Abstract
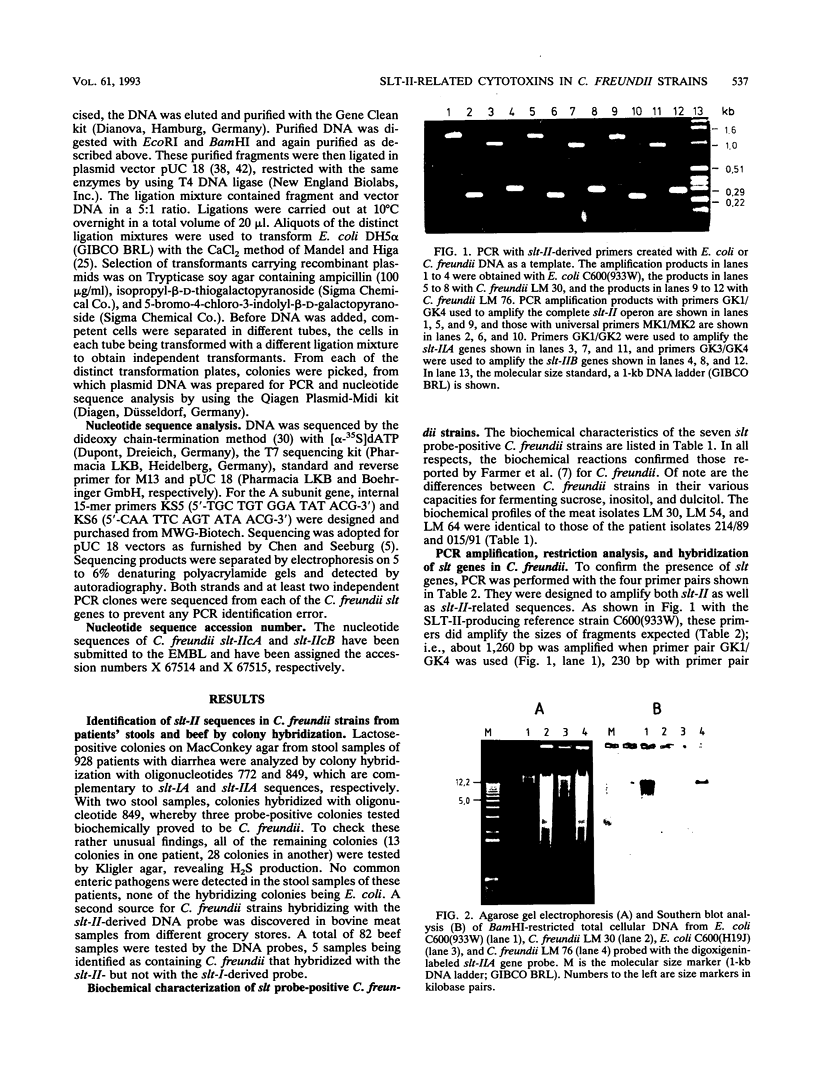
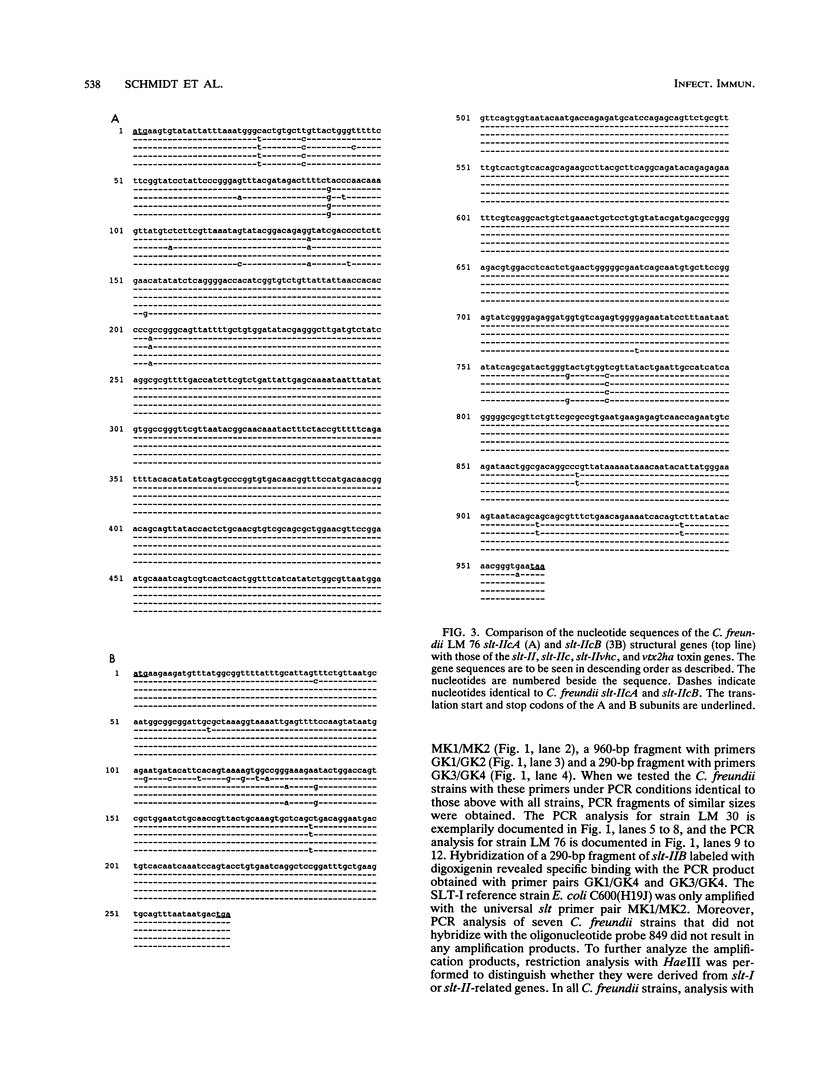
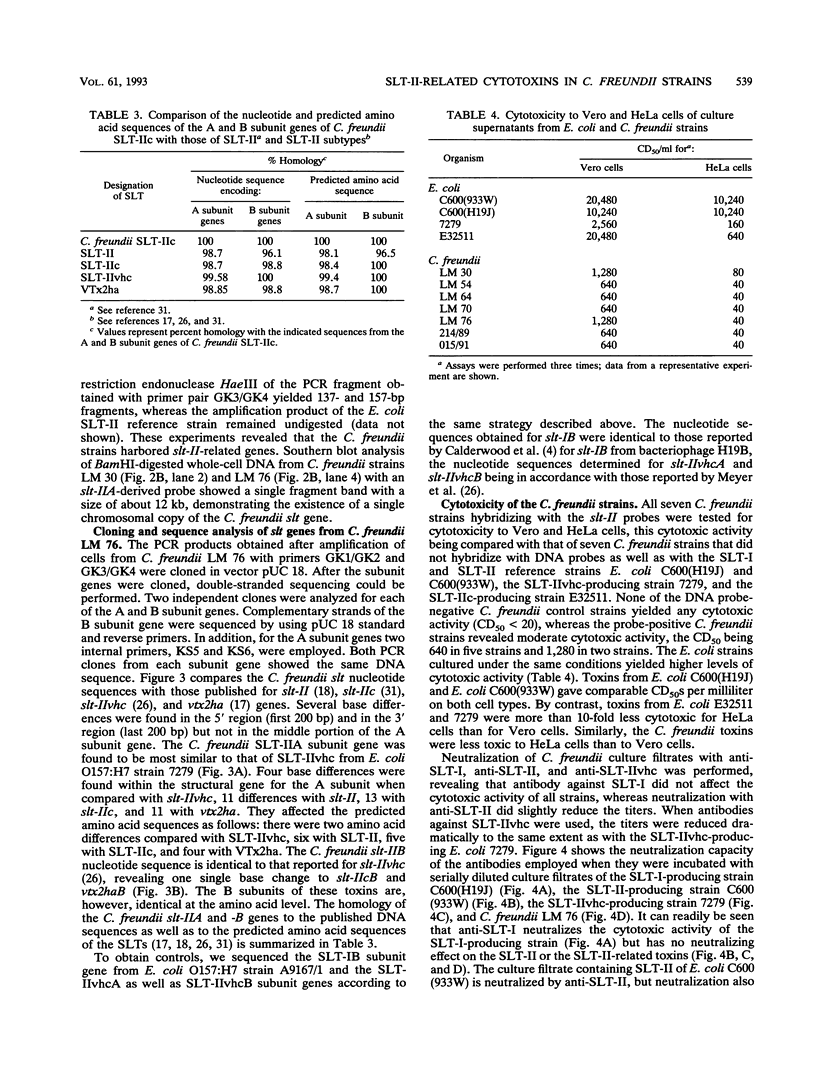
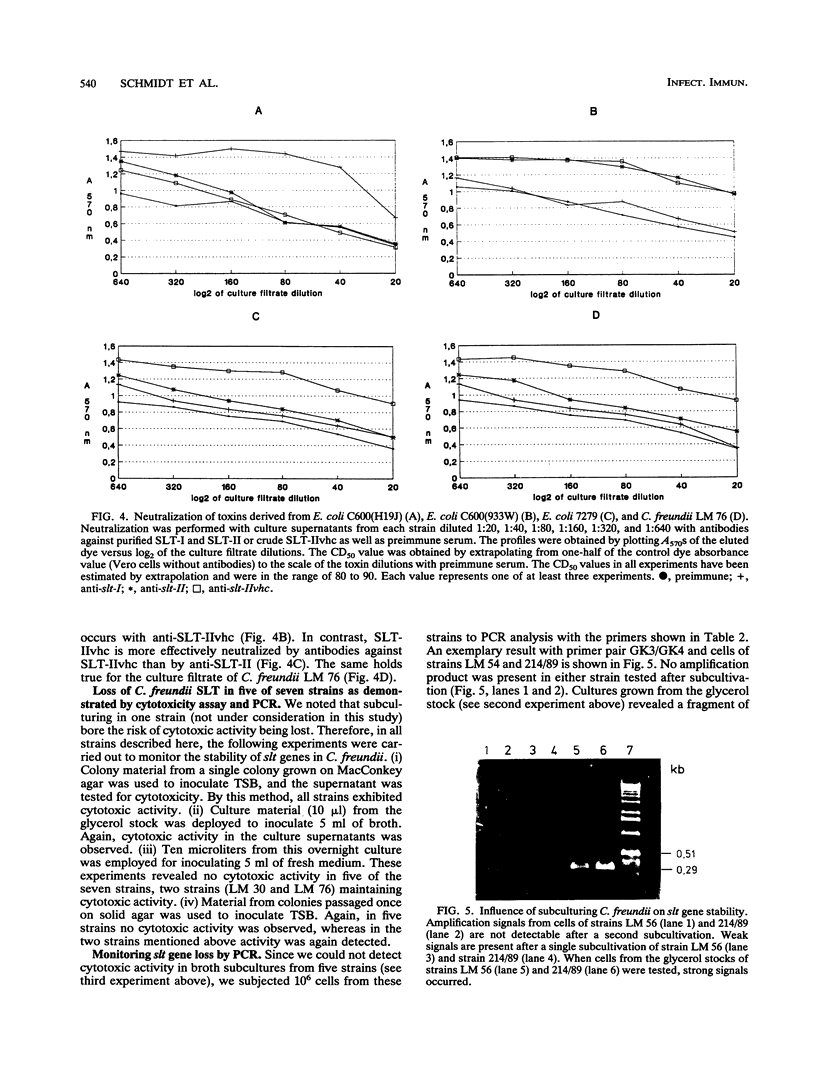
By hybridizing colonies grown from 928 individual stool samples of patients suffering from diarrhea with oligonucleotide probes 772 and 849 complementary to Shiga-like toxin I (SLT-I) and SLT-II gene sequences, respectively, we identified two strains that hybridized with probe 849, which biochemical identification revealed as Citrobacter freundii. An additional five slt-II probe-positive isolates were screened from 81 beef samples. Polymerase chain reaction analysis and restriction of amplified products provided evidence for slt-II-related genes in all seven strains. From C. freundii LM 76, the genes encoding the A and B subunits were cloned in pUC 18 vectors and sequenced. The gene encoding the A subunit differed from that of Escherichia coli slt-IIvhc in 4 bases, resulting in two amino acid residue differences. In 11, 13, and 11 nucleotides, differentiation of slt-IIA, slt-IIcA, and vtx2haA, respectively, was found. These differences affected the predicted amino acid sequence as follows: there were six amino acid differences with SLT-IIA, five with SLT-IIcA, and four with VTx2haA. The nucleotide sequence of the gene encoding the B subunit is identical to slt-IIvhcB and differed from slt-IIcB and vtx2haB by only a single nucleotide base, but this resulted in a predicted amino acid sequence identical to that reported for these toxins. We therefore termed the toxin genes C. freundii slt-IIcA and slt-IIcB. Culture filtrates inoculated with material from the colonies from primary cultures were cytotoxic to Vero cells. Neutralization assays with antisera to E. coli SLT-I, SLT-II, and SLT-IIvhc revealed that antibodies against SLT-IIvhc reduced the C. freundii cytotoxic activity specifically and to the same degree as with the E. coli SLT-IIvhc control strain. In five of the seven strains tested, subcultivation on both a liquid or solid medium resulted in loss of cytotoxic activity. With polymerase chain reaction, we demonstrated that loss of cytotoxic activity ran parallel with the loss of slt genes. These data demonstrate the intergeneric occurrence of SLT-II-related toxins, which may well be a new marker of enteropathogenicity in C. freundii. Our findings that the toxin genes belong to the slt-II family plus their evident instability in the majority of strains should help pave the way to a better understanding of their role in diarrhea or food poisoning.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Böhm H., Karch H. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1992 Aug;30(8):2169–2172. doi: 10.1128/jcm.30.8.2169-2172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Auclair F., Donohue-Rolfe A., Keusch G. T., Mekalanos J. J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Gannon V. P., Teerling C., Masri S. A., Gyles C. L. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J Gen Microbiol. 1990 Jun;136(6):1125–1135. doi: 10.1099/00221287-136-6-1125. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A., Capano G., Malamisura B., Alessio M., Guandalini S., Rubino A. Production of Escherichia coli STa-like heat-stable enterotoxin by Citrobacter freundii isolated from humans. J Clin Microbiol. 1987 Jan;25(1):110–114. doi: 10.1128/jcm.25.1.110-114.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A., Giannella R., Thompson M. R. Citrobacter freundii produces an 18-amino-acid heat-stable enterotoxin identical to the 18-amino-acid Escherichia coli heat-stable enterotoxin (ST Ia). Infect Immun. 1989 Feb;57(2):649–652. doi: 10.1128/iai.57.2.649-652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Dickens M. D., Wenzel R. P., Kapikian A. Z. Toxigenic bacterial diarrhea: nursery outbreak involving multiple bacterial strains. J Pediatr. 1976 Dec;89(6):885–891. doi: 10.1016/s0022-3476(76)80591-4. [DOI] [PubMed] [Google Scholar]
- Gunzer F., Böhm H., Rüssmann H., Bitzan M., Aleksic S., Karch H. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992 Jul;30(7):1807–1810. doi: 10.1128/jcm.30.7.1807-1810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., De Grandis S. A., MacKenzie C., Brunton J. L. Cloning and nucleotide sequence analysis of the genes determining verocytotoxin production in a porcine edema disease isolate of Escherichia coli. Microb Pathog. 1988 Dec;5(6):419–426. doi: 10.1016/0882-4010(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Hii J. H., Gyles C., Morooka T., Karmali M. A., Clarke R., De Grandis S., Brunton J. L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991 Dec;29(12):2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. R., Degener C. E., Barnes W. G. Clinical significance of citrobacter isolates. Am J Clin Pathol. 1978 Jul;70(1):37–40. doi: 10.1093/ajcp/70.1.37. [DOI] [PubMed] [Google Scholar]
- Ito H., Terai A., Kurazono H., Takeda Y., Nishibuchi M. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb Pathog. 1990 Jan;8(1):47–60. doi: 10.1016/0882-4010(90)90007-d. [DOI] [PubMed] [Google Scholar]
- Karch H., Bitzan M., Pietsch R., Stenger K. O., von Wulffen H., Heesemann J., Düsing R. Purified verotoxins of Escherichia coli O157:H7 decrease prostacyclin synthesis by endothelial cells. Microb Pathog. 1988 Sep;5(3):215–221. doi: 10.1016/0882-4010(88)90024-1. [DOI] [PubMed] [Google Scholar]
- Karch H., Meyer T. Evaluation of oligonucleotide probes for identification of shiga-like-toxin-producing Escherichia coli. J Clin Microbiol. 1989 Jun;27(6):1180–1186. doi: 10.1128/jcm.27.6.1180-1186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Meyer T., Rüssmann H., Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992 Aug;60(8):3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Meyer T. Single primer pair for amplifying segments of distinct Shiga-like-toxin genes by polymerase chain reaction. J Clin Microbiol. 1989 Dec;27(12):2751–2757. doi: 10.1128/jcm.27.12.2751-2757.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky B. A., Hook E. W., 3rd, Smith A. A., Plorde J. J. Citrobacter infections in humans: experience at the Seattle Veterans Administration Medical Center and a review of the literature. Rev Infect Dis. 1980 Sep-Oct;2(5):746–760. doi: 10.1093/clinids/2.5.746. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meyer T., Karch H., Hacker J., Bocklage H., Heesemann J. Cloning and sequencing of a Shiga-like toxin II-related gene from Escherichia coli O157:H7 strain 7279. Zentralbl Bakteriol. 1992 Jan;276(2):176–188. doi: 10.1016/s0934-8840(11)80004-6. [DOI] [PubMed] [Google Scholar]
- Nestorescu N., Szégli L., Popovici M., Dima V. Untersuchung einiger hoch-pathogener atypischer Citrobacter-Stämme. 2. Cytotoxische Wirkung der Kulturfiltrate. Zentralbl Bakteriol Orig. 1966 Jan;199(1):46–52. [PubMed] [Google Scholar]
- Newland J. W., Strockbine N. A., Miller S. F., O'Brien A. D., Holmes R. K. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985 Oct 11;230(4722):179–181. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C. K., McKee M. L., O'Brien A. D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect Immun. 1991 Mar;59(3):1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlák J. Present knowledge and aspects of Citrobacter. Curr Top Microbiol Immunol. 1973;62:41–59. doi: 10.1007/978-3-642-65772-6_2. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Jackson M. P., Sung L. M., Holmes R. K., O'Brien A. D. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J Bacteriol. 1988 Mar;170(3):1116–1122. doi: 10.1128/jb.170.3.1116-1122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler S. D., Johnson W. M., Lior H., Wang G., Rozee K. R. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1991 Jul;29(7):1339–1343. doi: 10.1128/jcm.29.7.1339-1343.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wadström T., Aust-Kettis A., Habte D., Holmgren J., Meeuwisse G., Möllby R., Söderlind O. Enterotoxin-producing bacteria and parasites in stools of Ethiopian children with diarrhoeal disease. Arch Dis Child. 1976 Nov;51(11):865–870. doi: 10.1136/adc.51.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., Jackson M. P., Samuel J. E., Holmes R. K., O'Brien A. D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988 Sep;170(9):4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willshaw G. A., Smith H. R., Scotland S. M., Rowe B. Cloning of genes determining the production of vero cytotoxin by Escherichia coli. J Gen Microbiol. 1985 Nov;131(11):3047–3053. doi: 10.1099/00221287-131-11-3047. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]