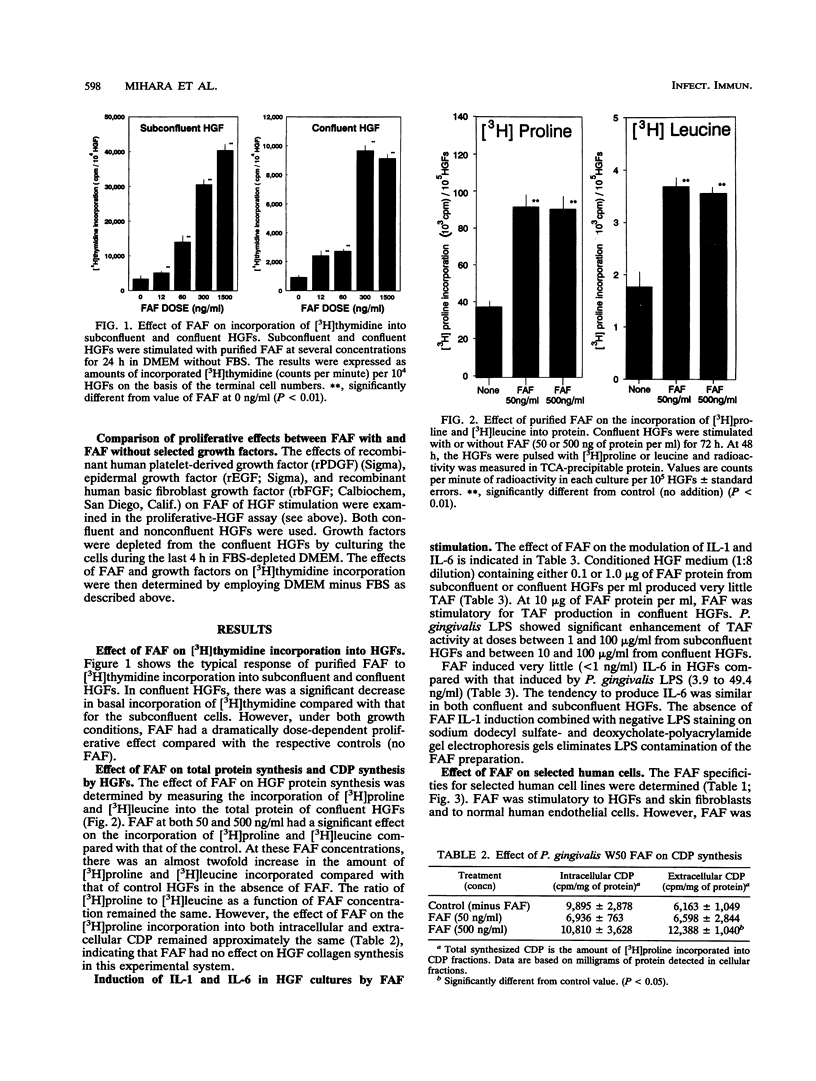
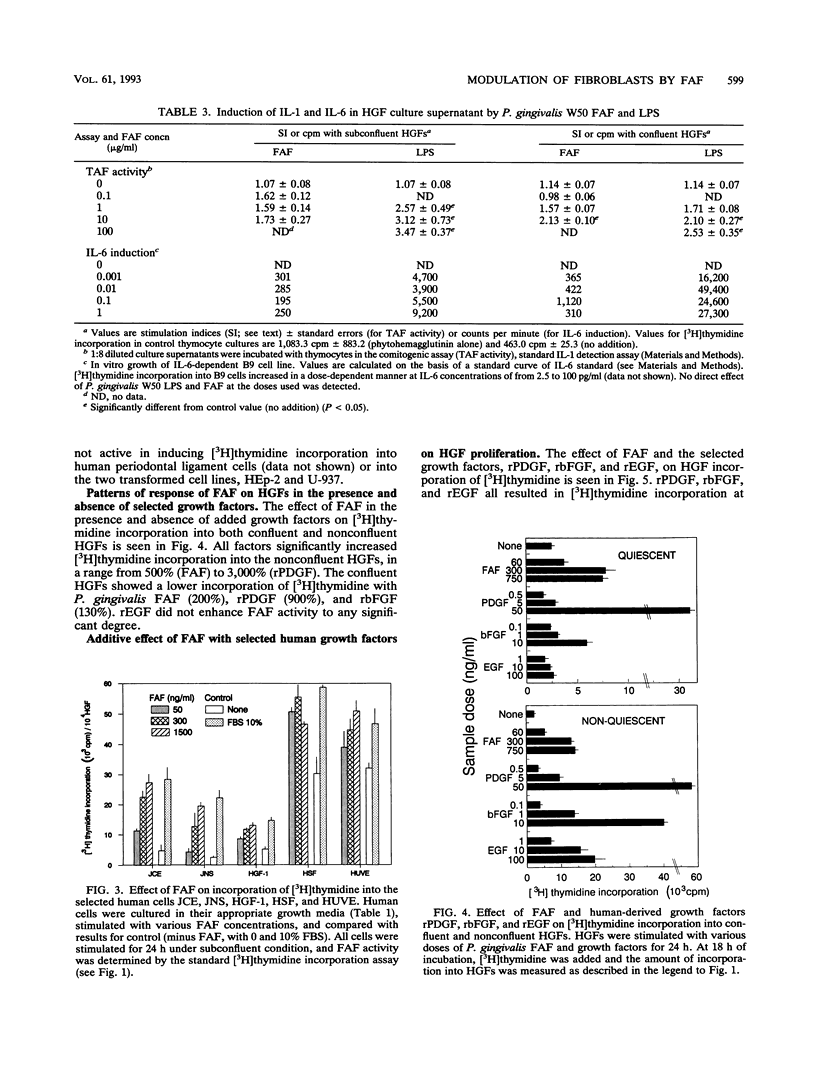
Abstract
The effect of a 24-kDa fibroblast-activating factor (FAF) isolated from outer membrane vesicles of Porphyromonas gingivalis W50 on the modulation of [3H]thymidine uptake and cell proliferation was examined in selected fibroblast and transformed cell lines. FAF enhanced the proliferation of human gingival fibroblasts, human skin fibroblasts, and human umbilical vein endothelial cells in subconfluent and confluent cells, suggesting that FAF might be functioning as a competence factor. The transformed cell lines, U-937 and HEp-2, were not responsive. FAF and several human-derived growth factors showed a synergistic effect on proliferation. [3H]proline and [3H]leucine were rapidly incorporated into fibroblasts in the presence of FAF; however, there was no selective induction of collagen synthesis. FAF was not active in the induction of interleukin-1 and interleukin-6. It is hypothesized that FAF from P. gingivalis functions as a growth factor for human fibroblasts but is without activity for transformed cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Robbins P. W. Effect of proteases on activation of resting chick embryo fibroblasts and on cell surface proteins. Cell. 1975 Oct;6(2):137–147. doi: 10.1016/0092-8674(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kudlow J. E., Lazar C. S., Gill G. N. Altered epidermal growth factor (EGF)-stimulated protein kinase activity in variant A431 cells with altered growth responses to EGF. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2574–2578. doi: 10.1073/pnas.79.8.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C. B., Proper J. A., Tucker R. F., Moses H. L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. COLLAGEN AND CELL PROTEIN SYNTHESIS BY AN ESTABLISHED MAMMALIAN FIBROBLAST LINE. Nature. 1964 Oct 24;204:347–349. doi: 10.1038/204347a0. [DOI] [PubMed] [Google Scholar]
- GROSSFELD H., MEYER K., GODMAN G. Differentiation of fibroblasts in tissue culture, as determined by mucopolysaccharide production. Proc Soc Exp Biol Med. 1955 Jan;88(1):31–35. doi: 10.3181/00379727-88-21484. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Rudland P., Lindstrom J., Benirschke K. Fibroblast growth factor: its localization, purification, mode of action, and physiological significance. Adv Metab Disord. 1975;8:301–335. doi: 10.1016/b978-0-12-027308-9.50026-3. [DOI] [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S., Posner B. I., Guyda H. Decreased sensitivity of old and progeric human fibroblasts to a preparation of factors with insulinlike activity. J Clin Invest. 1981 Oct;68(4):988–994. doi: 10.1172/JCI110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Hunkapiller M. W., Korant B. D., Hardy R. W., Hood L. E. Human fibroblast interferon: amino acid analysis and amino terminal amino acid sequence. Science. 1980 Feb 1;207(4430):525–526. doi: 10.1126/science.7352259. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Fiers W., Pober J. S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987 Oct 1;139(7):2317–2324. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Le J. M., Weinstein D., Gubler U., Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987 Apr 1;138(7):2137–2142. [PubMed] [Google Scholar]
- Mihara J., Holt S. C. Purification and characterization of fibroblast-activating factor isolated from Porphyromonas gingivalis W50. Infect Immun. 1993 Feb;61(2):588–595. doi: 10.1128/iai.61.2.588-595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Pledger W. J. A model of cell cycle control: sequential events regulated by growth factors. Mol Cell Endocrinol. 1983 Aug;31(2-3):167–186. doi: 10.1016/0303-7207(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P. Proteases stimulate proliferation of human fibroblasts. J Cell Physiol. 1977 Jun;91(3):387–392. doi: 10.1002/jcp.1040910308. [DOI] [PubMed] [Google Scholar]
- RIESER P., RIESER C. H. ANABOLIC RESPONSES OF DIAPHRAGM MUSCLE TO INSULIN AND TO OTHER PANCREATIC PROTEINS. Proc Soc Exp Biol Med. 1964 Jul;116:669–671. doi: 10.3181/00379727-116-29339. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Sugamura K., Nakai S., Fujii M., Hinuma Y. Interleukin 2 inhibits in vitro growth of human T cell lines carrying retrovirus. J Exp Med. 1985 May 1;161(5):1243–1248. doi: 10.1084/jem.161.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Mihara J., Morisaki I., Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991 Jan;59(1):295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally M., Li C. H., Hall K. IGF-2 stimulated growth mediated by the somatomedin type 2 receptor. Biochem Biophys Res Commun. 1987 Oct 29;148(2):811–816. doi: 10.1016/0006-291x(87)90948-x. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]



