Abstract
We investigated factors controlling the development of connections between muscle spindle afferents, spinal motor neurons and inhibitory Renshaw cells. Several mutants were examined to establish the role of muscle spindles, muscle spindle-derived NT3 and excess NT3 in determining the specificity and strength of these connections. The findings suggest that although spindle-derived factors are not necessary for the initial formation and specificity of the synapses, spindle-derived NT3 seems necessary for strengthening homonymous connections between Ia afferents and motor neurons during the second postnatal week. We also found evidence for functional monosynaptic connections between sensory afferents and neonatal Renshaw cells although the density of these synapses decreases at P15. We conclude that muscle spindle synapses are weakened on Renshaw cells while they are strengthened on motor neurons. Interestingly, the loss of sensory synapses on Renshaw cells was reversed in mice over-expresssing NT3 in the periphery, suggesting that different levels of NT3 are required for functional maintenance and strengthening of spindle afferent inputs on motor neurons and Renshaw cells.
Keywords: Proprioceptor, muscle spindle, motor neuron, Renshaw, stretch reflex
Introduction
The monosynaptic spinal stretch reflex circuit is mediated by the synaptic connections between muscle spindle proprioceptive Ia afferents and spinal α-motor neurons. Ia afferents connect specifically with motor pools innervating homonymous muscles and establish weaker synapses with synergistic motor neurons, but avoid motor pools innervating strict antagonists. Because of its relative simplicity, this circuit has been the object of many developmental studies of synaptic specificity, patterns of connectivity and synaptic strength1,2. However, this simple circuit is embedded within a larger interneuronal network that patterns the motor output. Antagonistic motor neurons are not only excited by different sets of Ia afferents but also reciprocally inhibited via Ia inhibitory interneurons (IaINs) that receive input from specific sets of Ia afferents and project to antagonists. Moreover, the output of each motor pool is controlled by Renshaw cells which provide recurrent inhibition to the same motor neurons and also to motor pools that are coupled through broad muscle synergies, frequently with similar Ia excitation2.
For this network to function as a balanced output module of the motor circuit, it is essential that Ia afferents establish specific connections with defined sets of motor neurons and interneurons. In addition, the relative strength of these synapses needs to be matched to the appropriate level of Ia afferent excitation or inhibition of homonymous, heteronymous and antagonistic motor neurons. Little is known about the mechanisms responsible for the establishment of specific connectivity and the regulation of appropriate synaptic strength in most elements of this circuit. To date, most studies have focused on the development of specific, homonymous Ia-motor neuron connections1,2. Based on the extensive work of Frank and colleagues1,3,4,5, it is generally agreed that peripheral signals control the specificity of these connections in an activity-independent manner. The identity of these instructive signals is largely unknown, although a few recent studies have started to describe some aspects of the molecular mechanisms through which peripheral signals could specify Ia-motor neuron synaptic specifcity6,7. For example, neurotrophin-3 (NT3) has been shown to be important for the survival of Ia afferents8,9 and in determining the strength of monosynaptic connections both chronically10 and acutely11. Moreover, NT3 overexpression during development induces inappropriate synaptic strengthening and can result in loss of specificity of the Ia-motor neuron connection12.
Little is known about the formation, specificity or developmental strengthening of the connections between Ia afferents and interneurons. In this chapter, we review recent data from our laboratories analyzing the developmental maturation of central Ia connections on both motor neurons and interneurons. The results suggest that muscle spindles are not required for the specificity of the central connections of Ia afferents, but that spindle-derived NT3 is important in adjusting the final strength of central Ia synapses on motor neurons and interneurons. Moreover, NT3 actions are exerted at a relatively late postnatal period (second and third postnatal week in rodents) coincident with increased phasic and tonic firing in Ia afferents and the maturation of normal responses to muscle stretch13. Behaviorally this period is characterized by the beginning of coordinated, weight-bearing, adult-like locomotion14.
Functional specificity of connections between muscle spindle afferents and motor neurons does not depend on ErbB2 expression or muscle spindle integrity
In normal mice, proprioceptive afferents project monosynaptically to homonymous motor neurons, but do not make direct connections with functionally antagonist motor neurons15. To investigate the importance of muscle spindles in the establishment of these connections, we examined the strength and specificity of muscle afferent projections to identified motor neurons in mice whose muscle spindle induction was inhibited by the conditional elimination of the neuregulin 1 (Nrg1) receptor ErbB2 from muscle spindle precursors16. Since neuronal induction of muscle spindle development depends on Nrg1 signaling17 through the activation of ErbB2 receptors on primary myocytes18,19, conditional elimination of ErbB2 from intrafusal muscle precursors resulted in a 70% loss of muscle spindles and a 90% loss in the number of intrafusal fibers16. The remaining muscle spindles were rudimentary and never fully matured. Immunocytochemical analysis in the ErbB2 mutants using markers for proprioceptive central axons and synapses (VGluT1, parvalbumin)20 revealed that the density of primary afferent boutons on motor neuron somata at P21 was reduced by ~30%. Thus, in the absence of normal spindle development, there is only a modest reduction in the normal density of VGluT1 contacts on motor neurons. This led us to examine whether the anatomical contacts between muscle afferents and motor neurons were functional and target specifically homonymous motor pools.
For this purpose, we compared the sensorimotor connections between the functionally antagonist motor neurons innervating the lateral gastrocnemius (LG) and the tibialis anterior (TA) muscles in wild type and ErbB2Δ mice. In normal mice, intracellular recordings revealed that the latency of EPSPs evoked by antagonist nerve stimulation (presumed polysynaptic) was approximately 5 ms longer than that evoked by stimulation of the homonymous nerve (monosynaptic activation). In ErbB2 mutant animals, the difference in homonymous and antagonist latency was similar (fig.1.D), indicating that: i) monosynaptic inputs from muscle afferents are functional in the ErbB2 mutant animals, and ii) for the TA and LG motor neurons, the specificity of the connections is preserved16.
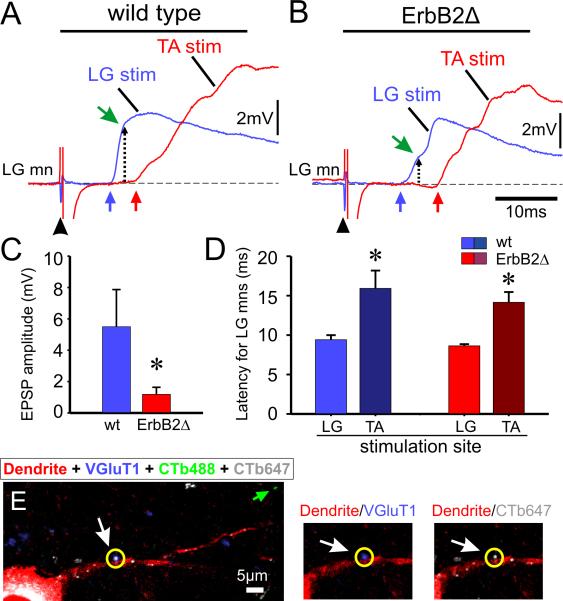
Figure 1. Monosynaptic connections between primary afferents and motor neurons are specific but reduced in strength in ErbB2Δ mice.
A, Comparison of the potentials recorded from an LG motor neuron evoked by homonymous (blue trace, LG) and antagonist (red trace, TA) muscle nerve stimulation at 5× T intensity averaged from five stimuli applied once every 10 s. The blue and red arrows indicate the onset of the EPSPs. B, Recordings of homonymous and antagonist evoked synaptic potentials in an ErbB2Δ LG motor neuron. The conditions and stimulation parameters were the same as for the wild-type records in A. The dotted lines with an arrow point to the amplitude of the EPSP at 3ms after the EPSP onset for both motoneurons. C, Bar graph showing the average amplitude of the intracellularly recorded monosynaptic EPSP in wild-type (blue bars, n=5 motor neurons) and ErbB2Δ animals (red bars, n=6 motor neurons) in response to stimulation of a peripheral nerve (* p<0.05, t-test). D, Bar graph showing the average latency of the synaptic potential evoked in LG motor neurons by stimulation of the homonymous, LG nerve, and the antagonistic, TA nerve, for wild-type (blue bars) and ErbB2Δ (red bars) mice. Error bars are SEM. Age range: P3-P5. E, image is from a wild-type LG motor neuron labeled in vivo by LG muscle injection with CTb647 (in white) and retrogradely filled (three days later) in vitro with Texas Red Dextran (in red) to reveal its somato-dendritic morphology. The CTb488 (in green) was injected in vivo into the TA muscle at the same time as CTb647 (see green arrow for a putative afferent fiber originating in the TA muscle). The motoneuron soma contains CTb647 (white) and Texas Red Dextran (red) confirming the identity of the motoneuron as LG. VGluT1-IR synaptic boutons were revealed immunohistochemically (shown in blue). The synaptic bouton circled in yellow (see white arrow) is shown in higher magnification on the right side. The bouton is VGluT1+ (blue) and the CTb647+ (white) conferring its identity as a primary afferent bouton from the LG muscle. Each image is a single optical scan at 0.69μm thickness. The green arrow points to a putative TA afferent bouton labeled with CTb488.
These results suggest that functional connections retained their specificity in the mutant animals, but we could not rule out the possibility that erroneous connections might be formed in the ErbB2 mice that were functionally silent or too weak to detect physiologically. To test this possibility, we used an anatomical assay for specificity. Cholera Toxin B subunit (CTb) conjugated to either Alexa-488 or Alexa-647 was injected in vivo into the TA and LG muscles respectively to label both motor neurons and proprioceptive afferent boutons and the tissue was later processed for VGlut1 immunoreactivity. Although the anterograde labeling methods we used do not label all afferent boutons, we failed to find any evidence in both normal and ErbB2Δ mice, of inappropriate projections either in the soma or proximal dendrites (~50-100μm in length) of LG afferents to TA motor neurons and vice versa (fig.1.E). The results therefore, suggest that spindle-derived factors are not required for the initial formation or the specificity of the central connections of muscle afferents with motor neurons.
We found however that the amplitude of the monosynaptic EPSPs was reduced by ~80% in the ErbB2Δ animals compared with wild-type animals (Fig. 1A,B) and this was paralleled by a similar reduction (80–85%) in the amplitude of ventral root potentials (from ErbB2Δ animals) generated in response to stimulation of the muscle nerves16. Thus in the ErbB2Δ mutant, a substantial number of specific Ia-motor neuron connections are formed (fig.1A, B and D), but these synapses are weaker than normal. One possible mechanism to account for these findings is a lack of spindle-derived NT310. Consistent with this idea, there was no detectable expression of NT3 mRNA in the muscles of Erb2Δ conditional mutants.
Effects of spindle-derived NT3 on the strength of monosynaptic Ia to motor neuron synapses
We studied the effects of selective elimination of NT3 expression from intrafusal fibers to investigate whether the reduction of the afferent evoked monosynaptic EPSPs in the ErbB2Δ mutant animals was due to the loss of spindle-derived NT3 rather than some other abnormality of spindle development. For this purpose, a novel transgenic mouse line carrying an Egr3-IRES-CRE allele was generated and crossed to animals with floxed alleles of NT321 to selectively eliminate NT3 expression from the intrafusal fibers of muscle spindles. In these animals, muscle spindle number and afferent innervation was normal16.
To monitor the strength of the afferent-motor neuron connections, we recorded the L5 ventral root responses following stimulation of the homonymous dorsal root. There was no difference between the amplitude of the ventral root potentials in the wild type and spindle-NT3 mutants in neonatal (P5) mice (Fig. 2A,B). However, in juveniles (P14), the amplitude of the monosynaptic reflex was significantly reduced (>80%) in animals that lacked spindle-derived NT3 (Fig. 2C,D). To rule out the possibility that the reduction in monosynaptic activation was due to NT3 deletion in non-spindle structures that also express Egr3 (Schwann cells and peripheral vasculature), we also investigated the strength of sensory afferent-motor neuron connections in animals in which NT3 was selectively eliminated from all muscle fibers (intrafusal and extrafusal) using a myf5CRE allele. The results were similar to those obtained with the selective elimination of NT3 expression from intrafusal fibers; there was no significant difference between myf5CRE mutants and wild type at P5, but at P14 there was a dramatic reduction in ventral root reflex amplitude (Fig. 2) and no significant difference in the latency of the ventral root responses. These results indicate that deficits of spindle derived NT3 cannot explain the reduction in the strength of the connections in the ErbB2Δ mutants during the first postnatal week. However, they reveal an important role for spindle-derived NT3 during later postnatal maturation. Interestingly, NT3 actions on the connections between Ia afferents and motor neurons coincides with the time that mice develop weight-bearing locomotion and maturation in the activity and responses of muscle spindle afferents13.
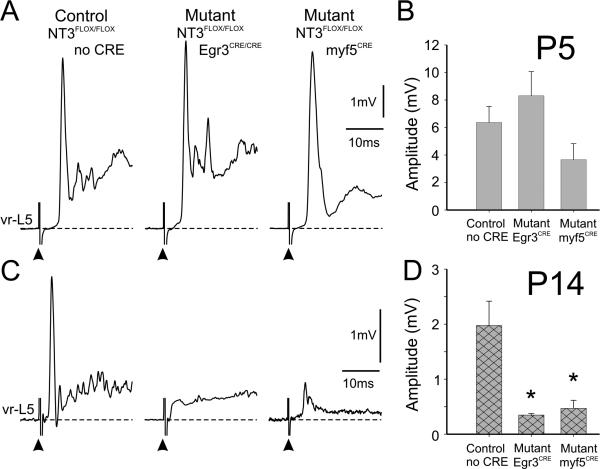
Figure 2. Muscle afferent-evoked monosynaptic ventral root potentials in animals deficient in muscle-spindle or muscle NT3 are not reduced at P5 but do show significant reductions at P14.
A, Averaged extracellular recordings from the L5 ventral root after stimulation of the L5 dorsal root at 5×T from three groups of animals (1 control and 2 mutants) at P5. The frequency of stimulation was 0.1 Hz. Note that the amplitude of the monosynaptic component the ventral root potential (arrows) did not differ significantly between the controls (NT3FLOX/FLOX; no Cre) and either of the two mutants (NT3FLOX/FLOX/Egr3CRE/CRE or NT3FLOX/FLOX/myf5CRE). B, Graph showing the average responses from three preparations from each group of animals. There was no significant difference between the groups (p=0.10, ANOVA). C, Recordings from the same groups as in A but at P14. Note that the monosynaptic reflex was greatly reduced in the mutants compared with the controls. D, Graph showing the averaged amplitude of the monosynaptic response from three animals per group. There was a significant reduction in the monosynaptic reflex for both mutants compared with that of the control group (*p<0.01, ANOVA Fisher's test). The latency of the onset of the responses was not significantly different between the mutant and control animals.
Previous work describing the presence of inappropriate connections in mice overexpressing NT3 in muscle proposed that a minor population of inappropriate but normally “silent” connections was strengthened by the excess NT312. Although it has been previously reported that inappropriate afferent inputs on motoneurons are present during embryonic development in the rat spinal cord, these connections are greatly suppressed within a few days after birth22. One possibility is that non-specific connections are located in distal dendritic regions that are not accessible for analysis. Alternatively, inappropriate connectivity in these mutants may result from excessive NT3-induced branching and extension of Ia afferent central terminals arbors within the cord23,24 and/or the retention of an excess of spindle afferents during development25. These NT3 effects are believed to result from overcoming inhibitory signals acting on sensory axon projections, particularly in the ventral part of the spinal cord24,26,27 and preventing normal cell death of a proportion of Ia afferents during development25. A larger than normal sensory afferent projection into lamina IX could then overwhelm repulsive molecular mechanisms during synaptogenesis that normally prevent the formation of inappropriate monosynaptic connections7. Indeed, by P21, in mlc::NT3 animals we observed a very large increase (~200%) in VGluT1+ contacts on motor neuron cell somata that was paralleled by a significant increase in the density of VGluT1-IR clusters in Laminae IX and VII28.
Primary afferents make functional monosynaptic contacts on Renshaw interneurons in the developing spinal cord
Ia afferents also make specific connections with Renshaw cells, Hb9 interneurons29 and other interneurons but little is known about the development of these connections. Most of our knowledge on the organization of proprioceptive inputs to interneurons comes from experiments in the adult cat spinal cord30,31. For example, cat Ia inhibitory interneurons (IaINs), which mediate reciprocal inhibition between antagonistic pools, receive inputs from Ia muscle afferents that appear to be stronger and more specific than on other interneurons (i.e., Ia/Ib interneurons, group II interneurons). This conclusion is based on the strong coupling between IaINs and Ia afferent inputs, their ability to follow relatively high frequencies of Ia afferent discharge in response to muscle stretch, and the fact that they do not receive convergent inputs from other proprioceptors31,32,33. In contrast, cat Renshaw cells, which mediate recurrent inhibition of homonymous and synergistic motor neurons, do not appear to receive direct connections from Ia fibers or any other low-threshold afferent fibers in the adult spinal cord34,35. The mechanisms by which Ia afferents select specific interneuronal targets within the ventral horn are unknown.
Although muscle afferent inputs to Renshaw cells cannot be detected functionally in the adult cat, work in the chick embryo demonstrated that muscle afferents make monosynaptic connections with R-interneurons, a class of avian embryonic inhibitory interneuron thought to be similar to the mammalian Renshaw cell36. However, because afferent inputs on mammalian Renshaw cells had not previously been studied in neonatal animals, it was not clear if these connections are also formed in mammalian development, or if they were unique to avians. To address this issue, we analyzed muscle afferent inputs to Renshaw cells in late embryonic, postnatal and adult rats and mice37.
Using a variety of anatomical methods to define proprioceptive primary afferents (VGluT1-IR, parvalbumin-IR, anterograde tracing from dorsal roots), we found that murine Renshaw cells receive few primary afferent inputs during late embryonic development when Ia afferent connections to motor neurons became functional (~E16-E17)15,38. However, the fraction of Renshaw cells that receive spindle afferent inputs gradually increases to ~50% at P0, 100% by P10, and these inputs are maintained in all adult Renshaw cells (Fig. 3C). This result was surprising since previous in vivo studies from adult cat had reported that Renshaw cells cannot be monosynapticaly activated by dorsal root stimulation.
Figure 3. Developmental changes in the number of synaptic inputs onto Renshaw cells derived from dorsal root and motor axon afferents.
A1, Confocal image of a P4 hemicord containing primary afferent sensory fibers labeled with Fluorescein Dextran (green) and motor neurons and motor axons labeled with Texas Red Dextran (red). The section was also immunolabeled with calbindin antibody (blue). A2, Higher magnification image of the area boxed in A1. Renshaw cells are positioned at the exit of motoneuron axons from the cord (double arrowhead). Motor neurons (red) and calbindin-IR Renshaw cells (blue) are both surrounded and contacted by primary afferent axons (green, arrow). Renshaw cells are contacted in addition by motor axon varicosities (red). A3, High magnification image of the Renshaw cell indicated with an arrow in A2, receiving convergent contacts from motor axon (red) and primary afferent (green) varicosities. B, High magnification images from a P15 spinal cord triple labeled to show primary afferents (dorsal root fill, in green), VGluT1-IR varicosities (red) and calbindin-IR Renshaw cells (blue). Superimposition of VGluT1-IR shows that all primary afferent varicosities, including those in contact with Renshaw cells, contain VGluT1-immunoreactivity. Arrowheads point to VGluT1-IR dorsal root afferent varicosities (yellow) in contact with a Renshaw cell calbindin-IR dendrite (blue). C, Percentage of calbindin-IR cells (CB-IR; presumed Renshaw cells) receiving at least one contact from each of the markers used to identify dorsal and ventral root inputs. Almost all calbindin-IR Renshaw cells receive VAChT-IR contacts (motor axons) in embryo (age: E18). In contrast, dorsal root inputs are few in the embryo spreading to all Renshaw cells after birth. D, comparison of synaptic responses from a motor neuron (green) and a Renshaw cell (black) to a single dr-L5 stimulus at suprathreshold intensity. Both neurons responded robustly and fired action potentials superimposed on the EPSPs. Inset shows comparison of average latencies for Renshaw cells (black, n=10), L5 motor neurons (green, n=5) and the ventral root L5 extracellular recording (blue, n=4) following stimulation of the dorsal root L5 (age range: P2-P3). There were no significant differences in the latencies among these groups (p>0.05, One-Way ANOVA). Error bars indicate SEM. Scale bars; A1: 200 μm; A2: 40 μm; A3: 10 μm; B: 10 μm.
To investigate whether the anatomically identified inputs were functional in newborn animals, we obtained whole cell recordings from identified Renshaw cells in the isolated spinal cord of the neonatal mouse. Most (11 of 12) Renshaw interneurons displayed a short-latency response following dorsal root stimulation (Fig. 3D). This response was determined to be monosynaptic because the onset of the evoked EPSP exhibited very little jitter during high frequency stimulation and the average latency of the response (~6ms) was similar to the latency of monosynaptic afferent-evoked EPSPs recorded from motor neurons. In conclusion, our morphological and anatomical evidence demonstrates the presence of functional sensory afferent synaptic inputs onto neonatal Renshaw cells.
Because Renshaw cells are located in the ventral most regions of laminae VII and IX, we assumed these projections must come from Ia afferents since no other sensory afferent class is known to project this ventrally in the spinal cord39. This raises the question of why Renshaw cells do not seem to have functional monosynaptic Ia afferent inputs in the adult cat. One possible explanation is that Ia afferent inputs do not make synaptic contacts on Renshaw cells in all species. To date most physiological studies of adult Renshaw cells have been conducted in the cat, while our findings were obtained in neonatal mice. However, one study conducted in fetal cats40 provided evidence compatible with the presence of monosynaptic dorsal root inputs on embryonic Renshaw cells. Moreover, our morphological data revealed VGLUT1 sensory synapses on adult Renshaw cells in cats, similar to those found in rodents. Thus the available data seem to rule out the possibility of species differences. An alternative explanation is that Renshaw cells lose this functional input during postnatal development by weakening its synaptic effectiveness, despite maintaining a significant number of synaptic contacts.
Developmental changes in the primary afferent synaptic density on Renshaw cells
Electrophysiological recordings from interneurons during the second and third postnatal week are difficult to obtain in rodents, and for this reason we analyzed the postnatal maturation of sensory inputs onto the dendrites and somata of Renshaw cells in rat and mouse using VGluT1 as a marker of proprioceptive inputs37. We used synaptic density as an indirect estimate of input strength and investigated ultrastructural features37 that are known to correlate with synaptic efficacy at Ia synapses41.
VGluT1+ contact densities changed with development on both dendrites and somata. However, most VGluT1+ boutons were found on dendrites and therefore most of the analysis focused on three-dimensional reconstructions of the dendritic arbors37. On dendrites, VGluT1 densities increased five- to seven-fold from P0 to P15 and then decreased to less than half the P15 density in the adult. This contrasted with the developmental maturation of VAChT bouton numbers, which we used as a marker of motor axon synapses. VAChT density increased during the first two weeks but then was maintained at the same density from P15 to adulthood. These results suggest that Renshaw cells shift from integrating sensory and motor inputs in neonates to predominantly motor inputs in the adult and raise the possibility that VGluT1 immunoreactive terminals on adult Renshaw cells may be functionally silent. Although we could not obtain electrophysiological evidence for the weakening of this input in mature mice spinal cords, we found (using electron microscopy) a partial regression of both the number and size of synaptic active zones in VGluT1+ synapses on Renshaw cells. A similar regression of the synaptic apparatus was not observed on other VGluT1 targets in the ventral spinal cord. These ultrastructural changes and the decreased synaptic density are consistent with a progressive weakening of VGluT1+ positive synapses on Renshaw cells during postnatal maturation.
Weakening or perhaps even silencing VGluT1+ inputs on Renshaw cells might be responsible for the de-selection of these interneurons as a major target of Ia afferents in the adult spinal cord. The mechanisms by which this “late” developmental synaptic adjustment on Renshaw cells occurs are unknown. To investigate whether the loss of muscle spindle afferent inputs onto Renshaw cells could be reversed by NT3, we analyzed this connection in mlc::NT3 transgenic mice25 in which exogenous NT3 is overexpressed in embryonic and postnatal muscle (Fig. 4). Using immunohistochemistry, we found that the density of VGluT1+ boutons on Neurolucida reconstructed Renshaw cell dendritic arbors increased by approximately 28% in mlc::NT3+/- animals with respect to controls at P15. More importantly, the densities of these contacts were maintained into adulthood, or even slightly increased (Fig. 4D). As a result, adult Renshaw cells displayed a large (99%) significant increase in VGluT1+ contact density compared to wild-types (in WT = 0.98 ± 0.03 contacts per 50 μm of dendrite; mlc::NT3+/- = 1.97 ± 0.1; p < 0.001, t-test).
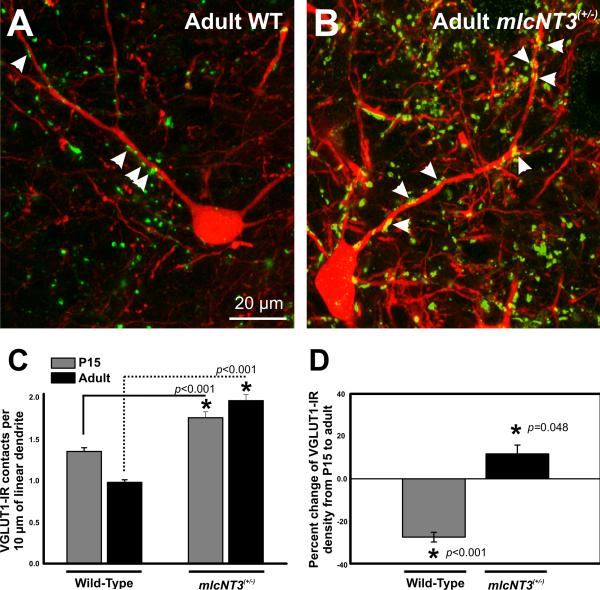
Figure 4. Density of VGluT1-immunoreactive contacts on postnatal Renshaw cells in the ventral horn of control and mlcNT3(+/-) mice.
A,B, High magnification images of VGluT1-immunoreactivity (FITC, green) on adult Calbindin-immunoreactive (CB-IR; Cy3, red) Renshaw cells (RCs) of control and mlcNT3(+/-) mice. Arrowheads indicate VGluT1-IR contacts on RC dendrites. There are fewer contacts on RCs from Ia afferents in control mice compared to mlcNT3(+/-) mice. C, VGluT1-IR contacts per 10 μm of linear dendrite of P15 and adult CB-IR RCs in control and mlcNT3(+/-) mice. In control animals, VGluT1-IR density on RCs decreases significantly from P15 (1.35 ± 0.05 VGluT1+ contacts per 10 μm of linear dendrite, number of RCs=57; number of animals=5) to adult (0.98 ± 0.03, # RCs=35, N=4; p<0.001, one-way ANOVA). In contrast, RCs in mlcNT3(+/-) mice showed a significant increase from P15 (1.76 ± 0.07, number of RCs=40, N=3) to adult (1.97 ± 0.10, number of RCs=24, N=3; p=0.041, oneway ANOVA). At all ages analyzed, RCs from mlcNT3(+/-) mice showed significant increases in the density of VGluT1-IR contacts when compared to age-matched controls (p<0.001, t-test). D, Percentage change in the density of VGluT1-IR contacts on RCs from P15 to adult in control and mlcNT3(+/-) mice. VGluT1-IR contact density decreases by ~30% from P15 to adult in control animals (p<0.001, t-test). In contrast, in mlcNT3(+/-) animals, the density of VGluT1-IR contacts increases by ~11% from P15 to adult. Therefore, it appears that increasing Ia afferent input density on RCs prevents their de-selection. Scale bars: A and B (in A), 20 μm.
In summary, the late postnatal adjustment of input densities seen normally on Renshaw cells does not occur in mlc::NT3+/- animals, suggesting that the late postnatal weakening of central sensory afferent connections on Renshaw cells is controlled by processes that can be overcome by an excess supply of peripheral NT3.
Summary and Conclusions
Our results suggest that spindle-derived factors are not responsible for specifying the connections between muscle spindle afferents and motor neurons, but that they do play a role in strengthening these connections during development. They also indicate that spindle-derived NT3 is important in this process when the animal begins to walk and afferent activity is significantly enhanced. Interestingly, during this time period, Ia synapses on Renshaw cells start to weaken, a process that can be overcome by excess NT3. It is therefore tempting to speculate that NT3-strengthening of Ia connections is differentially modulated depending on the target or its activity. For example, during postnatal maturation, Ia afferent activity might become tightly coupled with postsynaptic firing in motor neurons but not with Renshaw cells, leading to differential NT3-dependent strengthening on different targets. Normal levels of spindle-derived NT3 are adequate to strengthen and maintain spindle afferent synapses on motor neurons but not Renshaw cells. In this context, it would be of great interest to establish if homonymous or antagonistic Ia afferents project onto coupled motor neuron-Renshaw cell pairs.
Our studies also suggest that spindle-derived signals, including NT3, are not necessary for the development of specific connections between afferents and motor neurons. Other target-derived factors, for example glial cell-derived neurotrophic factor (GDNF) acting through the ETS transcription factor Pea3 have been shown to regulate connectivity between Ia afferents and motor neurons in particular motor pools6. Although the nature and source of the instructive signals that specify these connections remains unknown, recent work implicates semaphorin 3e and plexin D1 receptors in the read-out of these peripheral cues, which generate repulsive signals that restrict connectivity between certain forelimb spindle afferents and brachial motor pools7. As the molecular mechanism involved in Ia afferent-motor target specification become elucidated, it will be possible to test further the role of activity, target-derived factors and molecular recognition signals in the process. A model for activity-dependent modulation of genetic and molecular programs of axonal targeting was recently described for the case of motor neuron projections to specific peripheral targets42. Similar fine regulatory mechanisms might be necessary to precisely control the patterns of synaptic connectivity and strength within the spinal cord, even for the “simple” case of Ia afferent projections to ventral horn neurons.
Acknowledgements
This work was supported by the intramural program of NINDS (G.Z.M., N.A.S. and M.J.O'D) and by the National Institutes of Health Grant NS047357 (F.J.A.).
REFERENCES
- 1.Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: Driven to function by genetic and experience-dependent mechanisms. Neuron. 2007;56:270–283. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Smith CL, Frank E. Peripheral specification of sensory neurons transplanted to novel locations along the neuraxis. J Neurosci. 1987;7:1537–1549. doi: 10.1523/JNEUROSCI.07-05-01537.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank E, Wenner P. Environmental specification of neuronal connectivity. Neuron. 1993;10:779–785. doi: 10.1016/0896-6273(93)90194-v. [DOI] [PubMed] [Google Scholar]
- 4.Wenner P, Frank E. Peripheral target specification of synaptic connectivity of muscle spindle sensory neurons with spinal motoneurons. J. Neurosci. 1995;15:8191–8198. doi: 10.1523/JNEUROSCI.15-12-08191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HH, et al. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 6.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Pecho-Vrieseling E, et al. Specificity of sensory–motor connections encoded by Sema3e–Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernfors P, et al. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 9.Fariñas I, et al. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci. 2002;22:3512–3519. doi: 10.1523/JNEUROSCI.22-09-03512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvanian VL, et al. Chronic neurotrophin-3 strengthens synaptic connections to motoneurons in the neonatal rat. J Neurosci. 2003;23:8706–8712. doi: 10.1523/JNEUROSCI.23-25-08706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, et al. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci. 2007;27:3686–3694. doi: 10.1523/JNEUROSCI.0197-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vejsada R, et al. The postnatal functional development of muscle stretch receptors in the rat. Somatosens Res. 1985;2:205–222. doi: 10.3109/07367228509144564. [DOI] [PubMed] [Google Scholar]
- 14.Gramsbergen A. Posture and locomotion in the rat: independent or interdependent development? Neurosci Biobehav Rev. 1998;22:547–553. [PubMed] [Google Scholar]
- 15.Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci. 1997;17:3128–3135. doi: 10.1523/JNEUROSCI.17-09-03128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shneider NA, et al. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: The Role of spindle-derived Neurotrophin 3. J. Neurosci. 2009;29:4719–4735. doi: 10.1523/JNEUROSCI.5790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hippenmeyer S, et al. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 18.Andrechek ER, et al. ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol. 2002;22:4714–4722. doi: 10.1128/MCB.22.13.4714-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leu M, et al. ErbB2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130:2291–2301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez FJ, et al. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- 21.Bates B, et al. Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci. 1999;2:115–117. doi: 10.1038/5669. [DOI] [PubMed] [Google Scholar]
- 22.Seebach BS, Ziskind-Conhaim L. Formation of transient inappropriate sensorimotor synapses in developing rat spinal cords. J Neurosci. 1994;14:4520–4528. doi: 10.1523/JNEUROSCI.14-07-04520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradbury EJ, et al. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramer MS, et al. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- 25.Taylor MD, et al. Postnatal regulation of limb proprioception by muscle-derived neurotrophin-3. J Comp Neurol. 2001;432:244–258. doi: 10.1002/cne.1100. [DOI] [PubMed] [Google Scholar]
- 26.Patel TD, et al. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- 27.Li LY, et al. Neurotrophin-3 ameliorates sensory-motor deficits in Er81-deficient mice. Dev Dyn. 2006;235:3039–3050. doi: 10.1002/dvdy.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CA, et al. Influence of sensory afferents and motoneurons on the developmental regulation of calbindin and parvalbumin expression in spinal interneurons. Soc. Neurosci. 2007 Abstracts 132.22. [Google Scholar]
- 29.Ziskind-Conhaim L, et al. Synaptic integration of rhythmogenic neurons in the locomotor circuitry: the case of Hb9 interneurons. N.Y. Acad. Sci. 2010 doi: 10.1111/j.1749-6632.2010.05533.x. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology. The Nervous System. II. Am. Physiol. Soc.; Bethesda: 1981. 1981. pp. 509–595. Motor control. Part 1. [Google Scholar]
- 31.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 32.Eccles JC, Fatt P, Landgren S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956;19:75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Hultborn H, Jankowska E, Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J. Physiol. 1971;215:613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renshaw B. Central effects of centripetal impulses in axons of spinal central roots. J Neurophysiol. 1946;9:191–204. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- 35.Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenner P, O'Donovan MJ. Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. J Neurosci. 1999;19:7557–7567. doi: 10.1523/JNEUROSCI.19-17-07557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mentis GZ, et al. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci. 1990;10:125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fyffe RE. Afferent fibers. In: Davidoff RA, editor. Handbook of the spinal cord. 2 and 3. Dekker; New York: 1984. pp. 79–135. Anatomy and physiology. [Google Scholar]
- 40.Naka KI. Electrophysiology of the fetal spinal cord. II. Interaction among peripheral inputs and recurrent inhibition. J Gen Physiol. 1964;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce JP, Mendell LM. Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships . J Neurosci. 1993;13:4748–4763. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson MG, Milner LD, Landmesser LT. Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res Rev. 2008;57:77–85. doi: 10.1016/j.brainresrev.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]