Abstract
The gene for an approximately 27-kDa outer membrane-associated, immunoreactive protein was cloned from the rickettsial pathogen Coxiella burnetii. The gene, designated com1 for Coxiella outer membrane protein 1, was expressed in Escherichia coli, presumably by its own promoter. The complete nucleotide sequence of the gene was determined. The deduced amino acid sequence of 252 residues includes a putative leader sequence. The leader sequence is recognized in and removed by E. coli on the basis of the difference in the molecular mass of the protein produced in an in vitro transcription-translation system (27.6 kDa) and that of the protein immunoprecipitated from an iodinated E. coli clone (25.7 kDa). The Com1 protein expressed in E. coli was proteinase K sensitive in nondisrupted cells and soluble in 1% Sarkosyl, suggesting a loose association with the outer membrane. While the complete predicted sequence of the Com1 protein does not show any overall similarity to those of previously described proteins, a region which includes the only two cysteines in Com1 is homologous to the catalytic site of protein disulfide oxidoreductases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baca O. G., Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev. 1983 Jun;47(2):127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsborg J. M., Cianciotto N. P., Hindersson P. Nucleotide sequence analysis of the Legionella micdadei mip gene, encoding a 30-kilodalton analog of the Legionella pneumophila Mip protein. Infect Immun. 1991 Oct;59(10):3836–3840. doi: 10.1128/iai.59.10.3836-3840.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. R., Kordová N., Paretsky D. Electron microscopic studies of the rickettsia Coxiella burneti: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can J Microbiol. 1971 Feb;17(2):143–150. doi: 10.1139/m71-025. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilbert H. F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986 Apr;52(1):337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix L. R., Samuel J. E., Mallavia L. P. Differentiation of Coxiella burnetii isolates by analysis of restriction-endonuclease-digested DNA separated by SDS-PAGE. J Gen Microbiol. 1991 Feb;137(2):269–276. doi: 10.1099/00221287-137-2-269. [DOI] [PubMed] [Google Scholar]
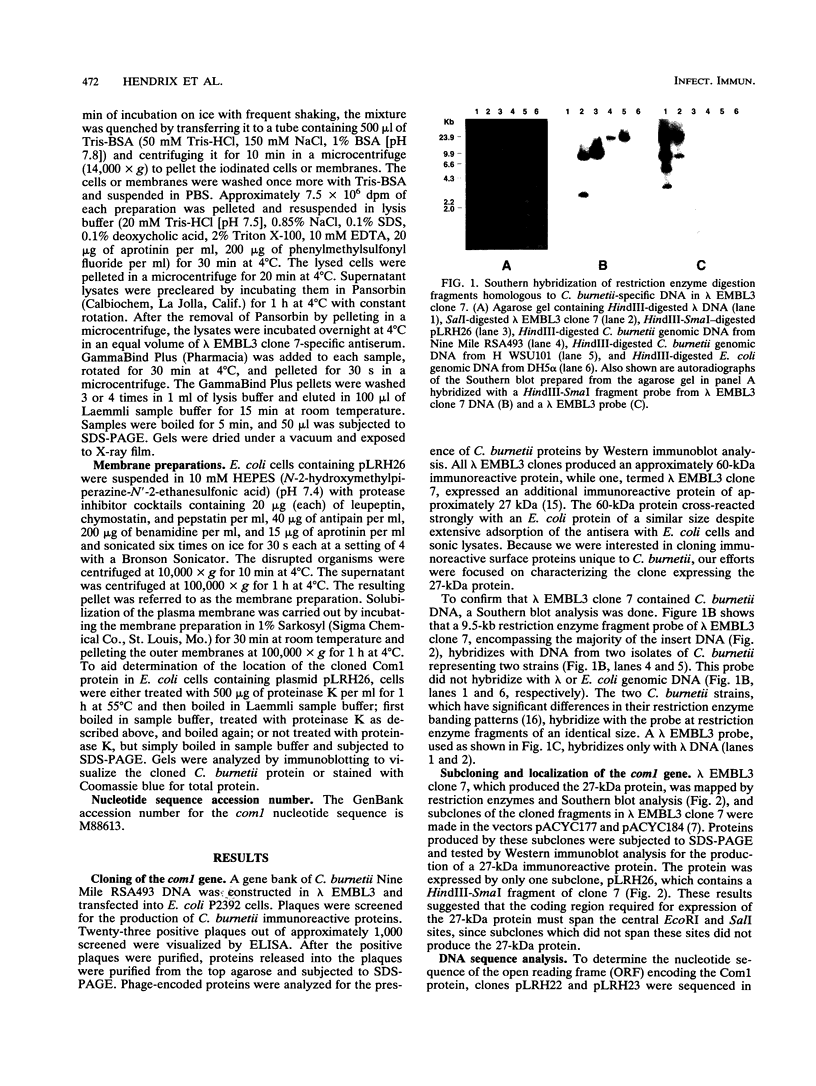
- Hendrix L. R., Samuel J. E., Mallavia L. P. Identification and cloning of a 27-kDa Coxiella burnetii immunoreactive protein. Ann N Y Acad Sci. 1990;590:534–540. doi: 10.1111/j.1749-6632.1990.tb42263.x. [DOI] [PubMed] [Google Scholar]
- Hendrix L., Mallavia L. P. Active transport of proline by Coxiella burnetii. J Gen Microbiol. 1984 Nov;130(11):2857–2863. doi: 10.1099/00221287-130-11-2857. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia L. P. Genetics of rickettsiae. Eur J Epidemiol. 1991 May;7(3):213–221. doi: 10.1007/BF00145669. [DOI] [PubMed] [Google Scholar]
- Minnick M. F., Heinzen R. A., Frazier M. E., Mallavia L. P. Characterization and expression of the cbbE' gene of Coxiella burnetii. J Gen Microbiol. 1990 Jun;136(6):1099–1107. doi: 10.1099/00221287-136-6-1099. [DOI] [PubMed] [Google Scholar]
- Minnick M. F., Heinzen R. A., Reschke D. K., Frazier M. E., Mallavia L. P. A plasmid-encoded surface protein found in chronic-disease isolates of Coxiella burnetti. Infect Immun. 1991 Dec;59(12):4735–4739. doi: 10.1128/iai.59.12.4735-4739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos A., Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987 May;55(5):1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Samuel J. E., Frazier M. E., Kahn M. L., Thomashow L. S., Mallavia L. P. Isolation and characterization of a plasmid from phase I Coxiella burnetii. Infect Immun. 1983 Aug;41(2):488–493. doi: 10.1128/iai.41.2.488-493.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel J. E., Frazier M. E., Mallavia L. P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985 Sep;49(3):775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeer N. Früherkennung eines 27-kDa-Membranproteins (MP27) bei mit Coxiella burnetii infizierten und vakzinierten Meerschweinchen. Zentralbl Veterinarmed B. 1988 Jun;35(5):338–345. [PubMed] [Google Scholar]
- Schmeer N., Müller H. P., Baumgärtner W., Wieda J., Krauss H. Enzyme-linked immunosorbent fluorescence assay and high-pressure liquid chromatography for analysis of humoral immune responses to Coxiella burnetti proteins. J Clin Microbiol. 1988 Dec;26(12):2520–2525. doi: 10.1128/jcm.26.12.2520-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. The biology of rickettsiae. Annu Rev Microbiol. 1982;36:345–370. doi: 10.1146/annurev.mi.36.100182.002021. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Hoover T. A., Waag D. M., Banerjee-Bhatnagar N., Bolt C. R., Scott G. H. Antigenic structure of Coxiella burnetii. A comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann N Y Acad Sci. 1990;590:370–380. doi: 10.1111/j.1749-6632.1990.tb42243.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]