bands in whole ticks (B) and dissected tick organs (D) were determined and normalized against tick actin, as described in the Materials and methods. For whole ticks (B), means and standard error of the means (s.e.m.) of the three PCR band densities per time point were calculated using the graph pad calculator (http://www.graphpad.com/quickcalcs/ttest1.cfm). In D, the filled circle denotes midgut, filled diamond denotes salivary glands, filled triangle denotes carcass and filled square denotes ovary.
Native AamS6 protein expression patterns directly correlate with transcription profiles
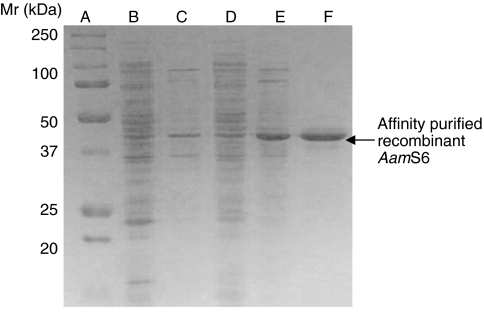
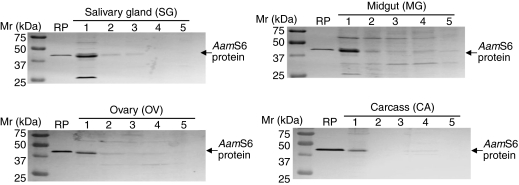
We successfully expressed and affinity purified a 6-His-tagged rAamS6 in E. coli cells (Fig. 2). The affinity-purified rAamS6 was then used to immunize rabbits to produce antibodies to rAamS6. To correlate native AamS6 protein production with mRNA expression profiles (see Fig. 1A,B), the antibody to rAamS6 was used to screen western blots of whole tick and dissected organ protein extracts as summarized in Figs 2 and 3. Consistent with mRNA expression patterns in whole animals (Fig. 1A), AamS6 was expressed in unfed ticks and during the first 5 days tested in this study (Fig. 3). Although the quantities of tick proteins that were subjected to western blot analysis were not determined and, thus, we may have loaded dissimilar protein quantities, it is apparent that in whole animals AamS6 was strongly expressed in unfed ticks, as well as during the first 24 h of feeding, and at the 48 and 72h feeding time points before fading from the 96 h time point (Fig. 3). Likewise at the organ level, the AamS6 protein was strongly expressed in organs that were dissected from 24 h fed ticks, followed by the 48–120 h feeding time points (Fig. 4). Consistent with the mRNA and protein expression profiles in whole animals (Figs 1, 2), the AamS6 protein expression profiles (Fig. 4) correlated with mRNA expression profiles at the salivary gland (SG) and midgut (MG) levels (Fig. 1D). Conversely, AamS6 mRNA expression profiles were not correlated with protein expression profiles at the ovary and carcass level. It is interesting to note here that, although the AamS6 protein band was barely detectable at the 96 and 120 h time points in whole animals (Fig. 3) and was only detectable up to the 72 h time point in SG (Fig. 4), it was detectable up to the 96 h feeding time point in the OV and through the five tested feeding time points in the midgut (Fig. 4).
Fig. 2.
Expression and affinity purification of insoluble recombinant (r) AamS6. The coding region for the mature AamS6 protein was subcloned into pRSET(a+) as described in the Materials and methods. Expression of rAamS6 was induced for 6 h at 37°C by adding IPTG to a 1 mmol l–1 final concentration. Samples were separated into soluble (supernatant) and insoluble (pellet) fractions. These fractions were subjected to SDS-PAGE electrophoresis with Coomassie Blue staining using a 12.5% acrylamide gel under denaturing conditions. Lanes A-F denote marker (A), uninduced soluble (B) and pellet (C), induced soluble (D) and pellet (E), and affinity-purified rAamS6 (F).
Fig. 3.
Temporal western blot expression analyses of the native AamS6 protein in whole ticks. Total tick proteins extracted from the same whole ticks that were used for total RNA extraction in Fig. 1A were subjected to western blot analysis using antibodies to rAamS6 or pre-immune sera as indicated. Arrowhead denotes the position of rAamS6 and the native AamS6 protein.
Fig. 4.
Spatial and temporal western blot analyses of native AamS6 protein in dissected tick organs. Total protein extracts of salivary gland, midgut, ovary and carcass (tick remnant after removal of SG, MG and OV) pooled from eight ticks per time point were subjected to western blot analyses using antibodies to rAamS6. Arrows denotes the position of the native AamS6 protein. Please note that membranes probed with pre-immune sera are not shown. Please refer to Fig. 3.
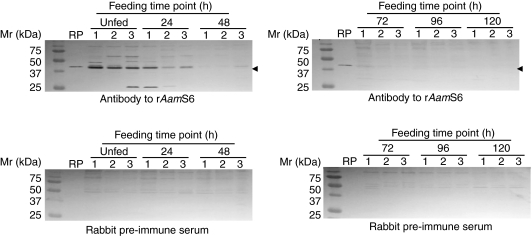
AamS6 is putatively injected into the host during tick feeding
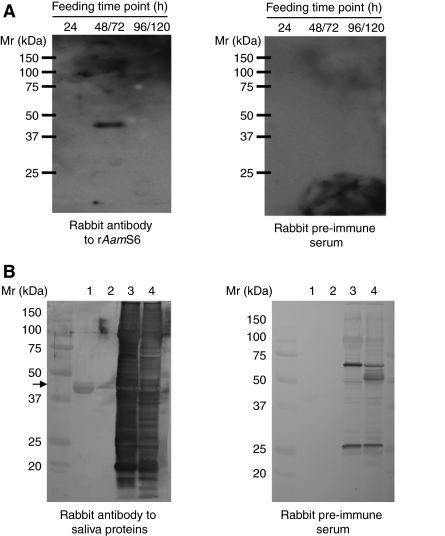
We successfully used pilocarpine hydrochloride dissolved in 95% ethanol to induce A. americanum tick salivation. The collected tick saliva was subjected to western blot analysis using antibodies to rAamS6, as summarized in Fig. 5A. Antibodies to rAamS6 specifically reacted with the expected ∼43/45 kDa AamS6 protein band on the 48/72 h tick saliva immunoblot (Fig. 5A). In agreement with the results in Fig. 5A, antibodies to tick saliva proteins that were generated by repeated 48 h infestations of rabbits specifically reacted with rAamS6. Taken together, the results in Fig. 5A,B suggest that AamS6 is among the tick saliva proteins that are injected into the host during tick feeding.
Fig. 5.
Validation of AamS6 protein secretion into tick saliva. (A) Pilocarpine-induced tick saliva harvested from ticks fed for the indicated time periods was subjected to western blot analysis using antibodies to rAamS6 or pre-immune sera as indicated. (B) Affinity-purified rAamS6 was subjected to western blot analysis using antibodies to tick saliva proteins raised by repeated 48 h infestations of rabbits with adult A. americanum or pre-immune sera as indicated. In B, lane 1 is affinity-purified rAamS6, lane 2 is a 10-fold dilution of lane 1, lanes 3 and 4 are protein extracts of unfed and 24 h partially fed ticks, respectively.
Dual silencing of AamS6/S17 or of AamS6 did not affect A. americanum tick feeding efficiency
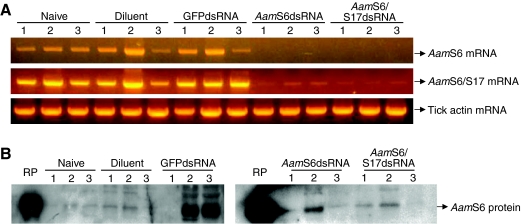
In vitro synthesized dsRNA was successfully injected into ticks, which were then allowed to feed on cattle. Qualitative two-step RT-PCR expression (Fig. 6A) and western blot (Fig. 6B) analyses were used to confirm if microinjections of dsRNA triggered the disruption of target AamS6 mRNA, and whether or not silencing of AamS6 mRNA resulted in depletion of the AamS6 protein. When PCR primers unique to AamS6 were used, AamS6 cDNA failed to be amplified from ticks that were injected with either dsRNA targeting the amino-terminal region of AamS6, which is identical to that of AamS17 (Mulenga et al., 2007), or dsRNA targeting the carboxy-terminal region, which is unique to AamS6 (Fig. 6A). However, when AamS6/S17 PCR primers based on the consensus region between AamS6 and AamS17 were used, only partial silencing was observed, as faint PCR bands were detectable (Fig. 6A). The amplification of AamS6 transcript in the three control groups indicates that neither injection trauma nor the diluent buffer affected the expression of the target gene. In order to determine whether or not silencing of the AamS6 mRNA caused the elimination of native AamS6 protein, total protein extracts isolated from the same animals that were used for the validation of AamS6 mRNA silencing were subjected to western blot analysis. The results summarized in Fig. 6B showed that, although the AamS6 transcript was completely disrupted, the native AamS6 protein that may have been expressed prior to the disruption of the AamS6 mRNA was not depleted.
Fig. 6.
Validation of RNAi-mediated silencing of AamS6 by RT-PCR and western blot analyses. At 48 h post attachment three ticks per treatment as indicated were manually detached and processed individually for total RNA and protein extraction as detailed in the Materials and methods. (A) Total RNA was subjected to two-step semi-quantitative RT-PCR to amplify the C-terminal transcript that is unique to AamS6, the N-terminal transcript that is conserved between AamS6 and AamS17, and the tick actin fragment (sample load control). (B) Total protein extracts from the same animals that were used for total RNA extraction in A were subjected to western blot analysis using antibodies to rAamS6.
Based on mortality and attachment rates, the dual silencing of AamS6/S17 or of AamS6 alone was not lethal and did not affect the ability of ticks to attach onto host skin, or to start and complete feeding (not shown). Likewise, one-way analysis of variance revealed that dual silencing of AamS6/S17 or of AamS6 alone did not affect the ability of ticks to acquire a full blood meal, as measured by engorgement mass, or their ability to convert the blood meal to eggs, as measured by egg mass conversion ratio (not shown).
DISCUSSION
Expression in tick salivary glands, being putatively extracellular and showing a high amino acid identity to other metastriata tick serpins (Mulenga et al., 2007) motivated our interest to gain further insight into the biology of A. americanum (Aam) serpin S6 in tick feeding regulation. Differential transcription patterns in which certain tick genes are down- or upregulated, induced or shut down in response to tick feeding activity, is a commonly observed phenomenon (Carvalho et al., 2010; Nene et al., 2002; Anisuzzamand et al., 2009). Broad interpretations of these data are that genes that are induced or upregulated in response to tick feeding activity are likely to be associated with blood meal feeding regulation. Contrastingly, the biological functions of genes that are shut down or downregulated in response to feeding are thought not to be associated with tick feeding regulation or to be restricted to a particular tick feeding stage (Almazán et al., 2003; de la Fuente et al., 2006; Hatta et al., 2010; Smith et al., 2009). Based on our RT-PCR expression data and western blot analyses, AamS6 fits the latter pattern. During the 24 and 48 h feeding time points when AamS6 is strongly expressed, the tick is involved in anchoring itself onto the host skin through the secretion of an adhesive substance called cement, creating the feeding lesion through the laceration of host tissues and small blood vessels, and creating a conducive environment for tick borne pathogens to colonize the host by suppressing the host's innate immunity (Sonenshine, 1993). Whether or not AamS6 is involved in regulating any of these functions remains to be investigated. Given that the mRNA and protein samples that were used in this study were extracted from the same individuals, we strongly believe the AamS6 mRNA and protein expression profiles reported in this study are consistent.
Although ticks can cause damage to their hosts, they are mostly known for their role as vectors of tick-borne diseases (TBDs) (Sonenshine, 1993). With the exception of tick-borne viruses, such as tick borne encephalitis (Nutall and Labuda, 2008) and Powassan (Ebel and Kramer, 2004) viruses, which are transmitted into the host within the first few minutes of the tick attaching onto host skin, data in several studies indicate that animal and human tick-borne pathogens such as Theileria parva (Bishop et al., 2004), Babesia bigemina (Bock et al., 2004), Rickettsia rickettsii (Burgdorfer, 1975), Borrelia burgdorferi (Burgdorfer, 1984) and B. microti (Bock et al., 2004) are transmitted after ticks have been feeding for 2–3 days. Thus, from the perspective of developing novel technologies to prevent ticks from transmitting TBD agents, tick proteins such as AamS6 that regulate tick feeding events prior to the inception of TBD agent transmission are desirable. The attraction is that, by blocking the functions of proteins such as AamS6, we will interfere with the ability of ticks to initiate feeding and/or to prepare conditions that are conducive to the transmitted TBD agent to colonize the host.
In hypothesizing that tick-encoded serpins play key role(s) in regulating the evasion of host defense reactions by ticks (Prevot et al., 2007; Mulenga et al., 2001a; Mulenga et al., 2003; Mulenga et al., 2008, Mulenga et al., 2009), the underlying assumption is that ticks inject serpins into the host during tick feeding. In previous studies the basis for this assumption has been the expression of putatively extracellular serpin mRNA in tick salivary glands (Prevot et al., 2007; Prevot et al., 2006; Mulenga et al., 2003; Mulenga et al., 2008; Mulenga et al., 2009). In this study, the observed specific reactivity of the antibody to rAamS6 with the native AamS6 protein band on the western blot of pilocarpine-induced tick saliva, coupled with antibodies to tick saliva proteins specifically binding to rAamS6, strongly suggests that the AamS6 protein is injected into the host during tick feeding. These data for the first time provide direct evidence that serpins are among the tick proteins that are injected into the host to regulate tick feeding. It is important to point out here that the AamS6 amino acid sequence has large segments in its amino-terminal region that are identical to those of AamS17 (Mulenga et al., 2007). Thus, there is a possibility that the antibody to rAamS6 may have cross-reacted with native AamS17 on tick saliva blots. However, given that AamS17 does not have a signal peptide and thus is putatively intracellular (Mulenga et al., 2007), it is less likely to be secreted into tick saliva. The potential of AamS6 to be secreted into the host during tick feeding strongly suggests that this protein is involved in the regulation of as yet unknown events at the tick-host interface. The specific biological functions of AamS6 at the tick-feeding site remain to be investigated.
The expression of AamS6 in multiple tick organs, as revealed by spatial RT-PCR and western blot analyses, is consistent with previous studies showing that both vertebrate and invertebrate serpins tend to be ubiquitously expressed (Gettins, 2002; Silverman et al., 2001; Huntington, 2006; Law et al., 2006; Irving et al., 2002; Irving et al., 2006). The ubiquitous expression pattern of AamS6 may underscore the significance of this protein in regulating tick physiology. To this end, a goal in this study was to use RNAi silencing to validate the biological significance of AamS6 in tick feeding regulation. On the basis of the RNAi silencing data presented here, the AamS6 protein appears not to be essential to A. americanum tick feeding success, in that the silencing of AamS6 did not affect A. americanum tick feeding success. However, we must interpret these findings with caution as, although RNAi-mediated silencing triggered the complete disruption of the AamS6 mRNA, our data validating RNAi silencing by western blot analysis show that the AamS6 protein that may have been expressed prior to silencing of the AamS6 mRNA was not depleted in those animals. The premise of RNAi silencing is that upon disruption of the target mRNA, the target protein will not be translated and thus its functions will be blocked. The failure to deplete the AamS6 protein means that the effectiveness of RNAi-mediated silencing is drastically reduced. Thus, whether or not AamS6 is functionally essential to tick feeding success remains to be ascertained. Given the apparent limitation caused by the slow turnover rates of proteins such as AamS6 on the effectiveness of RNAi-mediated gene silencing, we are of the opinion that immunologically blocking the functions of AamS6 will be the most appropriate approach to determine whether the AamS6 protein is important for tick feeding.
Acknowledgments
The authors thank Dr Pete Teel and Mr Otto Strey, III, for their help with the tick feeding experiments.
Footnotes
Funding for this work was provided by start up funds granted to A.M. by the Texas A&M College of Agriculture and Life Sciences and the Department of Entomology, as well as by an NIH grant [A1074789]. T.K.K. was a summer employment student supported by the NIH summer employment supplement (AI074789-01A1S1) to NIH A1074789 under the American recovery and reinvestment act. Deposited in PMC for release after 12 months.
REFERENCES
- Almazán C., Kocan K. M., Bergman D. K., Garcia-Garcia J. C., Blouin E. F., de la Fuente J. (2003). Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 21, 1492-1501 [DOI] [PubMed] [Google Scholar]
- Anisuzzaman M., Islam M. K., Miyoshi T., Alim M. A., Hatta T., Yamaji K., Matsumoto Y., Fujisaki K., Tsuji N. (2009). Longistatin, a novel EF-hand protein from the ixodid tick Haemaphysalis longicornis, is required for acquisition of host blood-meals. Int. J. Parasitol. 40, 721-729 [DOI] [PubMed] [Google Scholar]
- Bishop R., Musoke A., Morzaria S., Gardner M., Nene V. (2004). Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitol. 129, S271-S283 [DOI] [PubMed] [Google Scholar]
- Bock R., Jackson L., de Vos A., Jorgensen W. (2004). Babesiosis of cattle. Parasitol. 129, S247-S269 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. (1975). A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 12, 269-278 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. (1984). Discovery of the Lyme disease spirochaete and its relation to tick vectors. Yale. J. Biol. Med. 57, 515-520 [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Banach J. L., Grunwaldt E., Davis J. P. (1982). Lyme disease – a tick borne spirochaetosis. Science 216, 1317-1319 [DOI] [PubMed] [Google Scholar]
- Carvalho A. W., Maruyama S. R., Franzin A. M., Abatepaulo A. R. R., Anderson J. M., Ferreira B. R., Ribeiro J. M. C., Moré D. D., Maia A. A. M., Valenzuela J. G., et al. (2010). Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands. Exp. Parasitol. 124, 428-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs J. E., Paddock C. D. (2003). The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 48, 307-337 [DOI] [PubMed] [Google Scholar]
- de Castro J. J. (1997). Sustainable tick and tick-borne diseases control in livestock improvement in developing countries. Vet. Parasitol. 71, 77-97 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Kocan K. M. (2006). Strategies for development of vaccines for control of Ixodid tick species. Parasite Immunol. 28, 275-283 [DOI] [PubMed] [Google Scholar]
- Ebel G. D., Kramer L. D. (2004). Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 71, 268-271 [PubMed] [Google Scholar]
- George J. E., Pound J. M., Davey R. B. (2004). Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 129, S353-S366 [DOI] [PubMed] [Google Scholar]
- Gettins P. G. W. (2002). Serpin structure, mechanism, and function. Chem. Rev. 102, 4751-4803 [DOI] [PubMed] [Google Scholar]
- Ghosh S., Azhahianambi P., Yadav M. P. (2007). Upcoming and future strategies of tick control: a review. J. Vector Borne Dis. 44, 79-89 [PubMed] [Google Scholar]
- Goddard J., Varela-Stokes A. S. (2009). Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 160, 1-12 [DOI] [PubMed] [Google Scholar]
- Graf J. F., Gogolewski R., Leach-Bing N., Sabatini G. A., Molento M. B., Bordin E. L., Arantes G. J. (2004). Tick control: an industry point of view. Parasitology. 129, S427-S442 [DOI] [PubMed] [Google Scholar]
- Gratz N. (ed.) (2006). Tick-borne disease of the USA and Canada. In Vector-and Rodent-Borne Diseases in Europe and North America, pp. 185-188 Cambridge: Cambridge University Press; [Google Scholar]
- Hatta T., Tsuji N., Miyoshi T., Islam M. K., Alim M. A., Yamaji K., Anisuzzaman, Fujisaki K. (2010). Leucine aminopeptidase, HlLAP, from the ixodid tick Haemaphysalis longicornis, plays vital roles in the development of oocytes. Parasitol. Int. 59, 286-269 [DOI] [PubMed] [Google Scholar]
- Huntington J. A. (2006). Shape-shifting serpins – advantages of a mobile mechanism. Trends Biochem. Sci. 31, 427-435 [DOI] [PubMed] [Google Scholar]
- Imamura S., da Silva V. J., Sugino M., Ohashi K., Onuma M. (2005). A serine proteinase inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 23, 1301-1311 [DOI] [PubMed] [Google Scholar]
- Imamura S., Namangala B., Tajima T., Tembo M. E., Yasuda J., Ohashi K., Onuma M. (2006). Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine 24, 2230-2237 [DOI] [PubMed] [Google Scholar]
- Imamura S., Konnai S., da Silva Vaz I., Jr, Yamada S., Nakajima C., Ito Y., Tajima T., Yasuda J., Simuunza M., Onuma M., et al. (2008). Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus. Jpn. J. Vet. Res. 56, 85-98 [PubMed] [Google Scholar]
- Irving J. A., Pike R. N., Dai W., Bromme D., Worrall D. M., Silverman G. A., Coetzer T. H., Dennison C., Bottomley S. P., Whisstock J. C. (2002). Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochem. 41, 4998-5004 [DOI] [PubMed] [Google Scholar]
- Irving J. A., Pike R. N., Lesk A. M., Whisstock J. C. (2006). Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 10, 1845-1864 [DOI] [PubMed] [Google Scholar]
- James A. M., Liveris D., Wormser G. P., Schwartz I., Montecalvo M. A., Johnson B. J. B. (2001). Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183, 1810-1814 [DOI] [PubMed] [Google Scholar]
- Law H. P. R., Zhang Q., McCowan S., Buckle A. M., Silverman G. A., Wong W., Rosado C. J., Langendorf C. G., Pike R. N., Bird P. I., et al. (2006). An overview of the serpin superfamily. Genome Biol. 7, 216-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen E. N., Walker J., Peters A., Pastoret P. P., Jungersen G. (2007). Current status of veterinary vaccines. Clin. Microbiol. Rev. 20, 489-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A., Khumthong R. (2010). Silencing of three Amblyomma americanum (L.) insulin-like growth factor binding protein-related proteins prevents ticks from feeding to repletion. J. Exp. Biol. 213, 1153-1161 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Sugino M., Nakajima M., Sugimoto C., Onuma M. (2001a). Tick-encoded serine proteinase inhibitors (serpin); potential target antigens for tick vaccine development. J. Vet. Med. 63, 1063-1069 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Sugimoto C., Ingram G., Ohashi K., Onuma M. (2001b). Characterization of two cDNAs encoding serine proteinases from the hard tickHaemaphysalis longicornis. Insect Biochem. Mol. Biol. 31, 817-825 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Tsuda A., Onuma M., Sugimoto C. (2003). Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization. Insect Biochem. Mol. Biol. 33, 267-276 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Blandon M., Khumthong R. (2007). The molecular basis of the Amblyomma americanum tick attachment phase. Exp. Appl. Acarol. 41, 267-287 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Khumthong R., Chalaire K. C., Strey O., Teel P. (2008). Molecular and biological characterization of the Amblyomma americanum organic anion transporter polypeptide. J. Exp. Biol. 211, 3401-3408 [DOI] [PubMed] [Google Scholar]
- Mulenga A., Khumthong R., Chalaire K. C. (2009). Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics 10, 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V., Lee D., Quackenbush J., Skilton R., Mwaura S., Gardner M. J., Bishop R. (2002). AvGI, an index of genes transcribed in the salivary glands of the ixodid tick Amblyomma variegatum. Int. J. Parasitol. 32, 1447-1456 [DOI] [PubMed] [Google Scholar]
- Nicholson W. L., Sonenshine D. E., Lane R. S., Uilenberg G. (2009). Ticks (Ixodida). In Medical and Veterinary Entomology, 2nd edition (ed. Mullen G. R., Durden L. A.), p. 493-541 San Diego, CA: Academic Press; [Google Scholar]
- Nuttall P. A., Trimnell A. R., Kazimirova M., Labuda M. (2006). Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases Parasite Immunol. 28, 155-163 [DOI] [PubMed] [Google Scholar]
- Nuttall P. M., Labuda M. (2008). Saliva-assisted transmission of tick-borne pathogens. In Ticks: Biology, Disease and Control, p. 205-219 Cambridge: Cambridge University Press; [Google Scholar]
- Parola P., Raoult D. (2006). Tropical rickettsioses. Clin. Dermatol. 24, 191-200 [DOI] [PubMed] [Google Scholar]
- Prevot P. P., Couvreur B., Denis V., Brossard M., Vanhamme L., Godfroid E. (2007). Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 25, 3284-3292 [DOI] [PubMed] [Google Scholar]
- Prevot P. P., Adam B., Zouaoui B. K., Brossard M., Lins L., Cauchie P., Brasseur R., Vanhaevebeek M., Vanhamme L., Godfroid E. (2006). Anti-hemostatic effects of a serpin from saliva of the tick Ixodes ricinus. J. Biol. Chem. 281, 26361-26369 [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Zeidner N. S., Ledin K., Dolan M. C., Mather T. N. (2004). How much pilocarpine contaminates pilocarpine-induced tick saliva? Med. Vet. Entomol. 18, 20-24 [DOI] [PubMed] [Google Scholar]
- Silverman G. A., Bird P. I., Carrell R. W., Church F. C., Caughlin P. B., Gettins P. G. W., Moyer R. W., Pemberton P. A., Remold-O’Donnell E., Salvesen G. S. (2001). The serpins are expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 276, 33293-33296 [DOI] [PubMed] [Google Scholar]
- Smith A., Guo X., de la Fuente J., Naranjo V., Kocan K. M., Kaufman W. R. (2009). The impact of RNA interference of the subolesin and voraxin genes in male Amblyomma hebraeum (Acari: Ixodidae) on female engorgement and oviposition. Exp. Appl. Acarol. 47, 71-86 [DOI] [PubMed] [Google Scholar]
- Sonenshine D. E. (1993). Biology of Ticks. Oxford: Oxford University Press; [Google Scholar]
- Sonenshine D. E., Kocan K. M., de la Fuente J. (2006). Tick control: further thoughts on a research agenda. Trends Parasitol. 22, 550-551 [DOI] [PubMed] [Google Scholar]
- Sugino M., Imamura S., Mulenga A., Nakajima N., Tsuda A., Ohashi K., Onuma M. (2003). A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine 2, 2844-2851 [DOI] [PubMed] [Google Scholar]
- Uilenberg G., Thiaucourt F., Jongejan F. (2004). On molecular taxonomy: what is in a name? Exp. Appl. Acarol. 32, 301-312 [DOI] [PubMed] [Google Scholar]
- Willadsen P. (2004). Anti-tick vaccines. Parasitology 129, S367-S387 [DOI] [PubMed] [Google Scholar]