Abstract
Here we report a patient with Zellweger syndrome, who presented at the age of 3 months with icterus, dystrophy, axial hypotonia, and hepatomegaly. Abnormal findings of metabolic screening tests included hyperbilirubinaemia, hypoketotic dicarboxylic aciduria, increased C26:0 and decreased C22:0 plasma levels, and strongly reduced plasmalogen concentrations. In fibroblasts, both peroxisomal α- and β-oxidation were impaired. Liver histology revealed bile duct paucity, cholestasis, arterial hyperplasia, very small branches of the vena portae, and parenchymatic destruction. Immunocytochemical analysis of cultured fibroblasts demonstrated that the cells contain peroxisomal remnants lacking apparent matrix protein content and PEX14, a central membrane component of the peroxisomal matrix protein import machinery. Transfection of fibroblasts with a plasmid coding for wild-type PEX14 restored peroxisomal matrix protein import. Mutational analysis of this gene revealed a genomic deletion leading to the deletion of exon 3 from the coding DNA (c.85-?_170+?del) and a concomitant change of the reading frame (p.[Ile29_Lys56del;Gly57GlyfsX2]).
BACKGROUND
Human peroxisomes harbour a number of essential metabolic functions including ether phospholipid biosynthesis, fatty acid β-oxidation, fatty acid α-oxidation, and glyoxylate detoxification.1,2 Loss of one or several of these functions leads to a wide variety of genetically heterogeneous metabolic disorders which are usually subdivided into two groups: the single peroxisomal enzyme deficiencies, and the peroxisome biogenesis disorders (PBDs).2–4 Until now, 10 single peroxisomal enzyme deficiencies have been identified.2 In addition, 13 genes (termed PEX genes) known to be associated with PBDs have been (at least partially) molecularly characterised:3,4 PEX5 and PEX7 encode the cycling signal recognition factors for newly synthesised peroxisomal matrix proteins containing a C-terminal (PTS1) and N-terminal (PTS2) peroxisome targeting signal, respectively; PEX13 and PEX14 encode the peroxisomal membrane proteins (PMPs) constituting the PTS receptor docking complex; PEX2, PEX10, and PEX12 encode PMPs acting downstream of the PTS receptor docking event; PEX1, PEX6, and PEX26 encode peroxins involved in the release of PEX5 from the peroxisomal membrane to the cytosol; and PEX3, PEX16, and PEX19 encode key players in PMP biogenesis.
The severe and often fatal cerebro-hepato-renal syndrome of Zellweger (ZS) (MIM 214100 [OMIM]) can be considered as the archetypical PBD (MIM 601539 [OMIM]).5 Patients suffering from this disease clinically manifest craniofacial abnormalities, severe hypotonia, psychomotor retardation, hepatomegaly with prolonged jaundice, liver dysfunctions, renal cysts, bone stippling of multiple joints, and neuronal migration defects.3,4 Biochemically these patients are usually characterised by elevated very long chain fatty acid (VLCFA) and low plasmalogen levels.4 Mutations in PEX1 are the genetic cause of PBDs in approximately 70% of all patients.4 Here we report the identification of a novel PEX14 mutation. Until now, only one patient with a PEX14 deficiency (c.553C>T; p.Q185X; MIM 601791 [OMIM]) has been documented.6
PEX14 was initially identified as a peroxisomal docking factor for the PTS1 receptor PEX5.7–9 More recently, it was proposed that PEX14 is also the site from which PEX5 leaves the peroxisomal compartment.10 The PEX14 gene has been assigned to chromosome 1p36.22,11 and in silico mapping of PEX14 by alignment of the reference sequence NM_004565 [GenBank] to the UCSC chromosome 1 draft sequence displayed a gene extending over a large genomic area (approximately 155.8 kb) consisting of nine different exons.12
CASE PRESENTATION
The boy was born after 36 weeks of pregnancy to related Pakistani parents. Pregnancy was established by in vitro fertilisation and was uneventful except for a moderate third trimester vaginal blood loss. Birth weight, length and head circumference were 2120 g (<p3), 48 cm (p3) and 35 cm (<p3), respectively. The mother has a mentally retarded sib (no further details available) and her sister had three children (also from a consanguineous marriage), who all died (one girl in the first postnatal days, one boy at the age of 3–4 months, and one boy at the age of 10 months with icterus and intracerebral haemorrhage). The patient showed prolonged neonatal hyperbilirubinaemia responding to phototherapy.
At 3 months of age, the patient presented with icterus, dystrophy, facial dysmorphy (slight dolichocephaly, triangular face, and large normotonic fontanel) (fig 1A), striking axial hypotonia, and hepatomegaly (5 cm below the costal margin). His gaze fixed and followed normally, he had normal spontaneous movements in all limbs, but deep tendon reflexes were absent. Biochemical testing showed hyperbilirubinaemia (total/direct bilirubin: 5.35/3.73 mg/dl; normal 0.2–1.0/⩽0.5 mg/dl) without evidence for haemolysis. Serum alkaline phosphatase was slightly increased (1466 U/l; normal <1107 U/l) and transaminases were about five times the upper limit of normal (ULN). Blood count, routine chemistry and karyotype were normal, and serology for hepatotropic viruses was negative. Metabolic screening at this age showed: (1) normal plasma amino acids, phytanic acid, pristanic acid, and C24:0 levels; (2) increased C26:0 (11.45 μM; normal 0.30–1.30 μM) values, and (3) decreased C22:0 (17.0 μM; normal 28.0–96.0 μM) and plasmalogen concentrations. The plasmalogen levels were measured as the ratio of C16:0 dimethylacetal (DMA) to C16:0 (0.02; normal: 0.065–0.095) and the ratio of C18:0 DMA to C18:0 (0.00; normal: 0.180–0.220). Urine organic acid analysis showed hypoketotic dicarboxylic aciduria, without glycine conjugates but with increased concentrations of adipic acid (>1.5× ULN), suberic acid (>39× ULN), sebacic acid (>109× ULN), 4-OH-phenyllactic acid (>9× ULN), 3-OH-sebacic acid (>6× ULN), 3-OH suberic acid (>5× ULN), 5-OH-hexanoic acid (>2× ULN), and 7-OH-octanoic acid (>82× ULN).
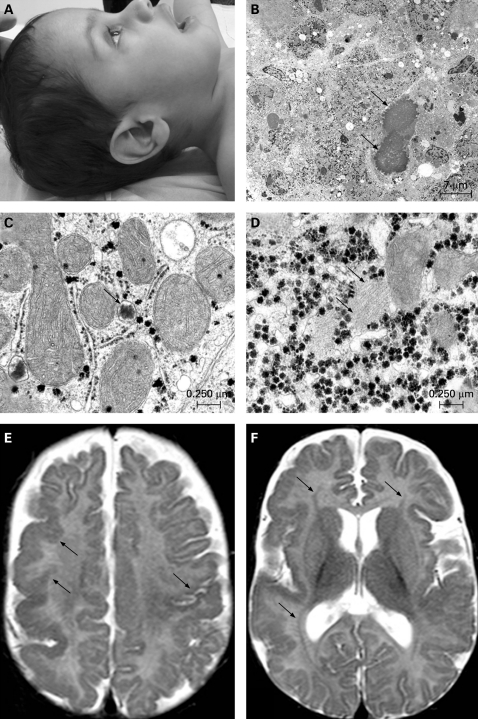
Figure 1. Facial profile, liver ultrastructure, and magnetic resonance imaging (MRI) brain scan of the patient.
(A) Lateral view of the patient’s head at the age of 20 months, showing slight megalocephaly and low implanted ears. (B) Electron microscopy of the liver showing severe parenchymatic destruction and cholestasis (dilated cholestatic bile canaliculi: arrows). (C) In the cytoplasm of most hepatocytes, peroxisomes were absent; only abnormal small bodies resembling incompletely developed peroxisomes or peroxisomal ghosts (arrow) are observed. (D) Rare areas with fibrillar material (arrow) are seen in the hyaloplasm of some hepatocytes. (E) Polymicrogyria is seen in the right frontal and parietal cortex and in the left rolandic cortex (arrows). (F) Bilateral symmetrical laminar heterotopia is seen as a thin line of grey matter between the ventricle and the cortex in the frontal and parietal lobe (arrows).
Radiological examination of the skeleton was normal. Ophthalmological examination showed posterior embryotoxon. Cerebral ultrasound and electroencephalography were normal. Histological examination of a liver biopsy showed paucity of intrahepatic bile ducts as well as arterial hyperplasia and very small branches of the vena portae. Electron microscopy analysis of the liver revealed severe parenchymatic destruction and cholestasis (fig 1B), and only abnormal small bodies resembling incompletely developed peroxisomes could be found (fig 1C). Rare areas with filamentous material were seen in the hyaloplasm of the hepatocytes (fig 1D). Percutaneous cholangiography was normal.
The patient was treated with fat soluble vitamin supplements, ursodeoxycholic acid (5–10 mg/kg) and prokinetics, and received Heparon as formula, initially through nasogastric tube feeding. Brain magnetic resonance imaging (MRI) at 5 months and computed tomography (CT) at 13 months showed a polymicrogyria-type of cortical developmental abnormality in the right frontal and parietal cortex and left Rolandic cortex (fig 1E), and bilateral zones of laminar heterotopia (fig 1F). Cerebellum and brain stem were normal.
At the age of 21 months, the patient’s weight was 9.3 kg (<p3), length 78 cm (p3) and head circumference 48 cm (p10–25). He had lost vision (from 16 months on), showed generalised hypotonia, and had no spontaneous movements anymore. He was still mainly drinking formula, and only occasionally eating fruit or vegetable mash (pristanic/phytanic low diet). He responded to pain and occasionally demonstrated a social smile. Biochemically, the cholestasis had completely disappeared (normal bilirubin and alkaline phosphatase) but transaminases had remained high (5–10× ULN). Plasma phytanic acid was at the upper limit of normal (8.61 μM; normal 0.37–8.62 μM) and pristanic acid increased (7.7 μM; normal 0–4 μM) from the age of 14 months on. Plasmalogen content of erythrocytes was still low (C16:0-dimethylacetal/C16:0 =0.011; C18:0-dimethylacetal/C18:0 =0.031; plasmalogen/total phospholipids =0.025). The patient was admitted to the hospital on two separate occasions since his diagnosis, once for suspected epileptic seizure (unresponsiveness, cyanosis) at 13 months, and once for pneumonia at 18 months. Currently, he is being cared for by his parents at home.
INVESTIGATIONS
The patient’s parents signed a written informed consent form, approved by the institution’s ethical committee. A percutaneous liver biopsy and a skin punch biopsy were taken from the patient. Control skin fibroblasts were kindly provided by Dr G Matthys (CME, K U Leuven). For electron microscopy, small fragments of the liver biopsy were immediately fixed in 2.5% glutaraldehyde (in 0.1 M phosphate buffer) at 4°C overnight. After 1 h of post-fixation in 1% osmium tetroxide (in 0.1 M phosphate buffer) at 4°C, the samples were dehydrated in graded series of alcohol and embedded in epoxy resin. Ultra-thin sections of 50–60 nm were cut, stained with uranyl acetate and lead citrate and examined at 50 kV using a Zeiss EM 900 electron microscope. Images were recorded digitally with a Jenoptik Progress C14 camera system operated by using Image-Pro express software.
Fibroblast studies
Degradation and esterification of fatty acids by cultured fibroblasts were measured essentially as described before.13,14 For transfections and (immuno)fluorescence microscopy, the cells were cultured and processed as described elsewhere.15,16 Fluorescence was monitored with a CellM imaging station (Olympus) equipped with U-MNUA2, U-MNIBA3, and U-MWIY2 fluorescence mirror units.
Antibodies
To raise a mouse polyclonal antiserum against catalase, the bovine protein (Boehringer) was purified by size exclusion chromatography using a Superdex HiLoad 16/60 column (GE Healthcare Life Sciences). Next, the purified protein was emulsified with an equal amount of Freund’s complete adjuvant (priming dose) or Freund’s incomplete adjuvant (successive boosting doses) and injected into the peritoneal cavity of a mouse (injection volume 0.2 ml; catalase concentration 500 μg/ml). Animal care approval was granted by the local institutional ethics committee. The rabbit antibodies against HsPEX13, HsPEX14, and peroxisomal thiolase are described elsewhere.15,17 The rabbit anti-PMP70 and anti-glutamate dehydrogenase (GDH) antisera were from Zymed Laboratories and Rockland, respectively. All secondary antibodies were from Sigma.
Plasmids and strains
The oligonucleotides (Eurogentec) synthesised for this study are compiled in supplementary table 1. The bicistronic expression plasmid coding for EGFP-PTS1 and non-tagged PEX14 was constructed by exchanging the EGFP-cassette in pIRES2-EGFP (Clontech) for EGFP-PTS1 (primers: pIRES2-EGFPfw, pIRES2-EGFP-KSKL), and inserting the cDNA coding for non-tagged PEX14 (template: pMF120;15 primers: HsPex14.2, HsPex14.3) into the multiple cloning site. The plasmid pKG122 has been described elsewhere.18
Table 1. Biochemical measurements in cultured skin fibroblasts.
| Parameter | Substrate | Patient | Mean (SE) for controls | |||
| Oxidation | Esterification | O/E | Oxidation | O/E | ||
| Mitochondrialβ-oxidation | (1-14C)palmitic acid | 3.52 | 19.10 | 0.185 | 2.64 (0.49) | 0.123 (0.024) |
| 2.98 | 18.20 | 0.164 | (12) | (8) | ||
| Peroxisomalβ-oxidation | 2-methyl-(1-14C)-hexadecanoic acid | 0.291 | 9.28 | 0.031 | 23.4 (2.36) | 2.43 (0.32) |
| 0.151 | 12.00 | 0.013 | (14) | (4) | ||
| (1-14C)-lignoceric acid | 0.141 | 1.24 | 0.113 | 0.603 (0.202) | 0.305 (0.046) | |
| 0.126 | 1.26 | 0.101 | (6) | (5) | ||
| Peroxisomalα-oxidation | 3-methyl-(1-14C)-hexadecanoic acid | NM | 15.90 | 0.000 | 2.40 (0.31) | 0.081 (0.015) |
| 0.035 | 12.80 | 0.003 | (15) | (4) | ||
Oxidation (in nmol/24 h/mg protein) represents the sum of both labelled CO2 and acid soluble metabolites formed during 20–24 h incubations with 4 µM 1-14C-labelled fatty acids or CO2 plus formate for 3-methylhexadecanoic acid; esterification (in nmol/24 h/mg protein) represents the label recovered in triglycerides and phospholipids. For the patient, values obtained from two separate incubations are given. For the controls, the number of separate incubations is given between brackets. As reported before,13 uptake of fatty acids by monolayers is not linearly related to the number of cells (confluency), hence the ratio oxidation/esterification (O/E) is more accurate to document deficiencies. NM, not measurable; SE, standard error of the mean.
RNA isolation and RT-PCR
The RNAqueous-4PCR Kit (Ambion) was used to isolate total RNA from patient and control fibroblasts. The HsPEX14 mRNA was copied into its complementary DNA sequence by employing the RETROscript Kit (Ambion) in combination with oligo(dT) primers. Subsequently, the PEX14 open reading frame was amplified by polymerase chain reaction (PCR) using Pfx DNA polymerase (Invitrogen) and four pairs of PEX14-specific primers. The corresponding PCR products were cloned yielding the following plasmids: pSH17, a pEGFP-C1 derivative encoding full length PEX14 (primer pair: HsPex14.1, HsPex14.rvSalI); pSH18, a pEGFP-C1 derivative encoding PEX14(1-200) (primer pair: HsPex14.fwSalI, HsPex14.rv1); pSH19, a pGAD424 derivative encoding PEX14(138-377) (primer pair: HsPex14.fw1, HsPex14.2); and pSH20, a pGEX-4T-3 derivative encoding full length PEX14 (primer pair: HsPex14.fwSalI, HsPex14.rvNotI). The cDNA inserts were analysed by DNA sequencing (Agowa).
Mutation analysis of genomic DNA
The high Pure PCR Template Preparation Kit (Roche) was used to isolate genomic DNA from cultured fibroblasts. A nested PCR strategy was used to map the mutation in the PEX14 gene (see supplementary table 2). PCR products were analysed using standard agarose gel electrophoresis. The secondary structure outputs of the single stranded genomic DNA breakpoint region were generated by the Mfold web server (http://frontend.bioinfo.rpi.edu/applications/mfold/).19
Other methods
Bilirubin, transaminases, alkaline phosphatases, blood counts, chemistry and viral serology were performed at the central clinical laboratory of the Leuven University Hospital, using standard techniques. Plasma and urine amino acids were analysed on a Biochrom 20-plus amino acid analyser (Biochrom Ltd) using cation exchange chromatography with lithium citrate based buffers. Very long chain fatty acids, pristanic acid, and phytanic acid were measured by capillary GC/MS after methylation. Urinary organic acids were measured by GC/MS in selected ion mode after acidic extraction and derivatisation with N,O-bis(trimethylsilyl)trifluoroacetamide containing 1% of trimethylchlorosilane. Plasmalogen content of lipid extracts of red blood cells was deduced from GC-analysis of the fatty acid methyl esters or by quantifying the acid released fatty aldehydes as p-nitro-phenylhydrazones by RP-HPLC.20 For karyotyping, cultured white blood cells were treated with 10 μg/ml colcemid for 120 or 240 min before trypsinisation, treated hypotonically (0.75 M KCl) at 37°C and fixed with 3:1 methanol:acetic acid. G-banded metaphases were analysed and karyotyped.
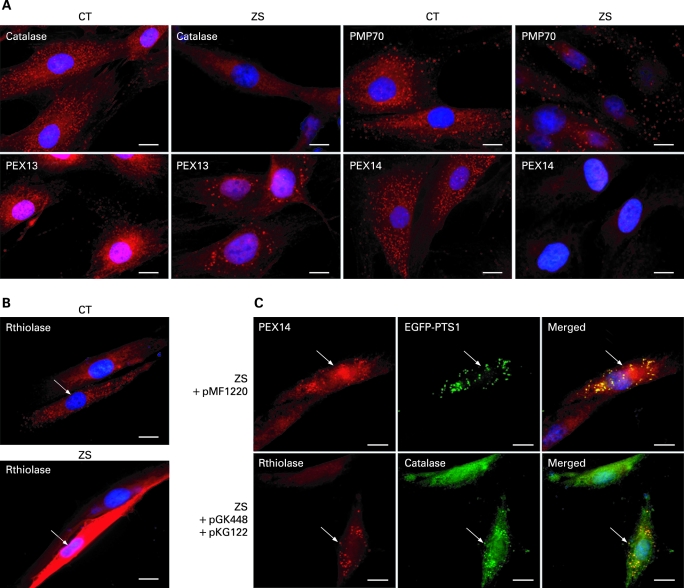
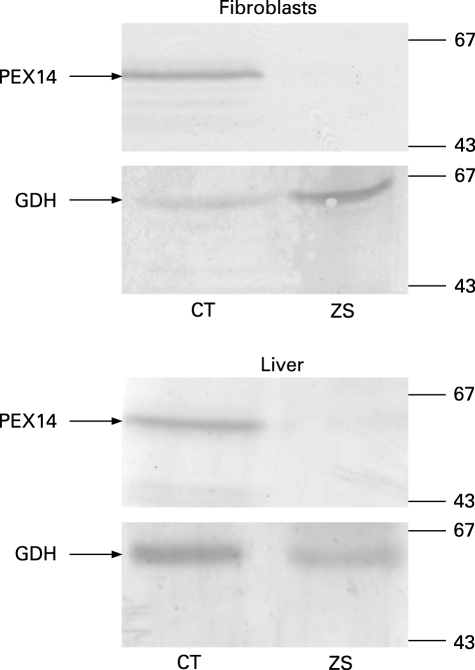
As the clinical phenotype, the biochemical testing and the liver ultrastructure of the patient were compatible with Zellweger syndrome, cultured skin fibroblasts were used to study peroxisome function and biogenesis. Functional measurements demonstrated impaired α-oxidation, defective β-oxidation of both 2-methyl branched long chain fatty acids and very long chain fatty acids (table 1), and a plasmalogen deficiency (data not shown). Immunofluorescence microscopy analysis revealed the absence of catalase- and thiolase-positive particles (fig 2A,B). On the other hand, the cells contained PMP70- and PEX13-positive vesicular membrane structures, the so-called “ghosts”, which appear somewhat larger in size and less abundant than normal peroxisomes (fig 2A). Interestingly, incubation of the cells with an antibody against the peroxisomal membrane protein PEX14 yielded negative staining results indicating that this PMP was absent (fig 2A), which was confirmed by immunoblot analysis of total lysates from liver and skin fibroblasts (fig 3). In addition, expression of wild-type PEX14 in these fibroblasts restored peroxisomal matrix protein import in virtually all transfected cells (fig 2C), while transfection with all other known human cDNAs encoding PEX proteins did not (data not shown).
Figure 2. Immunocytochemical localisation of peroxisomal proteins in patient and control fibroblasts.
(A) Control (CT) and Zellweger patient (ZS) fibroblasts were processed for immunostaining with antibodies specific for catalase, PEX13, PMP70, or PEX14 (red). (B) As we experienced difficulty detecting endogenous thiolase in human skin fibroblasts, the cells were transfected with a plasmid coding for rat thiolase B (rthiolase) 2 days before immunostaining with anti-thiolase B antibodies. (C) Cultured patient fibroblasts were (co)transfected with pMF1220, a bicistronic plasmid encoding non-tagged human PEX14 and EGFP-PTS1, or pGK448 and pKG122, two monocistronic plasmids encoding non-tagged human PEX14 and rat thiolase B, respectively; after 2 days, the cells were processed for fluorescence microscopy using rabbit anti-PEX14 antibodies (upper row) or rabbit anti-thiolase B (red) and mouse anti-catalase (green) antibodies (lower row); note that catalase and EGFP-PTS1, two PTS1-containing proteins, as well as thiolase B, a PTS2-containing protein, display a punctate staining pattern indicating the reconstitution of functional peroxisomes. The nuclei were counterstained with DAPI (blue), and transfected cells are marked by arrows. Scale bar: 20 μm.
Figure 3. Immunoblot analysis of PEX14 in patient and control fibroblasts.
Equal amounts of total protein extracted from cultured skin fibroblasts (two upper panels) or liver (two lower panels) from a control (CT) or the Zellweger patient (ZS) were subjected to SDS-PAGE and transferred to nitrocellulose. The membranes were then probed with antisera raised against PEX14 and GDH (loading control). The migration of relevant molecular mass markers (expressed in kDa) is indicated.
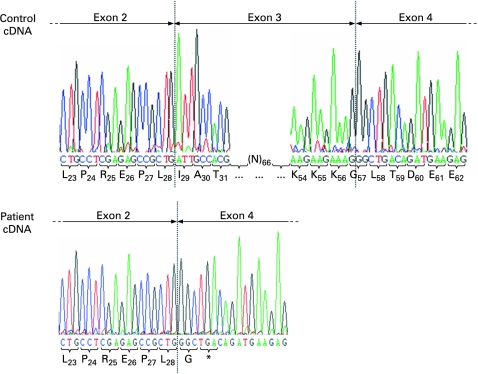
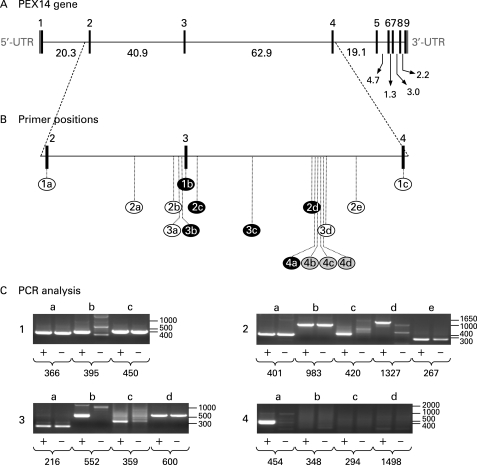
In order to identify the causative genetic defect in the patient, the coding sequence of PEX14 was amplified by RT-PCR using four distinct primer sets. Cloning and sequence analysis of the amplified sequences revealed that all cDNA fragments covering the 5′-end of the PEX14 mRNA transcript were lacking exon 3 (c.85_169del85) (fig 4). In the deduced protein sequence, the deletion of exon 3 (p.Ile29_Lys56del) causes a frameshift leading to a premature stop codon immediately downstream of exon 2 (p.Gly57GlyfsX2) (fig 4). To identify the underlying mutation at the genomic level, we used a nested PCR strategy (supplementary table 2, fig 5). By employing this approach, we detected a deletion of approximately 41 kilobase pairs containing exon 3 (fig 5B, C). Unfortunately, as we were not able to amplify a genomic PCR fragment spanning the putative breakpoint region, the precise breakpoint positions could not be identified. In this context, it should be noted that Mfold predictions for the single-stranded genomic DNA 3′-breakpoint region (fig 5B, sample series 4) pointed towards the presence of two strong stem-loop structures in the region covered by the PCR reactions 4b, 4c, and 4d (folding parameters: temperature =68°C; [Na+] =100 mM; [Mg2+] =1.3 mM) (data not shown). Also, as DNA samples from the parents were not available for further studies, we were unable to confirm that the same deletion was found in both parents in a heterozygous state.
Figure 4. Automated sequence chromatogram of the 5′- and 3′-flanking region of exon 3 from control and patient cDNA.
The deduced amino acids (one-letter code) are presented below the nucleotide sequences. The subscript numbers refer to the position of the amino acid in the wild-type protein. Note that a deletion of exon 3 in the patient’s cDNA results in a reading frame shift leading to a premature stop codon (*) in exon 4.
Figure 5. Mutation analysis of the PEX14 gene.
(A) Genomic organisation of the human PEX14 gene with schematic representations of the exon-intron structures (adapted from Krona et al12). Black boxes represent the coding regions, and grey boxes represent the 5′- and 3′-untranslated regions (UTRs) of the gene. The intron sizes are indicated in kilobase pairs. (B) Schematic representation of the nested PCR strategy. Each circled number represents a nested PCR reaction carried out with the primer pair combinations listed in supplementary table 2: black characters on a white background indicate that a specific PCR product was obtained from both the control and the Zellweger patient’s genomic DNA; white characters on a black background indicate that a specific PCR product could only be obtained from the control patient’s genomic DNA; and black characters on a grey background indicate that no specific PCR products could be obtained. (C) DNA fragment analysis of the nested PCR reactions listed in panel B (+, control; –, patient). The numbers under each lane refer to the expected size (in bp) of each PCR product. The molecular mass markers (in bp) are also indicated.
DISCUSSION
PEX14 functions as a central component of the peroxisomal matrix protein import machinery.10 So far, only one PEX14 mutation in exon 6 (c.553C>T; p.Q185X) has been characterised in Zellweger spectrum patients.6 The previously described patient died at 10 days of age, and clinical, morphological or radiological data were not reported.6 Here we report the identification and clinical presentation of a second Zellweger patient with PEX14-deficiency.
The first reported PEX14-deficient Zellweger patient was of Japanese origin; our patient was born to Pakistani parents. The diagnosis in both reported cases was based on clinical characteristics, increased very long chain fatty acids and low plasmalogen levels. In the first reported case, phytanic acid was normal. In our case, phytanic and pristanic acid remained normal until 14 months of age. Liver ultrastructure confirmed the absence of normal peroxisomes, and brain MRI showed polymicrogyria and laminar heterotopia.
At this moment, treatment for patients suffering from PBDs is mainly supportive. In addition, therapeutic interventions mostly target individual biochemical defects, and their effects on clinical outcome have not yet been proven.4 The patient we describe was treated with oral administration of bile acids and a phytanic/pristanic acid-low diet. Bile acid supplements have been reported to improve hepatobiliary function in infants with Zellweger syndrome.21,22 In our patient, normalisation of cholestasis was achieved and hepatomegaly regressed, but neurological function deteriorated significantly during follow-up.
Mutation analysis of the PEX14 gene revealed a homozygous deletion causing an out-frame skipping of exon 3 in the mRNA transcript. The peptide encoded by exon 3, corresponding to the amino acid residues 29 to 56 of the full length protein, is located in the most conserved region of PEX14.10 This region is known to be essential for binding to PEX5, PEX13, and PEX1923–27 and has also been shown to be part of the topogenic sequence.26,27 Note also that two PEX14-deficient Chinese hamster ovary (CHO) cell lines have been identified.28,29 In these cells, the PEX14 mRNA transcripts contained frameshift deletions of the exons 2 and 4, respectively.8,28 In addition, it has been shown that the minimal functional region of PEX14 required for restoring impaired matrix protein import in these cell mutants lies between amino acid residues 21 and 260.27 In our patient, the deletion of exon 3 led to a deficiency of PEX14 and the absence of normal peroxisomes from cultured skin fibroblasts, supporting the data mentioned above. These abnormalities could be entirely reversed by transfection of wild-type PEX14 in the fibroblasts. We therefore consider it proven that the described mutation is causative of the PBD and the described clinical picture.
The fact that the first reported patient died at 10 days of age, while our patient is currently surviving beyond 21 months, with both patients demonstrating a total absence of functional PEX14, is difficult to explain. The exact cause of death of the first reported patient is not documented, but theoretically genetic modifiers, degree of brain malformation or liver failure and access to supportive treatment could be invoked to explain the difference in survival. In any case, the fact that the clinical picture is not necessarily as severe as first reported is noteworthy, although variability of the clinical picture and survival are not surprising in Zellweger spectrum patients.
In conclusion, we report that a novel deletional frameshift mutation in exon 3 of PEX14 is associated with Zellweger syndrome, comprising severe but regressing cholestasis and hepatomegaly, radiological indications of a neuronal migration defect and cortical developmental anomaly, and rapidly progressive hypotonia.
LEARNING POINTS
We report the detailed clinical presentation and follow-up of a patient with the cerebro-hepato-renal syndrome of Zellweger.
Mutation analysis revealed a novel deletional frameshift mutation in exon 3 of PEX14, a gene encoding a central component of the peroxisomal matrix protein import machinery.
As this patient represents the second case of Zellweger syndrome caused by a defect in PEX14, the study of this patient contributes to a better understanding of the clinical picture associated with PEX14 gene mutations.
Acknowledgments
This article has been adapted with permission from Huybrechts SJ, Van Veldhoven PP, Hoffman I, Zeevaert R, de Vos R, Demaerel P, Brams M, Jaeken J, Fransen M, Cassiman D. Identification of a novel PEX14 mutation in Zellweger syndrome. J Med Genet 2008;45:376–83.
Footnotes
Competing interests: none.
REFERENCES
- 1.Mannaerts GP, Van Veldhoven PP, Casteels M. Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals. Cell Biochem Biophys 2003; 32: 73–87 [DOI] [PubMed] [Google Scholar]
- 2.Wanders RJ, Waterham HR. Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 2006; 1763: 1707–20 [DOI] [PubMed] [Google Scholar]
- 3.Shimozawa N. Molecular and clinical aspects of peroxisomal diseases. J Inherit Metab Dis 2007; 30: 193–7 [DOI] [PubMed] [Google Scholar]
- 4.Steinberg SJ, Dodt G, Raymond GV, et al. Peroxisome biogenesis disorders. Biochim Biophys Acta 2006; 1763: 1733–48 [DOI] [PubMed] [Google Scholar]
- 5.Goldfischer S, Moore CL, Johnson AB, et al. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 1973; 182: 62–4 [DOI] [PubMed] [Google Scholar]
- 6.Shimozawa N, Tsukamoto T, Nagase T, et al. Identification of a new complementation group of the peroxisome biogenesis disorders and PEX14 as the mutated gene. Hum Mutat 2004; 23: 552–8 [DOI] [PubMed] [Google Scholar]
- 7.Fransen M, Terlecky SR, Subramani S. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc Natl Acad Sci USA 1998; 95: 8087–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu N, Itoh R, Hirono Y, et al. The peroxin Pex14p. cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization, and functional analysis. J Biol Chem 1999; 274: 12593–604 [DOI] [PubMed] [Google Scholar]
- 9.Will GK, Soukupova M, Hong X, et al. Identification and characterization of the human orthologue of yeast Pex14p. Mol Cell Biol 1999; 19: 2265–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo JE, Schliebs W. Pex14p, more than just a docking protein. Biochim Biophys Acta 2006; 1763: 1574–84 [DOI] [PubMed] [Google Scholar]
- 11.Gavva NR, Wen SC, Daftari P, et al. NAPP2, a peroxisomal membrane protein, is also a transcriptional corepressor. Genomics 2002; 79: 423–31 [DOI] [PubMed] [Google Scholar]
- 12.Krona C, Ejeskär K, Abel F, et al. Screening for gene mutations in a 500 kb neuroblastoma tumor suppressor candidate region in chromosome 1p; mutation and stage-specific expression in UBE4B/UFD2. Oncogene 2003; 17: 2343–51 [DOI] [PubMed] [Google Scholar]
- 13.Van Veldhoven PP, Huang S, Eyssen HJ, et al. The deficient degradation of synthetic 2- and 3-methyl-branched fatty acids in fibroblasts from patients with peroxisomal disorders. J Inherited Metab Dis 1993; 16: 381–91 [DOI] [PubMed] [Google Scholar]
- 14.Casteels M, Croes K, Van Veldhoven PP, et al. Aminotriazole is a potent inhibitor of α-oxidation of 3-methyl-substituted fatty acids in rat liver. Biochem Pharmacol 1994; 48: 1973–5 [DOI] [PubMed] [Google Scholar]
- 15.Fransen M, Wylin T, Brees C, et al. Human Pex19p binds peroxisomal integral membrane proteins at regions distinct from their sorting sequences. Mol Cell Biol 2001; 21: 4413–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fransen M, Van Veldhoven PP, Subramani S. Identification of peroxisomal proteins by using M13 phage protein VI phage display: molecular evidence that mammalian peroxisomes contain a 2,4-dienoyl-CoA reductase. Biochem J 1999; 340: 561–8 [PMC free article] [PubMed] [Google Scholar]
- 17.Antonenkov VD, Croes K, Waelkens E, et al. Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes. Eur J Biochem 2000; 267: 2981–90 [DOI] [PubMed] [Google Scholar]
- 18.Ghys K, Fransen M, Mannaerts GP, et al. Functional studies on human Pex7p: subcellular localization and interaction with proteins containing a peroxisome-targeting signal type 2 and other peroxins. Biochem J 2002; 365: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulon V, Sniekers M, Huysmans E, et al. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the α-oxidation of straight chain fatty acids. J Biol Chem 2005; 280: 9802–12 [DOI] [PubMed] [Google Scholar]
- 21.Setchell KD, Bragetti P, Zimmer-Nechemias L, et al. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology 1992; 15: 198–207 [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Kimura A, Yamato Y, et al. Oral bile acid treatment in two Japanese patients with Zellweger syndrome. J Pediatr Gastroenterol Nutr 2002; 35: 227–30 [DOI] [PubMed] [Google Scholar]
- 23.Schliebs W, Saidowsky J, Agianian B, et al. Recombinant human peroxisomal targeting signal receptor PEX5. Structural basis for interaction of PEX5 with PEX14. J Biol Chem 1999; 274: 5666–73 [DOI] [PubMed] [Google Scholar]
- 24.Sacksteder KA, Jones JM, South ST, et al. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol 2000; 148: 931–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen M, Brees C, Ghys K, et al. Analysis of mammalian peroxin interactions using a non-transcription-based bacterial two-hybrid assay. Mol Cell Proteomics 2002; 1: 243–52 [DOI] [PubMed] [Google Scholar]
- 26.Fransen M, Vastiau I, Brees C, et al. Potential role for Pex19p in assembly of PTS-receptor docking complexes. J Biol Chem 2004; 279: 615–24 [DOI] [PubMed] [Google Scholar]
- 27.Itoh R, Fujiki Y. Functional domains and dynamic assembly of the peroxin Pex14p, the entry site of matrix proteins. J Biol Chem 2006; 281: 10196–205 [DOI] [PubMed] [Google Scholar]
- 28.Tateishi K, Okumoto K, Shimozawa N, et al. Newly identified Chinese hamster ovary cell mutants defective in peroxisome biogenesis represent two novel complementation groups in mammals. Eur J Cell Biol 1997; 73: 352–9 [PubMed] [Google Scholar]
- 29.Fujiki Y, Okumoto K, Kinoshita N, et al. Lessons from peroxisome-deficient Chinese hamster ovary (CHO) cell mutants. Biochim Biophys Acta 2006; 1763: 1374–81 [DOI] [PubMed] [Google Scholar]