Abstract
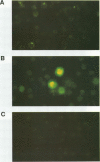
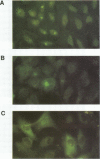
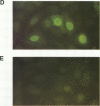
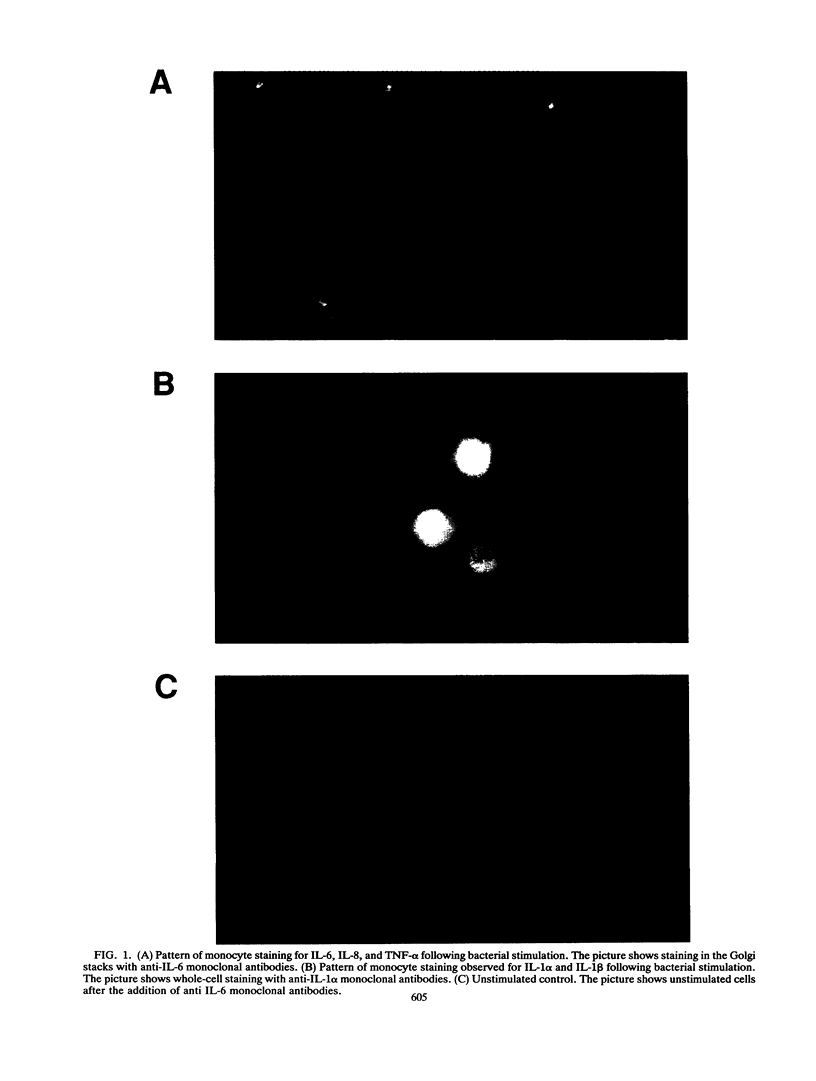
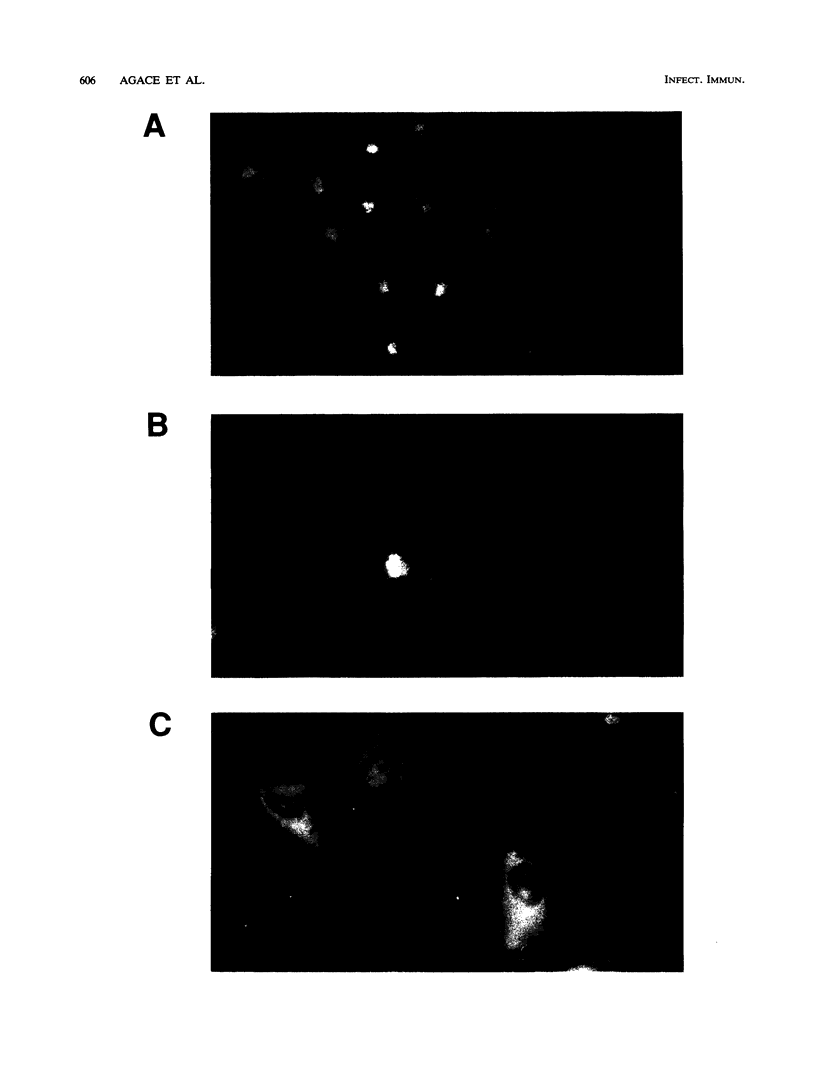
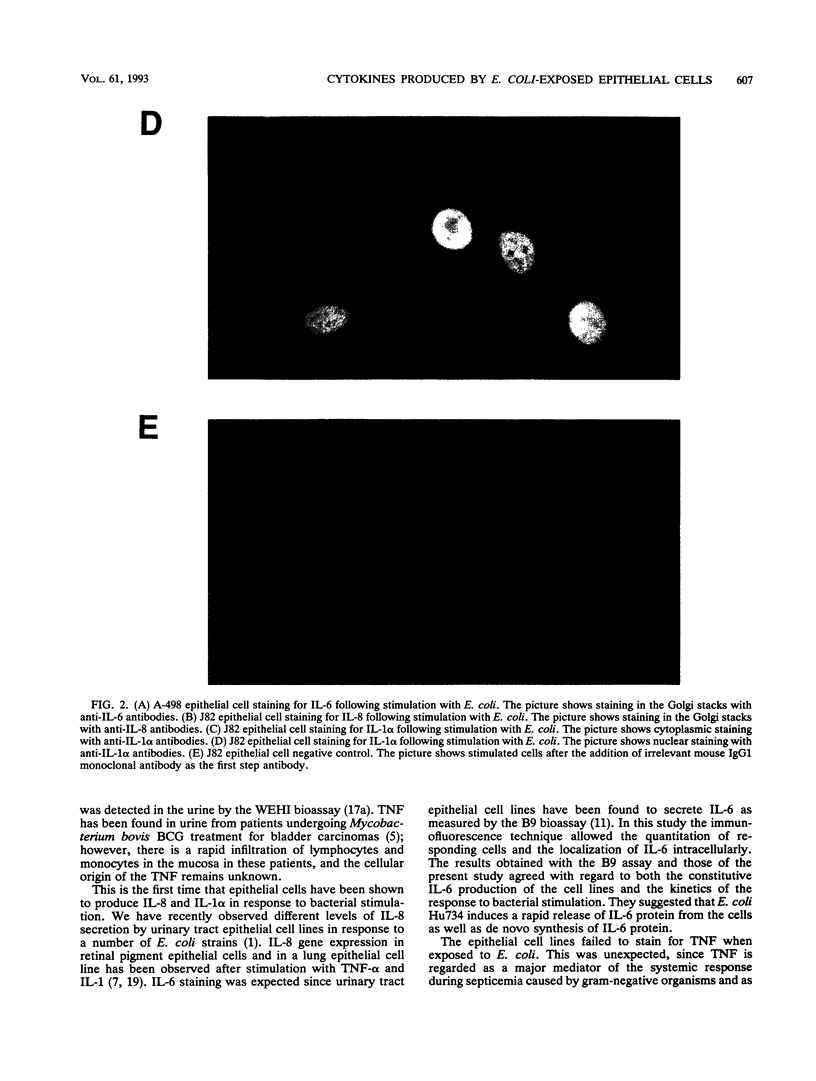
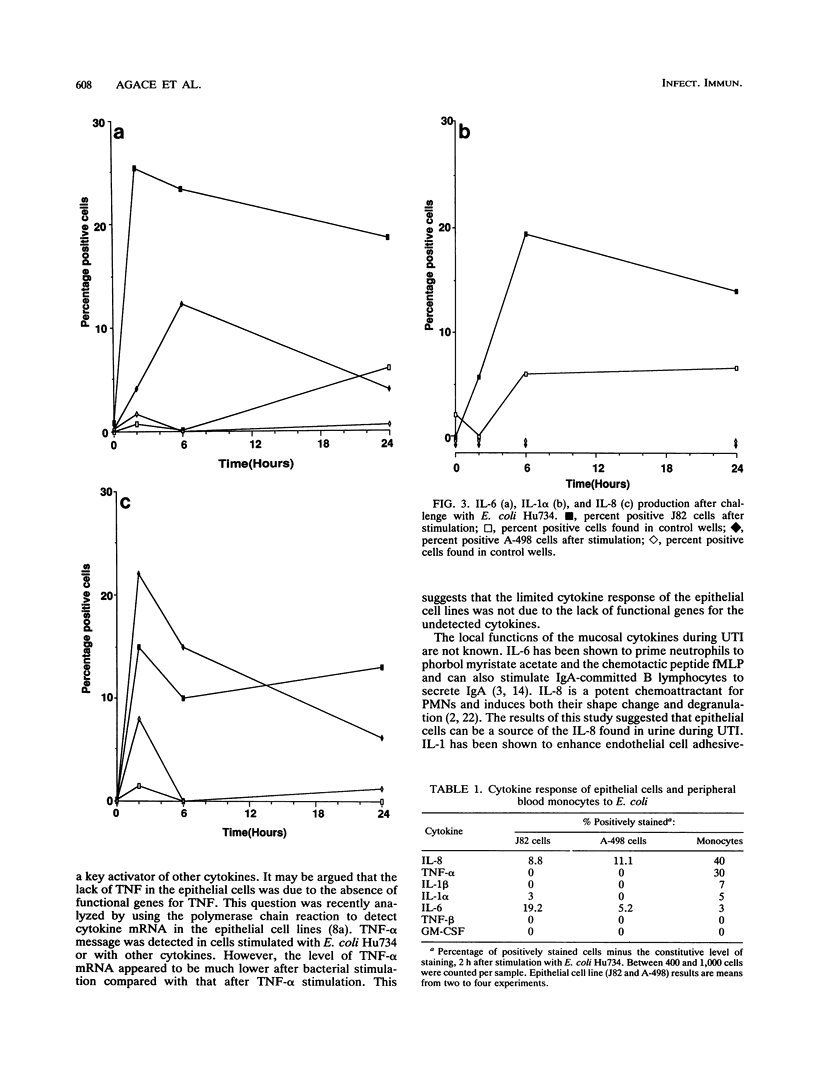
This study compared the repertoire of cytokines produced by epithelial cell lines and human peripheral blood monocytes in response to Escherichia coli. The A-498 and J82 urinary tract epithelial cell lines and human peripheral blood monocytes were exposed to E. coli Hu734. The cytokine content of single cells was detected by indirect immunofluorescence using monoclonal antibodies to interleukin-1 alpha (IL-1 alpha), IL-1 beta, tumor necrosis factor alpha (TNF-alpha), TNF-beta, IL-6, IL-8, and granulocyte macrophage-colony-stimulating factor, and the number of positive cells was used to quantitate the response. The J82 bladder cell line stained positive for IL-6, IL-8, and IL-1 alpha. The IL-8 and IL-6 response peaked at 2 h, while the number of IL-1 alpha-positive cells reached a peak 6 h after E. coli stimulation. The A-498 kidney cell line stained for IL-8 with a peak at 2 h and IL-6 with a peak at 6 h after E. coli stimulation. Peripheral blood monocytes stained for the cytokines IL-1 alpha, IL-1 beta, IL-8, IL-6, and TNF-alpha but not for TNF-beta and granulocyte macrophage-colony-stimulating factor after stimulation with E. coli. The results demonstrated that bacteria activated a cytokine response in the epithelial cell lines and monocytes. The epithelial cell lines had a more limited cytokine response profile than circulating monocytes, which may serve to limit the consequences of microbial exposure at the mucosal surface and help maintain the integrity of other tissue compartments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992 Jun;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley K. W., Eldridge J. H., Lee F., Kiyono H., Everson M. P., Koopman W. J., Hirano T., Kishimoto T., McGhee J. R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989 Jun 1;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhle A., Nowc C., Ulmer A. J., Musehold J., Gerdes J., Hofstetter A. G., Flad H. D. Detection of urinary TNF, IL 1, and IL 2 after local BCG immunotherapy for bladder carcinoma. Cytokine. 1990 May;2(3):175–181. doi: 10.1016/1043-4666(90)90013-j. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Nandoskar M., Walz A., Goh D. H., Kowanko I. C. Effects of tumour necrosis factor alpha and interleukin-1 alpha and beta on human neutrophil migration, respiratory burst and degranulation. Int Arch Allergy Appl Immunol. 1988;86(1):82–91. doi: 10.1159/000234610. [DOI] [PubMed] [Google Scholar]
- Hedges S., Anderson P., Lidin-Janson G., de Man P., Svanborg C. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect Immun. 1991 Jan;59(1):421–427. doi: 10.1128/iai.59.1.421-427.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S., Stenqvist K., Lidin-Janson G., Martinell J., Sandberg T., Svanborg C. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J Infect Dis. 1992 Sep;166(3):653–656. doi: 10.1093/infdis/166.3.653. [DOI] [PubMed] [Google Scholar]
- Hedges S., Svensson M., Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992 Apr;60(4):1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H., Takahashi C., Kanagawa K., Hirata H., Imai K., Yoshiki T. Cytokine regulation of ICAM-1 expression on human renal tubular epithelial cells in vitro. Transplantation. 1991 Jun;51(6):1272–1275. doi: 10.1097/00007890-199106000-00024. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Rechnitzer C., Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989 May;21(2):177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Linder H., Engberg I., van Kooten C., de Man P., Svanborg-Edén C. Effects of anti-inflammatory agents on mucosal inflammation induced by infection with gram-negative bacteria. Infect Immun. 1990 Jul;58(7):2056–2060. doi: 10.1128/iai.58.7.2056-2060.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Libby P., Freudenberg M., Krauss J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990 Nov;58(11):3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Mihara J., Morisaki I., Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991 Jan;59(1):295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems J., Joniau M., Cinque S., van Damme J. Human granulocyte chemotactic peptide (IL-8) as a specific neutrophil degranulator: comparison with other monokines. Immunology. 1989 Aug;67(4):540–542. [PMC free article] [PubMed] [Google Scholar]
- de Man P., van Kooten C., Aarden L., Engberg I., Linder H., Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989 Nov;57(11):3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]