Abstract
A boy aged 7.6 years presented to our Unit of Paediatric Endocrinology for evaluation of obesity. Progressive weight gain (10 kg) started 6 months earlier after an accidental penetrating orbital injury on the right eye. During this period the child has been treated with oral betamethasone (0.5 mg/day) for 1 month and dexamethasone 2% ocular drops (2 hourly by day) for 6 months. Physical examination showed he was 113.5 cm in height (−1.5 SD), weight 36.0 kg, blood pressure 110/90 mmHg (90th centile), body mass index 28 (+5 SD), truncal obesity, buffalo hump, “moon-face”, increased lanugo hair and supraclavicular fullness. Endocrinological work-up revealed undetectable levels of basal adrenocorticotropic hormone (ACTH), basal and ACTH-stimulated cortisol and 24 h urine excretion cortisol, confirming the diagnosis of iatrogenic Cushing syndrome. The abrupt withdrawal of ocular glucocorticoids by the parents evoked two adrenal crises; 4 months later the patient recovered. In conclusion, we would alert doctors that every formulation of glucocorticoids, no ocular drops excluded, can determine severe systemic side effects and iatrogenic Cushing syndrome.
BACKGROUND
Steroids are one of the most important drugs and since their introduction in 1948 they have never been given up or replaced.1 They are the treatment of choice for a lot of common pathologies due to their potent anti-inflammatory and immunosuppressive actions. Nonetheless, it is well-established that prolonged therapeutic schedules can determine severe side effects, especially with steroids administered by the systemic route (oral, intravenous and intramuscular).2 This is the unique limitation of these drugs and one way of avoiding the side effects and adrenal insufficiency, in particular, is to use alternate-day steroids. Another therapeutic option is the use of topic steroids in order to maximise the therapeutic effects in the site of inflammation and minimise the systemic absorption. But it is important to underline that every topic steroid has a systemic absorption and may potentially cause iatrogenic Cushing syndrome, although this is a very rare and unusual event.3 Most of these cases have been caused by rhinologic4 and dermatological5 preparations and only three reports of iatrogenic Cushing syndrome by ocular steroids have been described in paediatric literature.6–8
We discuss a very young child, aged 7.6 years, who developed a severe iatrogenic Cushing syndrome after long-term use of glucocorticoid ocular drops.
CASE PRESENTATION
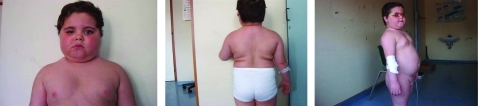
We describe the case of a male child, aged 7.6 yrs old, who presented to our Unit of Paediatric Endocrinology for evaluation of obesity. He was born at term by non-consanguineous parents. His birth weight was 2350 g (−2.3 SD) and birth length was 49.0 cm (−1.0 SD). His clinical history was unremarkable up to 7 years of age, when he had an accidental penetrating orbital injury on the right eye. Over the next 6 months he showed a progressive weight gain (10 kg). When he came to our observation, a clinical examination showed height 113.5 cm (−1.5 SD), target height 161.7 cm (−2.2 SD), weight 36.0 kg, ideal weight/height 185%, body mass index (BMI) 28 (+ 5 SD), blood pressure 110/90 mmHg (90th centile for age), cushingoid appearance, “moon face”, truncal obesity, “buffalo hump”, increased lanugo hair, supraclavicular fullness (fig 1), irritability and emotional lability. Over these 6 months he has been treated with oral betamethasone (0.5 mg/day) for 1 month and dexamethasone 2% ocular drops (2 hourly by day) up to now.
Figure 1.
The patient at the time of referral to our unit.
INVESTIGATIONS
The clinical appearance and the pharmacological history suggested the hypothesis of iatrogenic Cushing syndrome; endocrinological work-up was started and revealed a full suppression of pituitary-adrenal axis (table 1). His 24 h urine cortisol excretion was extremely low (<8 mcg) confirming severely impaired adrenal function. Glycometabolic status was investigated by oral glucose tolerance test that showed hyperinsulinism (basal insulin =22.30 mIU/ml, insulin peak =124 mIU/ml at 120 min after oral glucose load) but normal basal glucose level (80 mg/dl) and normal glucose tolerance (glycaemia =102 mg/dl at 120 min after oral glucose load).
Table 1.
Biochemical data at diagnosis
| Time (h) | 08.00 am | 04.00 pm | Midnight |
| Basal cortisol (mcg/dl) | <1.00 | <1.00 | <1.00 |
| ACTH (pg/ml) | <5.00 | <5.00 | <5.00 |
| Stimulated cortisol (mcg/dl) * | <1.00 | ||
*60 minutes after tetracosactrin stimulation test (synacthen, 250 mcg, intravenously). ACTH, adrenocorticotropic hormone.
OUTCOME AND FOLLOW-UP
These results confirmed the diagnosis of iatrogenic Cushing syndrome secondary to systemic absorption of ocular steroids. The ocular treatment was gradually discontinued and oral hydrocortisone was prescribed at a substitutive dose (10 mg/m2/day) with instructions to double the dose during acute infections or stressful events. The parents stopped the ocular treatment abruptly and did not administer the oral steroid in order to quicken the recovery, but 20 day after the boy experienced two adrenal crises. In that occasion, hydrocortisone by intramuscular route was necessary to normalise blood pressure and stopping vomiting.
The clinical and biochemical recovery was progressive and entirely reached 4 months after ocular steroid withdrawal (table 2; fig 2).
Table 2.
Clinical and biochemical data at diagnosis and during follow-up
| A | B | C | |
| Weight (SD) | +3.6 | +2.7 | +1.4 |
| Height (SD) | −1.5 | −1.6 | −1.4 |
| Weight reduction (kg) | – | 2.5 | 6.5 |
| Ideal weight/height (%) | 185 | 168 | 142 |
| Body mass index | 28 | 25 | 22 |
| Blood pressure (mmHg) | 110/90 | 100/70 | 95/55 |
| Basal cortisol (mcg/dl) * | <1.0 | 2.42 | 7.40 |
| Stimulated cortisol (mcg/dl)† | <1.0 | 3.02 | 20.00 |
| ACTH (pg/ml) | <5.0 | 36.70 | 39.00 |
| 17-OH-progesterone (ng/ml) | <0.1 | <0.1 | 0.20 |
| DHEAS (mcg/dl) | <0.20 | <0.10 | 0.20 |
| Delta-4-androstenedione (ng/ml) | <0.05 | <0.05 | 0.5 |
| Basal insulin (mIU/ml) | 22.30 | 6.57 | 4.27 |
*At 08.00 am; †60 minute after tetracosactrin stimulation test (synacthen 250 mcg, intravenously).
ACTH, adrenocorticotropic hormone; A, at diagnosis; B, 2 months after steroid withdrawal; C, 4 months after steroid withdrawal; DHEAS, dehydroepiandrosterone sulphate; SDS, standard deviation score.
Figure 2.
The patient 4 months after glucocorticoid ocular drops withdrawal.
DISCUSSION
Glucocorticoids are one of the most important and widely used drugs in clinical practice. The list of their indications is very long because they have a striking anti-inflammatory and immunosuppressive effect; they are the treatment of choice for common disorders such as allergic, dermatological, inflammatory and autoimmune diseases.9
Since their introduction in 1948, they have never been replaced with other drugs and new preparations have been synthesised over the years in order to strengthen the therapeutical power and minimise the side effects.2,10,11
Their successful use, in fact, may be limited by a broad range of adverse reactions. All the clinicians are aware that a prolonged use of systemic glucocorticoids at high doses can determine severe side effects and iatrogenic Cushing syndrome. This is a well-recognised clinical entity, whose diagnosis may be fairly obvious in the setting of treatment with high-dose, long-term and systemic administration of glucocorticoids but may be very difficult and may require high clinical suspicion in other clinical settings, such as local delivery of steroid (inhaled, dermatological, intra-articular and ocular treatment).12–14
Erroneously, most clinicians believe that the action of topical steroid is limited to the site of application/inflammation and that systemic absorption is very small or null. On the contrary, every topical steroid preparation may cause iatrogenic Cushing syndrome, especially when incorrectly used. Very few case reports regarding iatrogenic Cushing syndrome caused by topical steroids are reported in the paediatric literature and they are more frequently caused by dermatological and inhaled glucocorticoids.4,5,12–14 According to our knowledge, Cushing syndrome caused by ocular steroid drops is an exceptional event and only three case reports are cited in the Medline database to date6–8 in paediatric age and only one in adulthood.15
We believe that our case is worthy of mentioning because the awareness that ocular corticosteroids may produce severe systemic side effects is very scant among the clinicians.16 Ocular penetration of ocular corticoid drops depends upon drug concentration, chemical formulation and composition of the vehicle and, despite the development of new techniques and new vehicles, systemic adverse effects are still common. Mucous membranes are highly absorptive areas and conjunctiva, in particular, is extremely thin and vascular. Ocular drops can be absorbed by two different routes: the conjunctiva and the nasal mucosa where the lacrimal drainage system leads to tears. Moreover, the ocular corticosteroids do not undergo the metabolism by the portal system so intensive ocular treatment may reasonably produce greater effect than an equivalent dose administered by oral route.17
In our case, a very potent (dexamethasone) and high-concentrated agent (2%) has been used for a long time (6 months) and at an intensive regimen (2 hourly by day application); so it should be easy to suspect iatrogenic Cushing syndrome when the child started to gain weight but it did not happen and the correct diagnostic suspicion came late when most of the clinical signs had already developed.
The full suppression of pituitary-adrenal axis in our patient was confirmed by the hormonal status that showed undetectable levels of both basal and adrenocorticotropic hormone (ACTH)-stimulated cortisol, ACTH and urinary excretion cortisol levels but also by the clinical course; in fact, our patient experienced two adrenal crises when steroid treatment was stopped abruptly by the parents. The recovery was gradual and started from pituitary secretion, in fact ACTH was the first hormone that reached normal levels, followed by plasma cortisol and finally by urinary cortisol. This hormonal succession could suggest that adrenal gland is more sensitive to the action of steroids than pituitary is and its recovery is longer. The phase of pituitary-adrenal axis recovery has an unpredictable duration and is very unsteady: patients clinically stable might experience an adrenal crisis in the case of stressful situations. To prevent these life-threatening events, they should be carefully monitored, after steroid withdrawal, by regular biochemical follow-up until cortisol and ACTH levels return to baseline normal values.3 In case of stressful situations, the oral dose of steroid should be doubled or tripled and, if adrenal crisis or vomiting develop steroid, drugs should be administered by intravenous or intramuscular route.
Most clinicians recommend monitoring growth in children under steroid treatment in order to notice any change in growth velocity as a sign of steroid systemic absorption.18 In our case, height did not get worse because iatrogenic Cushing syndrome developed fast, over 6 months—a time span necessary to modify weight but not height percentile.
It is important to alert physicians that all forms of glucocorticoid delivery can cause Cushing syndrome, no ocular preparations excluded. This means that ocular drops should be used for a short time and under medical supervision and that withdrawal from long-term treatment can expose the patient to adrenal insufficiency; as a consequence the weaning-off should be carefully managed by skilful clinicians well-trained in endocrinology.
LEARNING POINTS
All formulations including ocular steroids can cause severe systemic side effects.
Glucocorticoid ocular drops should be used prudently because they can produce iatrogenic Cushing syndrome, in particular in paediatric age.
The weaning-off of topical steroid, ocular drops included, should be carefully managed for the potential risk of adrenal crisis.
Acknowledgments
We are grateful to Agostino Bartolone for excellent technical assistance.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Buttgereit F, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 2004; 50: 3408–17 [DOI] [PubMed] [Google Scholar]
- 2.Buttgereit F, Burmester GR, Lipworth BJ. Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet 2005; 365: 801–3 [DOI] [PubMed] [Google Scholar]
- 3.Hopkins RL, Leinung MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin N Am 2005; 34: 371–84 [DOI] [PubMed] [Google Scholar]
- 4.Homer JJ, Gazis TG. Cushing’s syndrome induced by betamethasone nose drops. BMJ 1999; 318: 1355. [PMC free article] [PubMed] [Google Scholar]
- 5.Atabek ME, Pirgon O, Unal E. Pituitary-adrenal axis suppression due to topical steroid administration in an infant. Pediatr Internat 2007; 49: 242–4 [DOI] [PubMed] [Google Scholar]
- 6.Ozerdem U, Levi L, Cheng L, et al. Systemic toxicity of topical and periocular corticosteroid therapy in an 11-year-old male with posterior uveitis. Am J Ophthalmol 2000; 130: 240–1 [DOI] [PubMed] [Google Scholar]
- 7.Chiang MY, Sarkar M, Koppens JM, et al. Exogenous Cushing’s syndrome and topical ocular steroids. Eye 2006; 20: 725–7 [DOI] [PubMed] [Google Scholar]
- 8.Steelman J, Kappy M. Adrenal suppression and growth retardation from ocular corticosteroids. J Pediatr Ophthalmol Strabismus 2001; 38: 177–8 [DOI] [PubMed] [Google Scholar]
- 9.Hudson AR, Roach SL, Higuchi RI. Recent developments in the discovery of selective glucocorticoid receptor modulators (SGRMs). Curr Top Med Chem 2008; 8: 750–65 [DOI] [PubMed] [Google Scholar]
- 10.Kirwan JR, Balint G, Anniversary Szebenyi B: 50 years of glucocorticoid treatment in rheumatoid arthritis. Rheumatology 1999; 38: 100–2 [DOI] [PubMed] [Google Scholar]
- 11.McMaster A, Ray DW. Drug insight: selective agonists and antagonists of the glucocorticoid receptor. Nat Clin Pract Endocrinol Metab 2008; 4: 91–101 [DOI] [PubMed] [Google Scholar]
- 12.Ozon A, Cetinkaya S, Alikasifoglu A, et al. Inappropriate use of potent topical glucocorticoids in infants. J Pediatr Endocrinol Metab 2007; 20: 219–25 [DOI] [PubMed] [Google Scholar]
- 13.Findlay CA, Macdonald JF, Wallace AM, et al. Childhood Cushing’s syndrome induced by betamethasone nose drops, and repeat prescriptions. BMJ 1998; 317: 739–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gen R, Akbay E, Sezer K. Cushing syndrome caused by topical corticosteroid: a case report. Am J Med Sci 2007; 333: 173–4 [DOI] [PubMed] [Google Scholar]
- 15.Afandi B, Toumeh MS, Saadi HF. Cushing’s syndrome caused by unsupervised use of ocular glucocorticoids. Endocr Pract 2003; 9: 526–9 [DOI] [PubMed] [Google Scholar]
- 16.Salminen L: Review: systemic absorption of topically applied ocular drugs in humans. J Ocular Pharmacol 1990; 6: 243–9 [DOI] [PubMed] [Google Scholar]
- 17.McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Safety 2002; 25: 33–55 [DOI] [PubMed] [Google Scholar]
- 18.Malozowski S, Purucker M, Worobec A. Cushing’s syndrome induced by betamethasone nose drops. Children taking intranasal corticosteroids should be monitored for growth retardation. BMJ 1999; 318: 1355. [PubMed] [Google Scholar]