Abstract
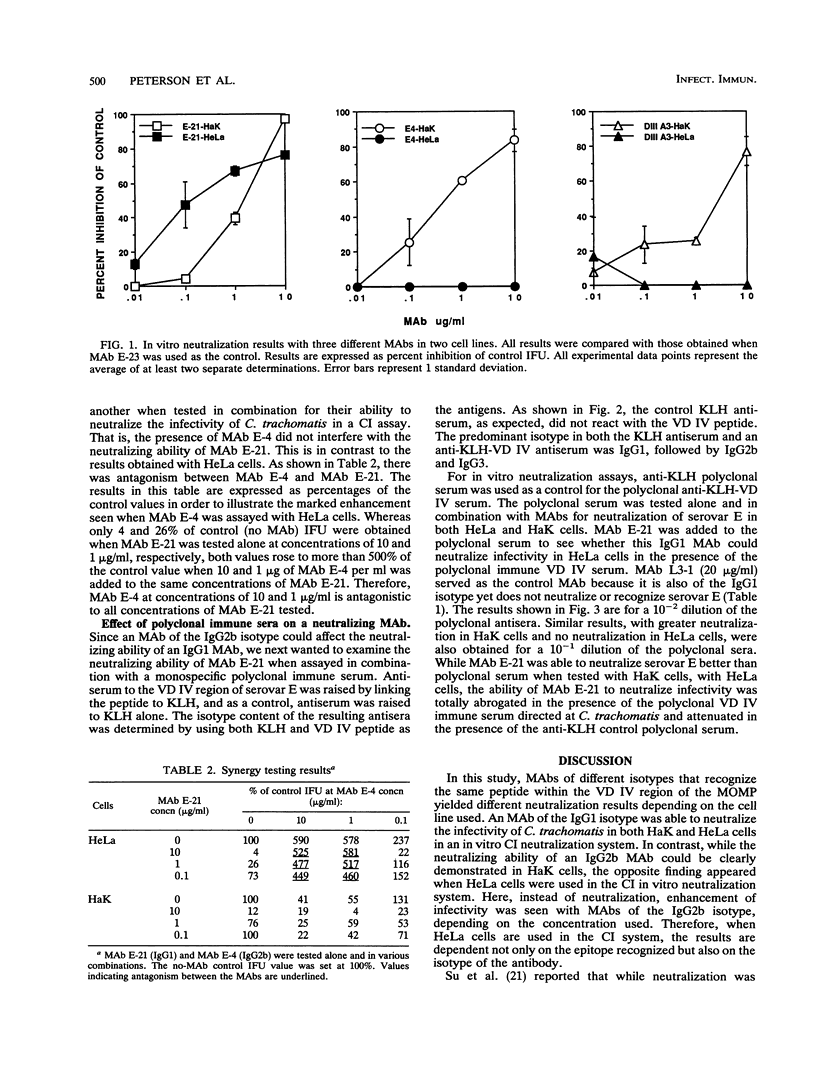
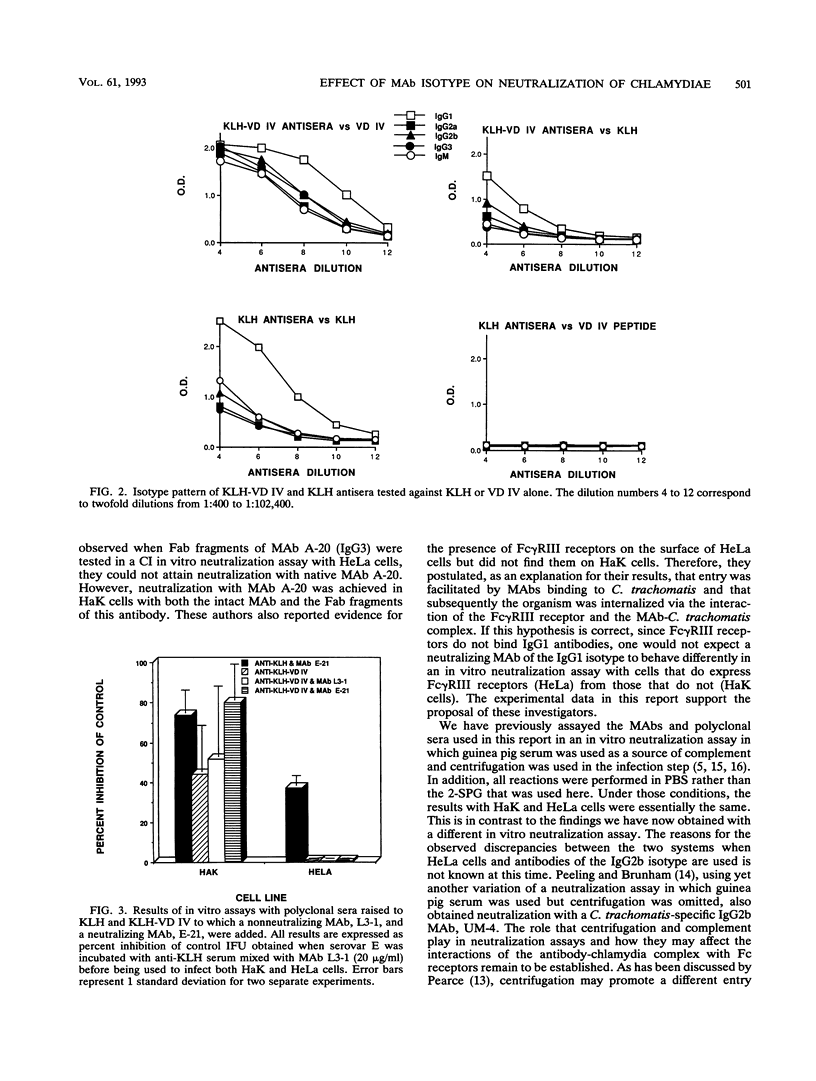
Monoclonal antibodies (MAbs) E-4, E-21, and DIII A3, which recognize the same or similar overlapping peptides in the variable domain IV of the major outer membrane protein of Chlamydia trachomatis but differ in isotype, were used in a complement-independent (CI) in vitro neutralization assay. These MAbs had previously been shown to neutralize chlamydial infectivity in HeLa 229 cells in a complement-dependent assay. In this report, all three MAbs neutralized chlamydial infectivity in HaK cells in a CI assay. However, when HeLa cells were used as the host cell, MAb E-4 (immunoglobulin G2b [IgG2b]) and MAb DIII A3 (IgG2b) failed to neutralize infectivity, while MAb E-21 (IgG1) neutralized chlamydial infectivity. These findings are consistent with the proposal that because of the presence of Fc gamma RIII receptors, HeLa cells facilitate infectivity and thus block neutralization through the uptake of an IgG2b-chlamydia complex. Since Fc gamma RIII receptors do not bind or bind poorly to IgG1, neutralization of C. trachomatis by MAb E-21 in HeLa cells is also corroborative evidence for the role of Fc gamma RIII receptors in this interaction. A fivefold enhancement of infectivity was seen when 10 and 1 micrograms of MAb E-4 per ml were tested in a CI assay with HeLa cells. In performing CI neutralization synergy studies in HeLa cells with MAbs E-4 and E-21, antagonism between MAbs E-4 and E-21 was observed at MAb E-4 concentrations of 10 and 1 micrograms/ml for all concentrations of MAb E-21 tested (10 to 0.1 micrograms/ml). When HaK cells were used in the same studies, no antagonism between the MAbs was found. In addition, when HeLa cells were used in a CI assay, polyclonal serum raised to a peptide representing variable domain IV of the major outer membrane protein inhibited the neutralizing ability of MAb E-21. The blocking of neutralization and the enhancement of infectivity by chlamydia-specific antibodies seen in this investigation with HeLa cells may have important clinical implications for developing preventive strategies for chlamydial infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Whiting C. V. Induction of MHC class II gene products in rat intestinal epithelium during graft-versus-host disease and effects on the immune function of the epithelium. Immunology. 1992 Feb;75(2):366–371. [PMC free article] [PubMed] [Google Scholar]
- Brandt W. E., McCown J. M., Top F. H., Jr, Bancroft W. H., Russell P. K. Effect of passage history on dengue-2 virus replication in subpopulations of human leukocytes. Infect Immun. 1979 Nov;26(2):534–541. doi: 10.1128/iai.26.2.534-541.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Cheng X., Pal S., de la Maza L. M., Peterson E. M. Characterization of the humoral response induced by a peptide corresponding to variable domain IV of the major outer membrane protein of Chlamydia trachomatis serovar E. Infect Immun. 1992 Aug;60(8):3428–3432. doi: 10.1128/iai.60.8.3428-3432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Mason T. J., Rodda S. J. Cognitive features of continuous antigenic determinants. J Mol Recognit. 1988 Feb;1(1):32–41. doi: 10.1002/jmr.300010107. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Homsy J., Meyer M., Tateno M., Clarkson S., Levy J. A. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science. 1989 Jun 16;244(4910):1357–1360. doi: 10.1126/science.2786647. [DOI] [PubMed] [Google Scholar]
- Mady B. J., Erbe D. V., Kurane I., Fanger M. W., Ennis F. A. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J Immunol. 1991 Nov 1;147(9):3139–3144. [PubMed] [Google Scholar]
- Pearce J. H. Early events in chlamydial infection. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):325–332. doi: 10.1016/s0769-2609(86)80043-6. [DOI] [PubMed] [Google Scholar]
- Peeling R. W., Brunham R. C. Neutralization of Chlamydia trachomatis: kinetics and stoichiometry. Infect Immun. 1991 Aug;59(8):2624–2630. doi: 10.1128/iai.59.8.2624-2630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Cheng X., Markoff B. A., Fielder T. J., de la Maza L. M. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect Immun. 1991 Nov;59(11):4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., de la Maza L. M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990 Jun 11;18(11):3414–3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet. 1988 Apr 9;1(8589):790–794. doi: 10.1016/s0140-6736(88)91657-1. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Kramer M. D., Eckerskorn C., Museteanu C., Müller-Hermelink H. K., Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991 Jul;164(1):123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- Su H., Spangrude G. J., Caldwell H. D. Expression of Fc gamma RIII on HeLa 229 cells: possible effect on in vitro neutralization of Chlamydia trachomatis. Infect Immun. 1991 Oct;59(10):3811–3814. doi: 10.1128/iai.59.10.3811-3814.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Ennis F. A. FcR-mediated enhancement of HIV-1 infection by antibody. AIDS Res Hum Retroviruses. 1990 Aug;6(8):999–1004. doi: 10.1089/aid.1990.6.999. [DOI] [PubMed] [Google Scholar]
- Takeda A., Tuazon C. U., Ennis F. A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988 Oct 28;242(4878):580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- el-Asrar A. M., Emarah M. H., Van den Oord J. J., Geboes K., Desmet V., Missotten L. Conjunctival epithelial cells infected with Chlamydia trachomatis express HLA-DR antigens. Br J Ophthalmol. 1989 May;73(5):399–400. doi: 10.1136/bjo.73.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]


