Abstract
This study reports the cardiac structure and function of a lifelong male endurance athlete, who has run over 148 000 miles, who presented with symptoms of chest discomfort, dyspnoea and loss of competitive running performance. Importantly, the athlete documented several periods of regular intensive endurance activity while suffering with flu-like symptoms. Cardiovascular MRI demonstrated a pattern of late gadolinium enhancement, which indicated myocardial scarring as a result of previous myocarditis.
Myocarditis is a non-ischaemic inflammatory disease of the myocardium associated with cardiac dysfunction and arrhythmogenic substrate. The clinical course of viral myocarditis is mostly insidious with limited cardiac inflammation and dysfunction. However, as in the present case, overwhelming inflammation may occur in a subset of patients leading to myocardial fibrosis due to recurrent inflammation.
BACKGROUND
This case study is an important clinical reminder for all medical staff dealing with competitive and recreational athletes suffering with viral or bacterial infections. Myocarditis should be suspected in athletes with unexplained cardiac arrhythmias and dysfunction, especially if preceded by flu-like symptoms.1,2 An early diagnosis is desirable in order to avoid the risk of a deleterious, possibly fatal, outcome, since physical activity can enhance the inflammatory process leading to myocardial fibrosis. This study offers advice, guidance and supporting literature to the medical community in dealing with this problem.
CASE PRESENTATION
A 68-year-old male veteran runner attended the CRY Centre for Sports Cardiology to investigate symptoms of chest discomfort, dyspnoea and loss of competitive running performance. The patient was a former Olympian competing in the 10 000 m and marathon. Since international retirement in the mid-seventies, the patient has run 115 marathons of which 103 were completed in under 2 h 45 mins. Of note, the patient has kept an accurate daily running diary documenting 43 years of continuous running. To date, the patient has not missed a single day of running (minimum distance of 2 miles) since 1964 and has documented running a total distance of 148 561 miles.
Presenting features
The patient reports a first presentation of cardiac symptoms in November 1986 with heart/chest discomfort that over time evolved to pain down the left arm while exercising. The patient did not seek medical attention as it was a one-off event. In October 1988, the patient first documented a short lived “chest flutter” that had a significant impact upon running performance.
In 2003, the patient collapsed twice. The initial episode occurred immediately following a summer race associated with extreme exertion and dehydration. The second collapse occurred following a very poor running performance at the end of the year, in which a nurse was unable to record a blood pressure. On both occasions the patient refused to seek medical attention.
Finally, throughout the 2006 running season, the patient documented an usual and sustained tachycardia (ca 190 bpm) during racing documented by a personalised signal Polar heart rate monitor, together with chest tightness and episodes of dyspnoea (particularly with a fast starting pace). On questioning, the patient reported several periods of sustained intensive exercise while suffering with flu-like symptoms. This was in order to maintain the patient’s record of 43 years of continuous daily running. There is no family history of note, including sudden cardiac death. The only cardiovascular examination on record was an electrocardiogram (ECG) following a road traffic accident in which the patient broke their sternum in 1993. The ECG was verbally reported as “normal”.
INVESTIGATIONS
Resting 12-lead ECG demonstrated sinus rhythm of 65 beats.min−1 and was otherwise entirely normal. The patient underwent an integrated maximal cardio-pulmonary exercise bicycle stress test. The patient attained a maximal measured heart rate of 160 bpm (152% of age predicted) and a O2max of 48.0ml.kg−1.min−1 which equated to 174% of age predicted maximum. There were no inducible arrhythmias during or post-exercise and no ST segment changes indicative of myocardial ischaemia. Blood pressure and heart rate response to exercise was normal throughout. Post-exercise blood pressure response was entirely normal; however, the patient demonstrated a high vagal tone with a very rapid reduction in heart rate to 68 beats.min−1 within 1 minute of cessation of exercise.
The patient underwent 2D, Doppler, Tissue Doppler and 2D strain echocardiography. Left ventricle (LV) ejection fraction (55%) was normal with normal longitudinal function. LV relaxation was impaired. All heart valves were entirely normal with normal colour flow. The patient had borderline left ventricular hypertrophy (LV posterior wall thickness of 12 mm) with a normal right ventricle (RV).
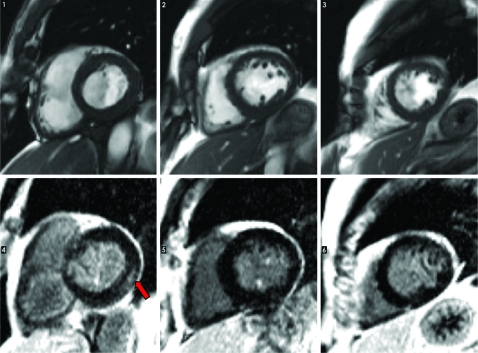
Cardiovascular magnetic resonance imaging (CMR) demonstrated no regional wall motion abnormality together with normal RV and LV wall thickness. A pattern of late gadolinium enhancement (LGE) indicated myocardial scarring in the basal, lateral wall as a result of previous myocarditis (fig 1).
Figure 1.
SA cine stack on the top. Late gadolinium enhancement images on the bottom row (arrow indicates mid-wall enhancement indicative of myocarditic scarring).
DIFFERENTIAL DIAGNOSIS
Myocarditis was the only differential diagnosis based on LGE.
DISCUSSION
Acute myocarditis is typically a viral or post-viral process, which may result in the acute onset of left ventricular systolic dysfunction. It can range from mild and clinically undetectable to fulfilment and fatal over a short time course.1 Clinically, patients with acute viral myocarditis present with tachycardia, hypotension and shortness of breath; all of which were presented twice by this patient in 2003 and 2006. The clinical course of myocarditis is highly variable with complete or near complete resolution occurring in a few weeks. The majority will experience some degree of recovery of function but are often left with a degree of left ventricular dysfunction.
Gadolinium-enhanced CMR provides a sensitive tool for detection of myocardial fibrosis, which is distinguished by bright late-enhancement regions where the contrast lingers in the extracellular spaces of scarred myocardium. Even when inflammatory changes of the myocardium are asymptomatic, as in most cases, myocardial fibrosis and dilated cardiomyopathy can occur resulting in heart failure.2 Although several diagnostic tools can be useful for the diagnosis of myocarditis, endomyocardial biopsy remains the gold standard.3 However, this case study demonstrates the usefulness of CMR in the identification of myocardial scarring.
Recently, our group has observed idiopathic interstitial myocardial fibrosis at post-mortem in the hearts of athletes that died suddenly during marathon running.4 The presence of idiopathic interstitial fibrosis could act as a pathological substrate in the development of fatal arrhythmias resulting in sudden cardiac death. Limited evidence reporting idiopathic fibrosis exists in the literature likely due to the absent histological examination of the hearts of veteran athletes’ post-mortem.5 Late gadolinium enhancement with CMR can accurately identify regions of myocardial fibrosis.6 The causes of interstitial fibrosis are not well understood; however, variable and dense interstitial fibrosis are observed in dilated cardiomyopathy,7 non-infarcted myocardium from hearts with ischaemic scars,8 dilated non-ischaemic myocardium9 and systemic hypertension.10 An increased collagen content following sirius red FB3 staining of the myocardium is also observed in the presence of inflammatory and amyloid cells and as a result of myocarditis.
Myocarditis should be suspected in athletes with unexplained cardiac arrhythmias and dysfunction especially if preceded by a flu-like syndrome. An early diagnosis is desirable in order to avoid the risk of fatal consequences since physical activity can enhance the inflammatory process.3 In patients with acute or chronic myocarditis, arrhythmia may be the only clinical symptom in the natural course of the disease. Factors responsible for the increased incidence of cardiac arrhythmias include structural changes, ventricular haemodynamics and vascular changes. The potentially malignant tachyarrhythmias and bradyarrhythmias caused by myocarditis are of particular concern. Acutely, inflammatory processes in the cardiac myocytes and interstitium can lead directly to fluctuations in membrane potential hence arrythmogenesis.3
Treatment is often difficult for highly competitive athletes to comprehend as initial treatment for athletes with myocarditis should be complete absence from all physical activity for at least 6 months. Athletes should only resume training when ventricular function and cardiac dimensions return to normal and the clinically relevant arrhythmias disappear.2,3 Adherence to such guidelines should be strongly advocated to reduce the potential of life-threatening arrhythmias or rapidly progressive cardiac dysfunction and the introduction of antiviral or an immunosuppressive treatment.2
LEARNING POINTS
The advice should be very clear: do not exercise while suffering from a viral or bacterial infection to avoid the potential for myocarditis and ensure that all symptoms have gone before re-engaging in low to moderate intensity physical activity.
Myocarditis can be life threatening and requires extensive recovery both of which are deleterious to athletic performance.
The potential for pathological cardiac remodelling as a result of a continued elevation in cardiac mechanical load associated with high-intensity exercise may result in dilated cardiomyopathy and congestive heart failure that may lead to a lifelong reduction in morbidity and an increased mortality risk.
Acknowledgments
The authors wish to express their thanks to Cardiac Risk in the Young and the British Olympic Association for their continued support towards the CRY Centre for Sports Cardiology.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Feigenbaum Feigenbaum's Echocardiography. 6th edition Lippincott Williams & Wilkins; 2004 [Google Scholar]
- 2.Babu-Narayan SV, McCarthy KP, Ho SY, et al. Images in cardiovascular medicine. Myocarditis and sudden cardiac death in the young: extensive fibrosis suggested by cardiovascular magnetic resonance in vivo and confirmed post mortem. Circulation 2007; 116: e122–5 [DOI] [PubMed] [Google Scholar]
- 3.Chimenti C, Pieroni M, Frustaci A: Myocarditis: when to suspect and how to diagnose it in athletes. J Cardiovasc Med (Hagerstown) 2006;7:301–6 [DOI] [PubMed] [Google Scholar]
- 4.Whyte G, Sheppard M, George K, et al. Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: is exercise the cause? Br J Sports Med 2008;42:304–5 [DOI] [PubMed] [Google Scholar]
- 5.Whyte GP, Sheppard M, George KP, et al. Arrhythmias and the athlete: mechanisms and clinical significance. Eur Heart J 2007; 28: 1399–401; author reply 401 [DOI] [PubMed] [Google Scholar]
- 6.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003; 108: 54–9 [DOI] [PubMed] [Google Scholar]
- 7.Marijianowski MM, Teeling P, Mann J, et al. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol 1995; 25: 1263–72 [DOI] [PubMed] [Google Scholar]
- 8.Volders PG, Willems IE, Cleutjens JP, et al. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol 1993; 25: 1317–23 [DOI] [PubMed] [Google Scholar]
- 9.Brooks A, Schinde V, Bateman AC, et al. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart 2003; 89: 1255–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardo Mindan FJ, Panizo A. Alterations in the extracellular matrix of the myocardium in essential hypertension. Eur Heart J 1993; 14: 12–4 [PubMed] [Google Scholar]